
Trends in epidemiology, clinicopathological characteristics and survival outcomes among patients with synchronous early-onset colorectal liver metastases in the United States from 2010 to 2019
Highlight box
Key findings
• The incidence of early-onset colorectal liver metastases (EO-CRLM) has increased rapidly, and represents a distinct population in terms of socioeconomic and clinicopathological characteristics.
What is known and what is new?
• Approximately 20% of colorectal cancer patients have synchronous colorectal liver metastases at the time of diagnosis, which becomes the primary cause of mortality in these patients. Little is known, however, regarding trends, demographic characteristics, clinicopathological features, and survival outcomes of patients with synchronous EO-CRLM compared to those with synchronous late-onset colorectal liver metastases.
• This study firstly present a comprehensive insight into the epidemiology, clinicopathological characteristics and survival outcome of synchronous EO-CRLM patients.
What is the implication, and what should change now?
• There is a rapid increase in the incidence and prevalence of patients with EO-CRLM, with notable variations in the disease burden across different socioeconomic strata and with distinct clinicopathological characteristics. These disparities reflect health care inequities within the overall population.
Introduction
Colorectal cancer (CRC) is a global health challenge, ranking as the third most prevalent cancer and the second leading cause of cancer-related mortality (1). In recent decades, there has been an increase in cases of early-onset colorectal cancer (EO-CRC), characterized by diagnoses in patients under the age of 50 (2). Such increases have led to lowering the age for CRC screening. Currently, EO-CRC accounts for approximately 10% of all new CRC cases, and there has been a simultaneous rise in CRC-related mortality among younger individuals (3). In stark contrast, there has been a consistent decline in the incidence and mortality of later-onset CRC worldwide (4).
Approximately 20% of CRC patients have synchronous colorectal liver metastases (CRLM) at the time of diagnosis, which becomes the primary cause of mortality in these patients (5). Little is known, however, regarding trends, demographic characteristics, clinicopathological features, and survival outcomes of patients with synchronous early-onset (younger than 50 years old) colorectal liver metastases (EO-CRLM) compared to those with synchronous late-onset (50 years old or older) colorectal liver metastases (LO-CRLM). Gaining insight into such differences may help identify at-risk patients, guide management, inform guidelines, and implement strategies to address the increasing burden of EO-CRC. Therefore, in this study we aimed to characterize EO-CRLM as a distinct disease utilizing data sourced from the National Cancer Database (NCDB). We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-66/rc).
Methods
Data source
Patients with synchronous CRLM diagnosed from 2010–2019 were identified in the NCDB (https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/). The NCDB encompasses approximately 70-80% of all new cancer diagnoses in the United States and monitors patient outcomes from over 1,400 accredited cancer treatment centers recognized by the American College of Surgeons Commission on Cancer (CoC). Relevant population data were sourced from the U.S. Census Bureau. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The study utilized morphology codes from the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) for CRC, specifically codes 8140, 8480, 8481, and 8490. The primary sites of tumors included the colon (C18.0-C18.9) and rectum (C20.9). Inclusion criteria targeted CRC patients with synchronous liver metastases at the initial diagnosis. Meanwhile, individuals under 18 years of age and those with metachronous CRLM were excluded. Data collected encompassed patient demographics (age, sex, race, year of diagnosis, insurance status, facility type, insurance type, median household income, high school education, rural-urban classification, and Charlson-Deyo score), tumor characteristics [primary tumor site, tumor grade, T stage, N stage, M stage, presence of bone, lung, or brain metastases, lymphovascular invasion, tumor deposits, perineural invasion, tumor size, carcinoembryonic antigen (CEA) levels, microsatellite instability (MSI) status, and Kirsten rat sarcoma viral oncogene homolog (KRAS) status], treatment modalities (primary tumor resection, metastasis resection, radiotherapy, chemotherapy, and immunotherapy) at any time during therapy, and patient survival characteristics. According to the age of onset of patients with synchronous CRLM, patients were stratified into 2 groups: EO-CRLM (younger than 50 years old) and LO-CRLM (50 years old or older).
