
The effective molecular characteristics of PD-1 inhibitor combination regimen as the first-line treatment for Chinese patients with HER2-positive gastric cancer: a real-world retrospective analysis study
Highlight box
Key findings
• The study indicated that programmed cell death protein 1 (PD-1) inhibitor combination regimen was an effective and well-tolerated therapy for the first-line treatment of patients with human epidermal growth factor receptor 2 (HER2)-positive gastric cancer (GC), especially those with HER2 fluorescence in situ hybridization (FISH) more than six and TP53 mutations.
What is known and what is new?
• The PD-1 inhibitor combined with trastuzumab and chemotherapy as a first-line regimen for advanced and metastatic GC patients with HER2-positive. However, there was no sufficient evidence of molecular characteristic regarding PD-1 blockade combined treatment in HER2-positive GC.
• Our study gave the underlining mechanism that HER2-positive GC patients with HER2 FISH more than six and TP53 mutations can benefit from the PD-1 inhibitor combined with trastuzumab and chemotherapy regimen.
What is the implication, and what should change now?
• The research can guide clinicians to provide individualized treatment and select more suitable patients.
Introduction
Gastric cancer (GC) is reported as the fifth frequent cancer in humankind and the third leading cause of tumor associated death worldwide (1). Human epidermal growth factor receptor 2 (HER2; also known as ERBB2) is a vital pathological feature of GC (2). HER2 belongs to a family of receptors involved in tumor cell proliferation, migration, and differentiation, and it promotes the malignant biological behavior of tumors (3). The first-line recommended systemic chemotherapy for advanced and metastasis GC is fluoropyrimidine plus platinum, combined with trastuzumab for patients with HER2-positive (4). Patients with advanced GC have a poor prognosis, with survival from 4 months to 12 months (5). The prognosis of GC patients with HER2-positive is even worse than that of GC patients with HER2-negative (2). However, when the anti-HER2 strategy, especially trastuzumab, was performed for HER2-positive GC, the survival time was even more prolonged than that for HER2-negative GC (6). Although we have clarified the role of HER2 in GC, we do not fully understand the molecular characteristics of HER2 in GC.
Recently, increasing evidence has shown that immunotherapy with immune checkpoint inhibitors (ICIs), especially programmed cell death protein-1 (PD-1) blockades, has revolutionized the therapeutic strategy of advanced and metastasis GC patients that are HER2-negative (7,8). However, for GC patients with HER2-positive, there remains a lack of relevant real-world clinical data on ICIs treatment. The ongoing KEYNOTE-811 trial showed the possibility of PD-1 inhibitors for GC patients with HER2-positive (9-11). The molecular characteristics of the beneficiary population have not yet been thoroughly elucidated. Thus, we designed this real-world retrospective study to draw a comparison of PD-1 inhibitor efficacy in GC patients with HER2-positive and attempted to identify the characteristics of the population with the most benefit from PD-1 inhibitor treatment. We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-95/rc).
Methods
Study design
This real-world retrospective study of patients with HER2-positive advanced or metastasis GC between January 2019 and September 2022 was carried out at the Zhongshan Hospital of Fudan University and was approved by the Institutional Ethics Committee (No. B2020-253R2). Written informed consent for publication was obtained from all participants. All data were anonymized before processing. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Sample selection and data collection
Inclusion criteria for patients with HER2-positive GC with survival outcomes were as follows: (I) histologically confirmed for advanced or metastasis gastric adenocarcinoma; (II) diagnosed of positive HER2 expression in cancer tissues by immunohistochemical (IHC) 3+ or IHC 2+ and fluorescence in situ hybridization (FISH) positive (HER2:CEP17 ratio ≥2) or both or next generation sequencing (NGS) testing; (III) application of first-line PD-1 inhibitor plus trastuzumab and chemotherapy (PTC) or trastuzumab and chemotherapy (TC) regimen; (IV) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Data on gender, age, pathological information, and therapeutic regimen were collected. The dates of diagnosis, initiation of treatment, termination of treatment, progress and death of patients were all collected. National Cancer Institute Common Toxicity Criteria version 5.0 was used to assess adverse events.
