Experience with a simplified feeding jejunostomy technique for enteral nutrition following major visceral operations
Introduction
Nutritional deficits, commonly encountered in patients with gastrointestinal cancers, increase the risk for postoperative morbidity and are among those parameters that can be manipulated to achieve superior postoperative outcomes in complex surgical patients (1). The potential value of nutritional repletion in patients undergoing major operative procedures in order to minimize operative complications has been evaluated in numerous studies. Generally, a value of enteral nutrition support in this setting has been primarily demonstrated through preoperative or perioperative nutrition efforts where feasible (2,3), while there is less evidence for benefits from routine postoperative nutrition support (4-7). However, postoperative enteral nutrition may still be valuable in settings of major complications, benefit earlier discharge, lead to shortened recovery or improve the ability to undergo postoperative therapy (8,9). Any possible postoperative benefits of feeding tubes must be weighed against the morbidity related to their placement and subsequent use, both of which depend on several other factors. For instance, the literature is unclear on whether placement of prophylactic feeding tubes at the time of resection by itself does or does not contribute to increased postoperative morbidity (10,11). The decision to place a feeding tube at the time of major upper gastrointestinal or hepato-pancreato-biliary resection should thus be guided by the nutritional risk of the patient, the likelihood of major postoperative morbidity with nutritional support needs, the safety and utility of the feeding access device chosen, the availability of alternative, endoscopically placed postoperative feeding access if needed and the possible utility or value of parenteral nutrition support. A previously described simplified technique of jejunostomy tube (JT) placement that is thought to be characterized by technical ease, minimal additional operating time, maximal safety and minimal device-related morbidity is now evaluated for circumstances of clinical use and related outcomes (12).
Methods
Feeding jejunostomy technique
The technique was originally described in 2002, but a few minor amendments have been instituted since that time (12). Briefly, the key steps can be summarized as follows:
- A 16-Fr red rubber catheter is inserted through the abdominal wall in a lateral location in order to minimize the risk for any possible small bowel volvulus around the tube;
- Tunneling of the catheter through the abdominal wall musculature is directed in an oblique direction towards the pelvis to lengthen the ensuing tunnel within the abdominal wall soft tissue, and to facilitate any necessary tube replacement;
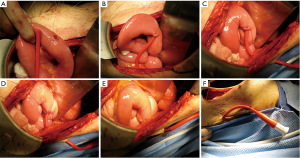
- A triangular seromuscular purse-string suture using 3-0 Vicryl or PDS on the antimesenteric jejunal border is placed approximately 10 centimeters distal to the lowest jejunal anastomosis (Figure 1A). The tip of the catheter is cut off to avoid any premature occlusion of side hole openings and to maximize the effect of flushes;
- The catheter is inserted into the jejunal lumen, and the purse-string suture is tied (Figure 1B). Injection of saline solution through the catheter eases its advancement into the bowel;
- A 3-0 PDS purse-string suture secures the seromuscular jejunal components to the parietal peritoneum; only four stitches are placed on each side in a continuous, alternating, circumferential suture line (Figure 1C,D). Exposure is optimized by starting in the caudad suture portion, advancing to the posterior area, and delaying suture tightening until all four stitches are placed (Figure 1E);
- The tube is sutured to the outside skin with 2-0 silk in two locations, flushed to ascertain patency, and capped (Figure 1F).
The original description also included approximation of a segment of small bowel the lateral abdominal wall in the left paracolic gutter, intended as a preventive measure against possible small bowel volvulus. This step has since been eliminated, with no resulting adverse events noted.
Clinical data analysis
Institutional Review Board approval was obtained for the prospective collection of data related to feeding JT placement. All clinical information was derived from a consecutive patient series of major abdominal operative procedures with curative or palliative intent by a single attending surgeon in an academic surgical oncology practice setting. Clinicopathological data for all patients had been prospectively collected. The series includes 343 consecutive patients collected over a 15-year period. The analysis focused on variables associated with JT placement and those related to feeding tube use postoperatively or after discharge from the hospital, in addition to device-related events and parameters affecting length of stay (LOS). Postoperative complications were graded according to the 5-grade scale proposed by Clavien and colleagues (13). In cases of multiple complications, that with the greatest severity determined the final grade. Postoperative lethal events were defined as those occurring during the in-hospital stay or within 30 days after the procedure. Patients experiencing lethal outcomes during their postoperative admission were excluded from the LOS calculation.