Statistical analysis
Incidence and limited-duration prevalence rates (over a 10-year period) (6) were calculated. To account for age differences, age-adjusted incidence rates were computed by applying weighted proportions of the corresponding age groups in the 2000 US standard population. The trend of the age-adjusted incidence rate was assessed using Joinpoint regression analysis (7), which enabled us to analyze data on a logarithmic scale within a specified time interval. To summarize the trend of the age-standardized rate, the average annual percentage change (AAPC) was calculated by utilizing the Joinpoint regression analysis. The AAPC serves as a widely recognized and comprehensive measure of the trend observed in the age-standardized rate (8-10). The Joinpoint regression analysis employed a Monte Carlo permutation method to test the significance of each group. This approach allowed us to select the most appropriate model and estimate the AAPC along with its corresponding 95% confidence interval (CI). The AAPC provides a concise and informative summary of the trend observed over the past 10 years. Joinpoint regression analyses were performed by Joinpoint regression program (Version 4.9.0.1–February, 2022; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute).
Overall survival (OS) time was defined as the interval from the date of diagnosis to the date of death or last follow-up. Based on various sub-variables, including patient demographics, tumor characteristics, and treatment, OS (median and 1-, 3-, and 5-year) was evaluated for all patients, as well as separately for patients with EO-CRLM versus LO-CRLM. Multiple imputation by chained equations (MICE) (11) was used to impute missing data and several imputed datasets were generated for subsequent analyses. To address missing data, we employed MICE (11), resulting in the generation of several imputed datasets for subsequent analyses. To mitigate selection bias between EO-CRLM and LO-CRLM, we applied inverse probability of treatment weighting (IPTW) (12). For each imputed dataset, we conducted multivariable logistic regression to derive the propensity scores. These scores were aggregated by averaging individual estimates from the imputed datasets, following Rubin’s rules (13). We then compared the baseline characteristics of EO-CRLM and LO-CRLM patients in our study population, utilizing the estimated propensity scores to weight each patient to achieve balance between the two groups. The balance of baseline characteristics was evaluated using the standardized difference (SD) method. OS was analyzed with adjusted Kaplan-Meier curves and log-rank tests that were based on IPTW. Additionally, an IPTW-adjusted Cox model was utilized to calculate the hazard ratio (HR). A two-sided significance threshold of P<0.05 was applied. The survival analyses were conducted using R software version 4.1.1 (Vienna, Austria).
Results
Epidemiology
Population
The study included a total of 115,422 patients with synchronous CRLM, including 17,536 patients with EO-CRLM (15.2%) and 97,885 patients with LO-CRLM (84.8%). The AAPC of incidence and prevalence for CRLM patients, EO-CRLM patients and LO-CRLM patients among sub-population stratified by demographics, tumor characteristics and treatment are shown in Table 1.
Table 1
| Variables | AAPC (95% CI) | ||
|---|---|---|---|
| CRLM | EO-CRLM | LO-CRLM | |
| Sex | |||
| Female | 1.5 (0.8, 2.2) | 4.8 (3.4, 6.2) | 0.8 (0.1, 1.4) |
| Male | 2.8 (2.1, 3.5) | 5.4 (4.0, 6.8) | 2.3 (1.5, 3.0) |