Therapeutic strategy
The first-line systemic chemotherapy regimen of GC patients with HER2-positive was constituted by the guideline of the National Comprehensive Cancer Network. The TC regimen included three-week cycles of trastuzumab (initially at 8 mg/kg then 6 mg/kg of body weight every 21 days intravenously) combined with chemotherapy regimen chosen by the investigator, such as XELOX (capecitabine and oxaliplatin), SOX (S-1 and oxaliplatin), AS (albumin-bound paclitaxel and S-1), etc. The PTC regimen involved a TC regimen combined with 3-week cycles of the PD-1 inhibitor intravenously, which the researchers chose. The types and dosages of PD1 inhibitors included 15 cases of keytruda (200 mg), 4 cases of nivolumab (360 mg), 6 cases of toripalimab (200 mg), 4 cases of tislelizumab (200 mg), 1 case of sintilimab (200 mg), 1 case of camrelizumab (200 mg), and 1 case of penpulimab (200 mg). There was no limitation on the secondary or posterior therapeutic schedule. The number of treatment cycles was decided by investigators according to the general conditions of patients. Treatment was discontinued as soon as the disease progressed, at a point of unacceptable toxicity, or upon patient refusal.
Endpoints
The primary endpoint was overall survival (OS), and the secondary endpoint was progression-free survival (PFS). OS was considered to be the time from the initiation of tumor treatment to the final follow-up or the death of GC patients. PFS was defined from the time of treatment initiation until disease progression. The disease assessment was executed by the Response Evaluation Criteria in Solid Tumors version 1.1.
Statistical analysis
The ages of patients with HER2-positive GC were divided into two groups based on the threshold of 65 years. Categorical data were analyzed with the chi-square test or Fisher’s exact test. OS and PFS were displayed by the Kaplan-Meier method. Propensity score matching (PSM) was executed at a ratio of 1:2 of PTC group and TC group. Efficacy was calculated as a hazard ratio (HR) with a 95% confidence interval (CI). Analysis was carried out using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA) and Statistical Package for the Social Sciences 26.0 (IBM, Armonk, NY, USA). P value <0.05 was considered statistically significant.
Results
Characteristics of patients with survival analysis
From January 2019 to September 2022, ninety-five GC patients with HER2-positive met the inclusion criteria, including 32 patients of the PTC regimen and 63 patients of the TC regimen (Figure 1). After PSM analysis, there were 32 cases in the PTC group and 43 in the TC group. The secondary or posterior therapeutic regimen was not restricted. All patients were comprehensively collected and monitored according to the experimental design. The final follow-up date was November 30, 2022. The baseline characteristics of the GC patients with HER2-positive before and after PSM were listed in Table 1. Patients in the PTC and TC groups were well balanced in gender, age, pathological Lauren type, HER2 expression, PD-L1 expression, cycles of trastuzumab, and other anti-HER2 regimens after PSM analysis. Up to the deadline of follow-up, 61 patients died (64.2%; 13 and 48 in each group, respectively). Before PSM, there was a statistical difference in Lauren types between the PTC and TC groups. The data showed that the mixed Lauren type was the major type in the PTC group, while the intestinal Lauren type was the main type in the TC group. However, the difference in Lauren type was well balanced between the two groups after PSM analysis. The expression levels of HER2 determined by IHC and FISH methods were not different between the two groups before and after PSM. PD-L1 expression in the tissues of 95 patients with HER2-positive GC was collected. In total, 85.7% of GC patients had PD-L1 expression data. The positive rate of PD-L1 expression was slightly higher in the PTC group than that in the TC group (59.4% vs. 31.7%). However, statistical difference was not discovered both before and after the PSM analysis. The mismatch repair status of all patients was proficient.