Continuous data between groups were compared via Student’s t-test, or Mann-Whitney analysis, based on the original data distribution. Chi square contingency testing was used for categorical data. Multiple logistic regression for feeding tube use was applied in a stepwise backward model, only entering covariates that met univariate significance. All calculations were performed using StatView software for Macintosh, version 5.0.1 (SAS Institute Inc., Cary, NC, USA). Statistical significance of group differences was assumed at a P value of <0.05.
Results
Patient demographics, clinical characteristics and early outcomes
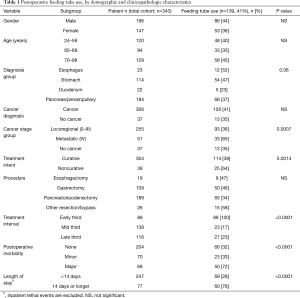
The described feeding tube placement technique was performed in 343 of 803 major hepatopancreatobiliary and upper gastrointestinal (GI) resections (43%). It was a standard recommendation for elective procedures involving resection of the esophagus, total or near-total stomach and upper or mid-duodenum, or was used widely for palliation of malignant bowel obstruction; it was utilized selectively for partial gastrectomies or other midgut resections. Of the 343 patients receiving a JT, 196 were male (57%) and 147 were female (43%), with a median age of 65.8 years (range: 24.0–98.0 years). Diagnoses included disorders of the pancreas or periampullary tissues (n=184, 54%), stomach (n=114, 33%), esophagus (n=23, 7%) and duodenum (n=22, 6%); a cancer diagnosis was confirmed in 306 (89%). Final pathologic cancer stage grouping yielded stages 0 (n=8, 3%), I (n=58, 19%), II (n=118, 38%), III (n=71, 23%) and IV (n=51, 17%). Operative procedures included pancreatoduodenectomy (PD, n=189, 55%), gastrectomy (n=109, 32%), esophagectomy (n=19, 6%) and others (n=26, 7%); the latter represented other intestinal resections (n=4, 1%), bypass procedures (n=15, 4%), and procedures without intestinal anastomosis (n=7, 2%). The operative intent was curative in 78%, palliative in 10%, or combined in 12% of patients.
The postoperative morbidity rate was 40%, with 204 patients remaining complication-free (60%), 70 developing a minor complication (grade 1 or 2, 20%) and 69 experiencing major morbidity (grades 3–5, 20%) including 19 lethal events (5.5%),
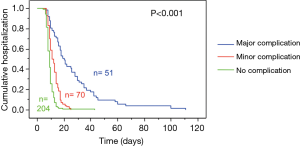
The median LOS of was 10 days (range, 4–111 days) and differed based on postoperative morbidity, with group medians of 9 days without, 11.5 days with minor and 20 days with major complications (Figure 2).
Feeding tube utilization
In the early part of this experience, tube feeds (TF) were routinely initiated on postoperative day 3, affecting 89 patients within the total cohort (26%). This practice was altered over time, and tube feeds were subsequently no longer routinely applied but reserved for nutrition support during major postoperative morbidity or in situations of severe preoperative nutrition compromise. Overall, tube feeds were administered postoperatively in 139 patients (41%) but only continued in 17% at time of discharge. With a selective tube feeding policy during the second and third study interval, 50 of 254 patients received postoperative enteral feeding support (20%), with feeds continued after discharge in 6%. Utilization of the feeding tube was linked to cancer stage group, noncurative treatment intent, treatment time interval, complication grade and LOS (Table 1), but not to age, gender, weight loss history, preoperative albumin, ASA grade, diagnostic group, history of preoperative chemotherapy or chemoradiation, type of resection performed or margin status. On multivariate analysis, LOS, complication grade and noncurative intent emerged as the only independent variables linked to tube feeding use (Table 2). Tube feeds maintained beyond discharge were ultimately only associated with time interval (P<0.0001), LOS (P=0.0006) and noncurative intent (P=0.014) (Table 2). The impact of diagnosis, cancer stage group, operative procedure (9% after PD, between 22% and 27% after all others) and the initial strategy for routine use of TF, all significant univariate factors, lost a significant association in the multivariate setting. There was no association between TF after discharge and age, gender, cancer diagnosis, margin status or postoperative complications in either analysis.