| Race | |||
| White | 1.8 (1.3, 2.4) | 4.6 (3.6, 5.6) | 1.2 (0.6, 1.8) |
| Black | 3.0 (2.0, 4.0) | 5.0 (3.0, 7.0) | 2.5 (1.6, 3.4) |
| Rural-urban grouping | |||
| Metro areas | 2.2 (1.6, 2.9) | 5.4 (4.2, 6.6) | 1.5 (0.9, 2.2) |
| Urban areas | 2.4 (1.6, 3.2) | 4.0 (2.4, 5.7) | 2.1 (1.2, 2.9) |
| Rural areas | 0.7 (−1.0, 2.5) | 0.8 (−6.0, 8.1) | 0.7 (−0.5,1.9) |
| Percent of no high school degree | |||
| ≥21% | 1.6 (1.1, 2.1) | 4.9 (3.7, 6.1) | 0.8 (0.2, 1.4) |
| 13.0–20.9% | 1.3 (0.6, 1.9) | 3.7 (1.8, 5.5) | 0.7 (0, 1.5) |
| 7.0–12.9% | 1.3 (0.5, 2.0) | 4.3 (2.6, 6.0) | 0.6 (−0.2, 1.3) |
| <7.0% | 0.9 (0.2, 1.7) | 4.2 (2.8, 5.6) | 0.1 (−0.7, 0.9) |
| Median income | |||
| <$38,000 | 1.0 (−0.1, 2.1) | 3.5 (1.9, 5.1) | 0.7 (0.2, 1.3) |
| $38,000–$47,999 | 1.0 (0.5, 1.6) | 4.2 (2.5, 5.9) | 0.3 (−0.4, 1.0) |
| $48,000–$62,999 | 1.3 (0.5, 2.2) | 4.2 (2.4, 6.1) | 0.6 (−0.3, 1.5) |
| ≥$63,000 | 1.4 (0.6, 2.3) | 4.8 (3.4, 6.2) | 0.6 (−0.3, 1.5) |
| Insurance status | |||
| Not insured | −2.2 (−5.0, 0.7) | −1.3 (−5.3, 2.9) | −2.6 (−5.2, 0.1) |
| Private insurance/Managed care | 2.6 (1.2, 3.9) | 5.8 (4.5, 7.1) | 1.6 (0.8, 2.3) |
| Medicaid | 5.6 (4.4, 6.7) | 6.7 (4.3, 9.2) | 4.7 (3.6, 5.9) |
| Medicare | 1.5 (0.6, 2.4) | 2.9 (0.2, 5.5) | 1.5 (0.6, 2.4) |
| Other government | 7.6 (5.5, 9.7) | 8.5 (0.8, 16.8) | 7.3 (5.3, 9.3) |
| Primary tumor site | |||
| Primary sites in colon | 2.0 (1.3, 2.6) | 4.9 (3.7, 6.1) | 1.4 (0.8, 1.9) |
| Primary sites in rectum | 3.4 (2.6, 4.2) | 5.9 (4.7, 7.0) | 2.6 (1.7, 3.6) |
| T stage | |||
| T1/T2 | −5.1 (−6.7, −3.4) | −2.1 (−8.8, 5.0) | −5.3 (−9.4, −1.0) |
| T3/4 | −1.5 (−2.6, −0.3) | 1.9 (0.9, 3.1) | −2.0 (−2.7, −1.2) |
| N stage | |||
| N0 | 3.3 (1.0, 5.7) | 6.2 (3.2, 9.3) | 3.9 (2.2, 5.7) |
| N1 | 1.3 (0.1, 2.5) | 4.0 (1.5, 6.5) | 1.3 (−0.6, 3.2) |
| N2 | −1.1 (−2.1, 0) | 2.8 (1.7, 3.9) | −2.2 (−3.4, −0.9) |
| M stage | |||
| M1a | 8.2 (5.9, 10.5) | 11.2 (8.1, 14.3) | 7.4 (5.3, 9.6) |
| M1b/M1c | 7.5 (4.9, 10.2) | 9.9 (8.0, 11.9) | 7.5 (5.6, 9.4) |
| MSI status | |||
| MSS | 25.9 (21.9, 30.1) | 22.4 (17.7, 27.3) | 27.6 (21.3, 34.2) |
| MSI-high | 35.3 (24.8, 46.8) | 28.7 (13.1, 46.3) | 38.3 (28.9, 48.4) |
| MSI-low | 17.3 (13.1, 21.6) | 15.6 (6.9, 25.0) | 17.9 (13.2, 22.9) |
| KRAS mutation | |||
| NO | 5.0 (3.6, 6.5) | 5.7 (3.8, 7.6) | 3.9 (1.5, 6.4) |
| Yes | 8.5 (6.6, 10.4) | 10.6 (8.8, 12.5) | 7.7 (6.2, 9.3) |
| Bone metastases | |||
| No | 2.3 (1.7, 2.9) | 5.2 (4.2,6.3) | 1.6 (1.0, 2.2) |
| Yes | 3.9 (2.8, 5.0) | 6.2 (2.6, 9.9) | 3.4 (2.1, 4.8) |
| Lung metastases | |||
| No | 1.8 (1.1, 2.5) | 4.8 (3.8, 5.9) | 1.1 (0.4, 1.7) |
| Yes | 4.3 (3.5, 5.1) | 6.9 (5.1, 8.8) | 3.7 (3.0, 4.4) |
| Treatment methods | |||
| Primary tumor resection | −3.4 (−4.0, −2.9) | −2.4 (−3.5, −1.3) | −3.7 (−4.2, −3.1) |
| Metastatic tumor resection | −1.0 (−1.5, −0.5) | 0.9 (−0.5, 2.4) | −1.7 (−2.5, −0.9) |
| Radiotherapy | 0.0 (−0.6, 0.7) | 0.0 (−1.3, 1.3) | −0.1 (−0.8, 0.6) |
| Chemotherapy | 0.4 (0.2, 0.7) | 0.3 (0, 0.5) | 0.5 (0.2, 0.8) |
| Endocrine therapy | 1.2 (−7.4, 10.5) | 3.7 (−7.3, 16.0) | −3.0 (−13.4, 8.6) |
| Immunotherapy | 64.5 (27.4, 112.4) | 63.5 (32.4, 101.8) | 64.6 (26.1, 115.0) |
AAPC, average annual percentage change; CI, confidence interval; CRLM, colorectal liver metastases; EO-CRLM, early-onset colorectal liver metastases; LO-CRLM, late-onset colorectal liver metastases; MSI, microsatellite instability; MSS, microsatellite stability; KRAS, Kirsten rat sarcoma viral oncogene homolog.