Table 1
| Variable | Before PSM, n (%) | After PSM, n (%) | |||||
|---|---|---|---|---|---|---|---|
| PTC | TC | P value | PTC | TC | P value | ||
| Gender | 0.15 | 0.81 | |||||
| Male | 20 (62.5) | 49 (77.8) | 20 (62.5) | 29 (67.4) | |||
| Female | 12 (37.5) | 14 (22.2) | 12 (37.5) | 14 (32.6) | |||
| Age (years) | 0.52 | 0.35 | |||||
| ≤65 | 19 (59.4) | 32 (50.8) | 19 (59.4) | 20 (46.5) | |||
| >65 | 13 (40.6) | 31 (49.2) | 13 (40.6) | 23 (53.5) | |||
| Lauren type | 0.003 | 0.35 | |||||
| Intestinal | 11 (34.4) | 42 (66.7) | 11 (34.4) | 22 (51.2) | |||
| Diffuse | 8 (25.0) | 13 (20.6) | 8 (25.0) | 8 (18.6) | |||
| Mixed | 13 (40.6) | 8 (12.7) | 13 (40.6) | 13 (30.2) | |||
| HER2 IHC | 0.94 | 0.97 | |||||
| 2+ | 9 (28.1) | 17 (27.0) | 9 (28.1) | 11 (25.6) | |||
| 3+ | 21 (65.6) | 43 (68.2) | 21 (65.6) | 29 (67.4) | |||
| Othera | 2 (6.3) | 3 (4.8) | 2 (6.3) | 3 (7.0) | |||
| HER2 FISH | 0.18d | 0.25d | |||||
| ≤6 | 9 (28.1) | 29 (46.0) | 9 (28.1) | 19 (44.2) | |||
| >6 | 17 (53.1) | 22 (34.9) | 17 (53.1) | 15 (34.9) | |||
| N/A | 6 (18.8) | 12 (19.0) | 6 (18.8) | 9 (20.9) | |||
| PD-L1 | 0.07e | 0.09e | |||||
| Positive | 19 (59.4) | 20 (31.7) | 19 (59.4) | 14 (32.5) | |||
| Negative | 13 (40.6) | 34 (54.0) | 13 (40.6) | 23 (53.5) | |||
| N/A | 0 | 9 (14.3) | 0 | 6 (14.0) | |||
| Trastuzumab | 0.009 | 0.17 | |||||
| ≤6 | 11 (34.4) | 40 (63.5) | 11 (34.4) | 22 (51.2) | |||
| >6 | 21 (65.6) | 23 (36.5) | 21 (65.6) | 21 (48.8) | |||
| Posterior lineb | 0.37 | 0.72 | |||||
| Yes | 3 (9.4) | 12 (19.0) | 3 (9.4) | 6 (14.0) | |||
| No | 29 (90.6) | 51 (81.0) | 29 (90.6) | 37 (86.0) | |||
| Other anti-HER2c | >0.99 | 0.74 | |||||
| Yes | 5 (15.6) | 9 (14.3) | 5 (15.6) | 5 (11.6) | |||
| No | 27 (84.4) | 54 (85.7) | 27 (84.4) | 38 (88.4) | |||
| Total | 32 (100.0) | 63 (100.0) | 32 (100.0) | 43 (100.0) | |||
a, HER2 positive expression by NGS test; b, trastuzumab in posterior line treatment of gastric cancer patients; c, other anti-HER2 treatment, including RC48 and pyrotinib; d, Fisher’s exact test between FISH ≤6 and >6; e, Fisher’s exact test between PD-L1 positive and negative. FISH, fluorescence in situ hybridization; IHC, immunohistochemical; N/A, not available; NGS, next generation sequencing; PSM, propensity score matching; PTC, PD-1 inhibitor plus trastuzumab and chemotherapy; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; TC, trastuzumab and chemotherapy.
The median cycles of trastuzumab treatment were 11 and 7 in the PTC and TC groups. Before PSM analysis, there were 11 patients (34.4%) in the PTC group and 40 patients (63.5%) in the TC group of trastuzumab therapeutic cycles less than 6 of the first-line treatment (P=0.009). The cases of trastuzumab cycles over 6 were more in the PTC group than in the TC group. However, the number of cycles of trastuzumab between ≤6 and >6 in each group was balanced after PSM analysis (P=0.25). We also analyzed the regimens after first-line treatment to minimize the effects of other variables. Three patients (9.4%) in the PTC group and 12 patients (19.0%) in the TC group continued to apply trastuzumab after the first-line treatment. Five patients in the PTC group and nine cases in the TC group implemented other anti-HER2 treatment, except trastuzumab, including RC48 and pyrotinib. Given the above, the two groups were also well balanced regarding the application of trastuzumab in the posterior line treatment and the other anti-HER2 regimen in the posterior line treatment.