Full table

Full table
Feeding tube-related events
Tube-related events in 38 patients (11%) included occlusion (n=14), drainage around the tube (n=17), pain (n=3), and accidental removal (n=4) but no intraabdominal leaks, infections or intestinal obstructions. None of the tube-specific morbidity events reached a severity grade of greater than 2, in fact all but two were rated as grade 1. No intravenous medications were required for device-related infections or symptoms. Patients with prolonged feeding tube needs, or those with significant drainage of succus around the initial tube underwent placement of a balloon catheter for intraluminal tract control. In three patients, a subsequent tube exchange for long-term access was performed in an interventional radiology procedure. Although 19 patients (5%) required suture placement at the cutaneous JT entry site after removal of the tube rather than the occluding adhesive paper strips otherwise used, all sites healed well without need for additional interventions. JT events were observed in 21 of 56 patients with tube feeds beyond discharge (38%), compared to 6% of patients who did not receive tube feeds at discharge, indicating a propensity to develop feeding tube related issues during a longer course of tube feedings administered (P<0.0001).
Discussion
The role for postoperative nutrition support in patients undergoing major visceral resections for gastrointestinal cancer remains controversial. Several studies have shown advantages to enteral over parenteral nutrition access, and have demonstrated that the use of intestinal postoperative feeds is feasible and safe (5,14). Aside from the potential for reduced postoperative complication rates and LOS, enteral nutrition supplements were found to be beneficial regarding adjuvant cancer therapy delivery as they allowed for a greater rate of successful treatment completion (8). However, a randomized control trial in 195 patients failed to show clear evidence for a postoperative enteral nutrition benefit (15). Interestingly, a trend for lower infectious morbidity in patients receiving postoperative tube feeds was offset in this trial by a greater rate of intestinal complications such as partial small bowel obstruction and prolonged ileus. Other trials have indicated improved outcomes when nutrition support was delivered perioperatively or preoperatively compared to just in the postoperative period (2,3,16). Attempts to allow for early postoperative oral nutrition have so far led to mixed results and have not eliminated the potential role for enteral nutrition support (17,18). Utilization of postoperative tube feeds in the current series has also changed over time. The initial practice of routine postoperative feeds has been disbanded in favor of selective feedings in cases of major complications or delayed oral diet tolerance. In addition, patient scheduled for high-risk procedures are encouraged to use preoperative oral immunonutrition supplements as supported by randomized trial data (3).
Irrespective of the actual benefit of enteral nutrition support in the postoperative setting, feeding tubes can still be reasonably placed as prophylactic measures at the time of major upper gastrointestinal and pancreatic resections (19-21). Although in some centers endoscopic percutaneous JTs can be placed postoperatively upon demand with a success rate as high as 86% (22), this special expertise is still of limited availability in those acutely ill patients suffering from severe postoperative complications, or challenging to implement in those individuals who fail to tolerate oral diets within 1 week or more after the operation. Parenteral nutrition support may be considered in these settings, but carries some clear disadvantages related to hepatic tolerance, infectious complications and costs (7,14). Most patients undergoing resective or palliative bypass procedures for cancer have to be considered at increased nutritional risk that can be linked to greater postoperative complication hazards. A reliable and validated nutritional assessment tool to predict postoperative nutrition support needs for this patient cohort does not yet exist, although a screening approach has been proposed (23) and some nutritional indices appear to fare better than others (24-26); this method could support the strategy to selectively provide nutrition access at the time of operation in those individuals considered at potential risk. Since in the current series postoperative complications and increased LOS were the dominant determinants for postoperative feeding tube use, validated predictors of postoperative morbidity such as the NSQIP or POSSUM scores could also be considered potential guides for selective operative placement of jejunostomies. However, it is still mandatory that ease of tube placement and overall safety can justify this maneuver.
The described technique has been used for over a decade now with good success in terms of absence of major morbidity, and has provided reliable access for enteral nutrition support whenever required in the postoperative period. Modification to the original technique have been minor, and the procedure remains technically easy and swift (12). Outcomes comparison to other published JT placement techniques provide sufficient evidence to consider this a valid or superior JT option due to its absence of any major tube-related complication as described by others at a low but appreciable frequency (5,27-34). It is acknowledged that some published reports on specific techniques or large single-institution series carry an inherent bias towards positive outcomes that does not guarantee equal results in other settings (32). For instance, morbidity rates related to various feeding tube techniques as reported in several trials for postoperative enteral feeds range from 10% to 35% (27,33,35), but may be as high as 50% or above (6). We believe that the technique presented here would lend itself to widespread use with good outcomes due to its simplicity and apparent safety. We have not observed a need for routine gastric decompression during JT use as reported by others (19); at least for pancreatectomy, postoperative nasogastric tube (NGT) reinsertion rates of around 20% were independent from early postoperative NGT decompression or subsequent JT use for enteral feeding (36). There also has not been a need to perform an interventional procedure to place a larger bore tube in cases of prolonged tube requirements such as after needle-catheter JTs (31). In fact, all tube exchanges into an equal-size, 16-Fr balloon-tipped catheter have been uncomplicated, and there is no longer any perceived need to obtain routine contrast radiographs to verify the new tube position as long as the tube is easily flushed and succus is obtained via aspiration.