Annual incidence
The annual age-adjusted incidence of EO-CRLM increased from 0.48 per 100,000 in 2010 to 0.69 per 100,000 in 2019 (AAPC: 5.1, 95% CI: 4.1 to 6.1) as shown in Figure 1. Alternatively, the annual age-adjusted incidence of LO-CRLM increased from 2.37 per 100,000 in 2010 to 2.62 per 100,000 in 2019 (AAPC: 1.6, 95% CI: 1.0 to 2.2). Although the incidence of synchronous liver metastasis increased in both the early onset and late onset cohort, the rate of increase was greater in EO-CRLM compared with LO-CRLM (AAPC difference: 3.5, 95% CI: 2.5 to 4.5; P<0.001).

Annual incidence among sub-population stratified by demographics
The annual incidence of EO-CRLM and LO-CRLM from 2010 to 2019 among sub-populations stratified by demographics is shown in Figure 2 and Figure S1, respectively. The AAPC of incidence among sub-population stratified by demographics is shown in Table 1. For EO-CRLM patients, the largest increase of age-adjusted incidence was observed in males (AAPC: 5.4, 95% CI: 4.0 to 6.8), those identifying as black race (AAPC: 5.0, 95% CI: 3.0 to 7.0) , those having other government insurance (AAPC: 8.5, 95% CI: 0.8 to 16.8), those with median income ≥$63,000 (AAPC: 4.8, 95% CI: 3.4 to 6.2) and those with percent of no high school degree ≥21.0% (AAPC: 4.9, 95% CI: 3.7 to 6.1). For geographical distribution, the incidence of EO-CRLM in metropolitan area, EO-CRLM in urban area, and EO-CRLM in rural area increased with AAPCs of 5.4 (95% CI: 4.2 to 6.6), 4.0 (95% CI: 2.4 to 5.7), and 0.8 (95% CI: −6.0 to 8.1), respectively. While the incidence of LO-CRLM in metropolitan area, urban area, and rural area increased with AAPCs of 1.5 (95% CI: 0.9 to 2.2), 2.1 (95% CI: 1.2 to 2.9), and 0.7 (95% CI: −0.5 to1.9), respectively. Geographically, the annual incidence of EO-CRLM was growing faster than the incidence of LO-CRLM, regardless of whether it is the metropolitan (P<0.001), or urban area (P<0.001).