Follow-up
The median age of the GC patients with HER2-positive was 63 years (range from 31 to 82 years). The median follow-up time was 26 months. Before PSM, we found the median OS is 24.67 and 17.70 months of patients receiving PTC and TC regimens, respectively, with a significant difference (P=0.02; HR =0.4980, 95% CI: 0.2928–0.8468) (Figure 2A, Table 1). Furthermore, we found a significant difference in median PFS (P=0.007; HR =0.4454, 95% CI: 0.2639–0.7519) between these HER2-positive GC patients receiving PTC and TC regimens (Figure 2B).
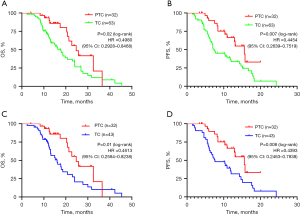
After further PSM analysis, the median OS of GC patients was 24.67 and 16.00 months (P=0.01; HR =0.4613, 95% CI: 0.2584–0.8238) of the PTC and TC groups, while the median PFS was 15.57 and 7.57 months (P=0.008; HR =0.4393, 95% CI: 0.2463–0.7838) respectively (Figure 2C,2D). Both OS and PFS were prolonged in the PTC group, indicating that a combined PD-1 inhibitor in first-line treatment can improve the therapeutic effect in GC patients with HER2-positive.
As we knew, HER2 status influence the treatment strategy of GC, while FISH testing results indicating the amplification of HER2. We further analyzed whether HER2-FISH results were relevant to clinical treatment efficiency. Totally, we have 72 patients has HER2-FISH testing results. The median HER2-FISH testing number is 6. Then we conduct an analysis of the survival time according to HER2-FISH results in all GC patients with HER2-positive, including both PTC and TC groups. It was a significant statistical difference of OS (P=0.02; 24.00 vs. 16.30 months) and PFS (P=0.0003; 15.57 vs. 7.00 months) in all GC patients with HER2-positive between FISH >6 and FISH ≤6 (Figure 3A,3B; Table 2). After a stratified analysis of HER2-FISH number in PTC and TC groups, we found the patients with HER2-FISH >6 has a better PFS outcome than HER2-FISH ≤6 in PTC group (undefined vs. 8.4 months, P<0.001), while no significant difference in other subgroups (Figure 3C-3H). The data suggested that patients with higher HER2 amplification may benefit from systemic therapy. The median number was not available in Table 2 may due to the death rate was no higher than 50%, which need to extend the duration of follow-up.

Table 2
| Variable | N | OS (months) | PFS (months) | |||
|---|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | |||
| Before PSM | ||||||
| PTC | 32 | 24.67 | 19.32–30.02 | 15.57 | 11.63–19.50 | |
| TC | 63 | 17.70 | 13.90–21.50 | 7.37 | 4.23–10.50 | |
| After PSM | ||||||
| PTC | 32 | 24.67 | 19.32–30.02 | 15.57 | 12.63–19.50 | |
| TC | 43 | 16.00 | 11.95–20.05 | 7.57 | 3.62–11.52 | |
| HER2 FISH | ||||||
| >6 | 38 | 24.00 | 17.05–30.95 | 15.57 | 14.44–16.70 | |
| ≤6 | 38 | 16.30 | 12.22–20.38 | 7.00 | 5.91–8.09 | |
| PTC >6 | 17 | 21.40 | N/A | N/A | N/A | |
| PTC ≤6 | 9 | 17.43 | 10.00–24.87 | 8.37 | 6.51–10.23 | |
| TC >6 | 21 | 24.00 | 12.88–35.12 | 10.40 | 0.00–21.27 | |
| TC ≤6 | 29 | 16.00 | 10.67–21.33 | 6.77 | 5.64–7.89 | |
| PD-L1 subgroup | ||||||
| PTC_PD-L1+ | 19 | 25.83 | 19.05–32.16 | 15.80 | N/A | |
| PTC_PD-L1− | 13 | 22.73 | 14.42–28.05 | 8.40 | 4.49–12.31 | |
| TC_PD-L1+ | 20 | 24.00 | 18.99–29.01 | 15.10 | 7.90–22.30 | |
| TC_PD-L1− | 34 | 16.00 | 11.34–20.66 | 6.97 | 6.53–7.41 | |
| TP53 subgroup | ||||||
| PTC_TP53m | 19 | 24.67 | 18.84–30.50 | 12.93 | 5.90–19.97 | |
| TC_TP53m | 15 | 12.10 | 10.29–13.91 | 6.77 | 4.87–8.66 | |
CI, confidence interval; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; N/A, not available by Kaplan-Meier analysis; OS, overall survival time; PFS, progress free survival time; PD-L1, programmed cell death ligand 1; PSM, propensity score matching; PTC, PD-1 inhibitor plus trastuzumab and chemotherapy; PD-1, programmed cell death protein 1; TC, trastuzumab and chemotherapy; TP53m, TP53 mutation.