It is concluded that the feeding JT technique described is a safe and reliable procedure with minimal additional operative time requirements. The technique is also suitable for laparoscopic procedures, blocked tubes are easily replaced without guidewire or imaging support, the potential for long-term access is given and tube removal is generally uncomplicated. A necessity for successful JT placement with this approach, however, is sufficient mobility of the proximal jejunum to reach to the abdominal wall. Avoidance of a Witzel tunnel is likely related to the fact that no intestinal obstructive events were observed. The overall tube-related morbidity is limited and of low severity, with no cases of obstruction, volvulus or intraabdominal leakage and therefore no need for any reoperation or interventional drainage. Although the majority of patients in this series did not require prolonged postoperative tube feeds, the author continues to perform this procedure after major upper GI resections to provide prophylactic feeding access to patients in case of significant postoperative morbidity or delayed gastrointestinal functional recovery with resulting enteral nutrition support needs. The technique can be recommended to surgeons who consider providing intraoperative enteral feeding access for the moderate to high nutritional risk patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was supported by IRB protocol No. 02200070019 of the University of Medicine and Dentistry of New Jersey, and by protocol No. STU 092010-072 of the University of Texas Southwestern Medical Center, with written informed consent obtained as required by the IRB.
References
- Fearon KC, Jenkins JT, Carli F, et al. Patient optimization for gastrointestinal cancer surgery. Br J Surg 2013;100:15-27. [Crossref] [PubMed]
- Braga M, Gianotti L, Nespoli L, et al. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg 2002;137:174-80. [Crossref] [PubMed]
- Gianotti L, Braga M, Nespoli L, et al. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002;122:1763-70. [Crossref] [PubMed]
- van Berge Henegouwen MI, Akkermans LM, van Gulik TM, et al. Prospective, randomized trial on the effect of cyclic versus continuous enteral nutrition on postoperative gastric function after pylorus-preserving pancreatoduodenectomy. Ann Surg 1997;226:677-85; discussion 685-77.
- Braga M, Gianotti L, Gentilini O, et al. Feeding the gut early after digestive surgery: results of a nine-year experience. Clin Nutr 2002;21:59-65. [Crossref] [PubMed]
- Lobo DN, Williams RN, Welch NT, et al. Early postoperative jejunostomy feeding with an immune modulating diet in patients undergoing resectional surgery for upper gastrointestinal cancer: a prospective, randomized, controlled, double-blind study. Clin Nutr 2006;25:716-26. [Crossref] [PubMed]
- Klek S, Kulig J, Sierzega M, et al. The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: a prospective, randomized, clinical trial. Ann Surg 2008;248:212-20. [Crossref] [PubMed]
- Daly JM, Weintraub FN, Shou J, et al. Enteral nutrition during multimodality therapy in upper gastrointestinal cancer patients. Ann Surg 1995;221:327-38. [Crossref] [PubMed]
- Gupta V. Benefits versus risks: a prospective audit. Feeding jejunostomy during esophagectomy. World J Surg 2009;33:1432-8. [Crossref] [PubMed]
- Dann GC, Squires MH 3rd, Postlewait LM, et al. An assessment of feeding jejunostomy tube placement at the time of resection for gastric adenocarcinoma: A seven-institution analysis of 837 patients from the U.S. gastric cancer collaborative. J Surg Oncol 2015;112:195-202. [Crossref] [PubMed]
- Sun Z, Shenoi MM, Nussbaum DP, et al. Feeding jejunostomy tube placement during resection of gastric cancers. J Surg Res 2016;200:189-94. [Crossref] [PubMed]
- Schwarz RE. Simple feeding jejunostomy technique for postoperative nutrition after major upper gastrointestinal resections. J Surg Oncol 2002;79:126-30. [Crossref] [PubMed]
- DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 2006;244:931-7; discussion 937-9. [Crossref] [PubMed]
- Baigrie RJ, Devitt PG, Watkin DS. Enteral versus parenteral nutrition after oesophagogastric surgery: a prospective randomized comparison. Aust N Z J Surg 1996;66:668-70. [Crossref] [PubMed]
- Heslin MJ, Latkany L, Leung D, et al. A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg 1997;226:567-77; discussion 577-80. [Crossref] [PubMed]
- Senkal M, Zumtobel V, Bauer KH, et al. Outcome and cost-effectiveness of perioperative enteral immunonutrition in patients undergoing elective upper gastrointestinal tract surgery: a prospective randomized study. Arch Surg 1999;134:1309-16. [Crossref] [PubMed]
- Han-Geurts IJ, Hop WC, Kok NF, et al. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br J Surg 2007;94:555-561. [Crossref] [PubMed]
- Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 2008;247:721-9. [Crossref] [PubMed]
- Mack LA, Kaklamanos IG, Livingstone AS, et al. Gastric decompression and enteral feeding through a double-lumen gastrojejunostomy tube improves outcomes after pancreaticoduodenectomy. Ann Surg 2004;240:845-51. [Crossref] [PubMed]
- Venskutonis D, Bradulskis S, Adamonis K, et al. Witzel catheter feeding jejunostomy: is it safe? Dig Surg 2007;24:349-53. [Crossref] [PubMed]
- Ramamurthy A, Negi SS, Chaudhary A. Prophylactic tube jejunostomy: a worthwhile undertaking. Surg Today 2008;38:420-4. [Crossref] [PubMed]
- Shike M, Latkany L, Gerdes H, et al. Direct percutaneous endoscopic jejunostomies for enteral feeding. Gastrointest Endosc 1996;44:536-40. [Crossref] [PubMed]
- Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36. [Crossref] [PubMed]
- Mohri Y, Inoue Y, Tanaka K, et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg 2013;37:2688-92. [Crossref] [PubMed]
- Tokunaga R, Sakamoto Y, Nakagawa S, et al. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum 2015;58:1048-57. [Crossref] [PubMed]
- Bachmann J, Muller T, Schroder A, et al. Influence of an elevated nutrition risk score (NRS) on survival in patients following gastrectomy for gastric cancer. Med Oncol 2015;32:204. [Crossref] [PubMed]
- Haun JL, Thompson JS. Comparison of needle catheter versus standard tube jejunostomy. Am Surg 1985;51:466-9. [PubMed]
- Gerndt SJ, Orringer MB. Tube jejunostomy as an adjunct to esophagectomy. Surgery 1994;115:164-9. [PubMed]
- Myers JG, Page CP, Stewart RM, et al. Complications of needle catheter jejunostomy in 2,022 consecutive applications. Am J Surg 1995;170:547-50; discussion 550-1. [Crossref] [PubMed]
- Schwaitzberg SD, Sable DB. Transverse Witzel-T-tube feeding jejunostomy. JPEN J Parenter Enteral Nutr 1995;19:326-7. [Crossref] [PubMed]
- Sarr MG. Appropriate use, complications and advantages demonstrated in 500 consecutive needle catheter jejunostomies. Br J Surg 1999;86:557-61. [Crossref] [PubMed]
- Tapia J, Murguia R, Garcia G, et al. Jejunostomy: techniques, indications, and complications. World J Surg 1999;23:596-602. [Crossref] [PubMed]
- Han-Geurts IJ, Hop WC, Verhoef C, et al. Randomized clinical trial comparing feeding jejunostomy with nasoduodenal tube placement in patients undergoing oesophagectomy. Br J Surg 2007;94:31-5. [Crossref] [PubMed]
- Patel SH, Kooby DA, Staley CA 3rd, et al. An assessment of feeding jejunostomy tube placement at the time of resection for gastric adenocarcinoma. J Surg Oncol 2013;107:728-34. [Crossref] [PubMed]
- Abu-Hilal M, Hemandas AK, McPhail M, et al. A comparative analysis of safety and efficacy of different methods of tube placement for enteral feeding following major pancreatic resection. A non-randomized study. JOP 2010;11:8-13. [PubMed]
- Roland CL, Mansour JC, Schwarz RE. Routine nasogastric decompression is unnecessary after pancreatic resections. Arch Surg 2012;147:287-9. [Crossref] [PubMed]
Cite this article as: Minarich MJ, Schwarz RE. Experience with a simplified feeding jejunostomy technique for enteral nutrition following major visceral operations. Transl Gastroenterol Hepatol 2018;3:44.