Increasing data showed that PD-L1 expression may be one of the factors predicting therapeutic effects in patients with HER2-negative GC. Therefore, we tried to conduct a stratified analysis of the survival time according to PD-L1 expression in GC patients with HER2-positive. It was no significant statistical difference of OS in the PTC group and TC group between PD-L1 positive (CPS ≥1) and negative (CPS <1) GC patients with HER2-positive. However, there was a significant difference of PFS in the sub-analysis (Figure 4A,4B). To further analyze the PFS outcome, we found that the difference mainly occurred between patients that were PD-L1 positive and PD-L1 negative sub-groups in the PTC regimen. Additionally, HER2-positive GC patients with PD-L1 positive expression in the PTC regimen showed a better PFS than patients that are PD-L1 negative in the TC regimen (Figure 4C,4D, Table 2). However, the advantage of PFS was not transferred to that of OS.

Among these, 19 patients in the PTC group and 17 cases in the TC group underwent NGS testing respectively. From the NGS results, we found that TP53 was the most common abnormal gene in patients with GC. After confirming the TP53 mutation status, we found 19 cases in the PTC group and 15 cases in the TC group with TP53 mutations. Although it was no statistically significant difference in the median PFS (12.93 vs. 6.77 months) between the PTC and TC groups (P=0.15; HR =0.5027, 95% CI: 0.1879–1.344), however, we found the median OS was 24.67 and 12.10 months (P=0.001; HR =0.3115, 95% CI: 0.1345–0.7218) with a significant statistical difference between the two groups (Figure 4E,4F, Table 2). The OS outcome was prolonged by approximately 12 months for the PTC regimen, compared with that of the TC regimen. The data showed that GC patients with HER2-positive could benefit more from ICI combination treatment.
Adverse events
Adverse events in both groups are displayed in Table 3. Myelosuppression including leukocytopenia, thrombocytopenia, and anemia was the most common hematological adverse event in the PTC and TC groups. Diarrhea, vomiting, hepatic dysfunction, and hand-foot syndrome also occurred in these patients. Vomiting occurred in approximately 20% of all GC patients. The data displayed that the incidence of peripheral neurotoxicity was slightly higher in the PTC group (21.88%) than that in the TC group (9.52%). However, there was no significant statistical difference. The incidences of heart failure were similar in the PTC and TC groups. There were no serous immune-related adverse events in PTC group.
Table 3
| Event | PTC (n=32) | TC (n=63) | P value | |||
|---|---|---|---|---|---|---|
| Grade1/2 (%) | Grade3/4 (%) | Grade1/2 (%) | Grade3/4 (%) | |||
| Anemia | 3 (9.38) | N/A | 5 (7.94) | N/A | >0.99 | |
| Leukocytopenia | 5 (15.63) | 1 (3.13) | 11 (17.46) | 2 (3.17) | >0.99 | |
| Thrombocytopenia | 5 (15.63) | 2 (6.25) | 11 (17.46) | 2 (3.17) | 0.57 | |
| Diarrhea | 5 (16.38) | 1 (3.13) | 2 (3.17) | 1 (1.59) | >0.99 | |
| Vomiting | 8 (25.00) | N/A | 12 (19.05) | N/A | >0.99 | |
| Hepatic dysfunction | 3 (9.38) | N/A | 4 (6.35) | N/A | >0.99 | |
| Hand-foot syndrome | 1 (3.13) | N/A | 2 (3.17) | N/A | >0.99 | |
| Peripheral neurotoxicity | 6 (18.75) | 1 (3.13) | 3 (4.76) | 3 (4.76) | 0.27 | |
| Heart failure | 3 (9.38) | N/A | 2 (3.17) | N/A | >0.99 | |
N/A, not available; PTC, PD-1 inhibitor plus trastuzumab and chemotherapy; PD-1, programmed cell death protein 1; TC, trastuzumab and chemotherapy.
Discussion
There is increasing evidence that HER2 is one of the most important biomarkers and a key driver of tumorigenesis in human GC. Overexpressed HER2 protein has been reported in 10–20% of patients with GC and was considered to be an inverse prognostic factor (12,13). Since HER2 is recognized as a crucial molecular biomarker, HER2-targeted treatment has drawn wide attention in GC patients with HER2-positive. A typical clinical trial showed that trastuzumab combined with systemic chemotherapy improved OS from 11.1 to 13.8 months and PFS from 5.5 to 6.7 months in GC patients with HER2-positive (6). Therefore, the recommended standard first-line regimen for GC patients with HER2-positive disease is trastuzumab combined with fluoropyrimidine and platinum-based chemotherapy. Although trastuzumab, one of the most important anti-HER2 target regimens, had improved the survival times of patients that are HER2-positive, many patients still could not benefit from the recommended treatment. Many HER2-positive patients have still not prolonged survival time through anti-HER2 therapy. Thus, it is urgent to elucidate the underlying reason for this phenomenon and to find a more useful strategy for these patients.
The Checkmate-649 and ATTRACTION-4 trials showed a promising survival outcome of PD-1 blockade combined with systemic chemotherapy in the first-line treatment of GC patients with HER2-negative (7,8). We were interested in whether a PD-1 inhibitor as the first-line treatment could lengthen the survival time of HER2-positive GC patients. The Phase III KEYNOTE-811 study was designed to assess the efficacy of pembrolizumab combined with trastuzumab and chemotherapy as a first-line regimen for advanced and metastatic GC patients with HER2-positive (9,11). However, there was no sufficient evidence of molecular characteristic regarding PD-1 blockade combined treatment in HER2-positive GC. Thus, we conducted a retrospective study to define the efficacy of PD-1 inhibitors, trastuzumab, and chemotherapy as first-line therapies in advanced and metastatic GC patients with HER2-positive. Patients with TP53 or KRAS mutations, especially those with TP53/KRAS mutation co-occurrence, showed remarkable clinical benefits with PD-1 inhibitors (14). Thus, we deduced that TP53 mutations might also influence the efficacy of PD-1 blockades in GC.
Our study found that the PTC regimen as the first-line therapeutic strategy for GC with HER2-positive can prolong OS and PFS times compared with the TC regimen with a significant difference. The data suggested that the patients with HER-2 positive GC who received PTC treatment reached a better median OS (24.67 vs. 16.00 months, P=0.01) and PFS (15.57 vs. 7.37 months, P=0.008) than TC treatment. The OS was extended for approximately 8 months after combining with PD-1 inhibitor in GC patients with HER2-positive in the first-line systemic treatment. From our data we found that the amplification number of HER2 by HER2-FISH testing can indicate the survival outcome of GC patients. GC patients with higher HER2-FISH numbers (>6) are more likely to benefit from combination therapy. We also conducted subgroup analyses for HER2 IHC2+ and 3+ of PTC group and TC group, respectively, but there was no statistically significant difference.
Increasing evidence supports that the expression level of PD-L1 is frequently abnormal in GC tissues and its upregulation may be a prognostic factor for better outcomes (15,16). We found that the PD-L1 positivity rate was 49.6% in our gene testing analysis data and 45.3% (39 cases positive and 47 cases negative) in our survival analysis of GC patients with HER2-positive. Previous studies have suggested that PD-L1 positive expression is a predictor of the benefit of PD-1 inhibitor therapy in GC patients with HER2-negative (7,17). Through the survival time analysis, we found that GC patients with HER2-positive could also benefit from PD-1 inhibitor therapy. However, whether PD-L1 is also a symbolic sign of immunotherapy effect in HER2-positive GC remains to be answered. We then conducted a stratified analysis of survival time by the expression of PD-L1. Whether a PD-L1 CPS of ≥1 or <1, the OS of GC patients with HER2-positive was not significantly different after PD-1 inhibitors combination treatment. Interestingly, during the PTC group analysis, we found that PFS was longer in the PD-L1 positive expression subgroup. However, no statistical difference was determined in OS among these subgroups. Overall, our data suggested that the expression of PD-L1 was not a major factor in the benefit of immunotherapy in patients with HER2-positive GC. Therefore, it remains a clinical challenge which needs to further explore the molecular characteristics that can benefit from immunotherapy of GC patients with HER2-positive.
Tumor suppressor gene tumor protein p53 (TP53) is reported as one of the most common mutated genes in various malignancies (18,19). As we found that the TP53 mutation is the most frequent gene in patients that are HER2-positive, we analyzed whether TP53 mutation could affect the treatment effect in these patients. We found the median OS was 24.67 and 12.10 months (P=0.001; HR =0.3115, 95% CI: 0.1345–0.7218) between the PTC group and TC group. The median OS was prolonged by approximately 12 months when PD-1 inhibitors were added in GC patients that are HER2-positive with TP53 mutations. These data indicated that TP53 mutation may be a potential indicator of ICIs in GC patients with HER2-positive. The higher frequency of TP53 mutations may affect the therapeutic effect of ICIs in GC patients with HER2-positive. Thus, the available evidence suggested that PD-1 inhibitors have durable efficacy in GC patients and PD-1 inhibitors plus trastuzumab and systemic chemotherapy is a promising treatment option in GC patients with HER2-positive, especially in those with TP53 mutations.
The study also has some limitations. This was a retrospective study, which was subject to bias. The number of patients who participated in the survival analysis was relatively small. The cycles of trastuzumab between PTC and TC groups were slightly different which may also influence the survival results. Inconsistencies in the timing of the selection of samples for genetic testing may also influence the analysis of results to some extent.
Conclusions
In conclusion, this study manifested that the PD-1 inhibitor combination regimen was an effective and well-tolerated therapy for the first-line treatment of patients with HER2-positive GC, especially those with HER2 FISH more than six and TP53 mutations.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing. We also thank the patients involved in this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-95/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-95/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-95/prf
Funding: The study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-95/coif). D.S. is from Genecast Biotechnology Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Zhongshan Hospital of Fudan University (No. B2020-253R2). All data were anonymized before processing. Written informed consent for publication was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523-9. [Crossref] [PubMed]
- Tanner M, Hollmén M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 2005;16:273-8. [Crossref] [PubMed]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021;71:264-79. [Crossref] [PubMed]
- Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654-64. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:234-47. [Crossref] [PubMed]
- Chung HC, Bang YJ, S, Fuchs C, et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol 2021;17:491-501. [Crossref] [PubMed]
- Janjigian YY, Kawazoe A, Yañez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021;600:727-30. [Crossref] [PubMed]
- Janjigian YY, Kawazoe A, Bai Y, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023;402:2197-208. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015;18:476-84. [Crossref] [PubMed]
- Dong ZY, Zhong WZ, Zhang XC, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res 2017;23:3012-24. [Crossref] [PubMed]
- Liu X, Choi MG, Kim K, et al. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol Res Pract 2020;216:152881. [Crossref] [PubMed]
- Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017;66:794-801. [Crossref] [PubMed]
- Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:1571-80. [Crossref] [PubMed]
- Giacomelli AO, Yang X, Lintner RE, et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat Genet 2018;50:1381-7. [Crossref] [PubMed]
- Hu J, Cao J, Topatana W, et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J Hematol Oncol 2021;14:157. [Crossref] [PubMed]
Cite this article as: Zhang L, Guan B, Li W, Yu S, Li Q, Yu Y, Cui Y, Sun D, Wang Y. The effective molecular characteristics of PD-1 inhibitor combination regimen as the first-line treatment for Chinese patients with HER2-positive gastric cancer: a real-world retrospective analysis study. Transl Gastroenterol Hepatol 2025;10:23.