Annual incidence among sub-population stratified by tumor characteristics
The annual incidence of EO-CRLM and LO-CRLM from 2010 to 2019 among sub-population stratified by tumor characteristics is shown in Figure 3 and Figure S2, respectively. The AAPC of incidence among sub-population stratified by tumor characteristics is shown in Table 1. The incidence of EO-CRLM patients with primary tumor site located in rectum had the sharpest increase, with the AACP of 5.9 (95% CI: 4.7 to 7.0). For T stage, the increased incidence in EO-CRLM with T3/T4 stage was from 0.32 per 100,000 population in 2010 to 0.35 per 100,000 population in 2019 (AAPC: 1.9, 95% CI: 0.9 to 3.1). For N stage, the largest increase in age-adjusted incidence was observed in EO-CRLM with N0 stage (AAPC: 6.2, 95% CI: 3.2 to 9.3). For M stage, the largest increase of age-adjusted incidence was observed in EO-CRLM with M1a stage (AAPC: 11.2, 95% CI: 8.1 to 14.3). For extra-hepatic metastases, the incidence increased faster in EO-CRLM patients with lung metastases than that in LO-CRLM patients with lung metastases (AAPC difference: 3.2, 95% CI: 1.6 to 4.9, P<0.001); and the increased incidence was faster in EO-CRLM patients without bone metastases than that in LO-CRLM patients without bone metastases (AAPC difference: 3.6, 95% CI: 2.6 to 4.6, P<0.001).

Across sub-type gene groups, EO-CRLM with MSI-high had the largest increase of incidence with AAPC of 28.7 (95% CI: 13.1 to 46.3) than MSI-low with AAPC of 15.6 (95% CI: 6.9 to 25.0) and microsatellite stability (MSS) with AAPC of 22.4 (95% CI: 17.7 to 27.3). EO-CRLM with KRAS mutation had the higher increase of incidence with AAPC of 10.6 (95% CI: 8.8 to12.5) than EO-CRLM with non KRAS mutation (AAPC: 5.7, 95% CI: 3.8 to 7.6) (P<0.001).
Treatment trends
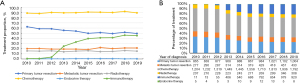
The treatment proportion of EO-CRLM and LO-CRLM from 2010 to 2019 is shown in Figure 4 and Figure S3, respectively. Chemotherapy was the primary therapy for EO-CRLM patients. From 2010 to 2019, at least 49.3% of patients were treated with chemotherapy. EO-CRLM patients undergoing immunotherapy had grown significantly faster since 2012, with AAPC of 63.5 (95% CI: 32.4 to 101.8). The proportion of EO-CRLM patients undergoing radiotherapy and chemotherapy showed a relatively stable trend. There was a downward trend in the proportion of EO-CRLM patients undergoing primary tumor resection from 62.50% to 49.46% (AAPC: −2.4, 95% CI: −3.5 to −1.3) (Figure 4).
Prevalence
Reflecting the rising incidence of CRLM, the 10-year limited-duration prevalence increased substantially, from 0.0032% in 2010 to 0.0102% in 2019 (AAPC: 14.2, 95% CI: 12.0 to 16.4) (Figure 5). The 10-year limited-duration prevalence for EO-CRLM increased from 0.0004% in 2010 to 0.0020% in 2019 (AAPC: 15.7, 95% CI: 10.2 to 21.5). The 10-year limited-duration prevalence for LO-CRLM increased from 0.0027% in 2010 to 0.0082% in 2019 (AAPC: 11.1, 95% CI: 6.9 to 15.4).

Clinicopathological characteristics analyses
Clinicopathological characteristics of CRLM, EO-CRLM and LO-CRLM were presented in Table 2. The populations were with a median age of 64.0 years old and 50,872 (44.1%) were female. The primary tumor sites were colon in 91,888 (79.6%) patients and rectum in 23,534 (20.4%) patients. Of 42,644 CRLM with a known KRAS status, 20,007 (46.9%) were KRAS mutation. Of 30,594 CRLM with a known MSI status, 1,371 (4.5%) were MSI-high, and 1,368 (4.5%) were MSI-low. Compared with LO-CRLM patients, EO-CRLM patients were more likely to be female, identify as black, have a Charlson-Deyo score of 0, reside in metropolitan areas, have a higher percentage of high school graduation, possess a median income of ≥$63,000, have private insurance or managed care, be diagnosed between 2016 and 2019. Patients with EO-CRLM were also more likely to have primary tumors located in the rectum, exhibit moderate differentiation, display perineural invasion, N2 stage, have no bone or lung metastases, have non KRAS mutation, exhibit MSS, undergo primary tumor resection, undergo metastatic tumor resection, receive chemotherapy, and receive immunotherapy.
Table 2
| Variables | CRLM, n=115,422 | EO-CRLM, n=17,536 | LO-CRLM, n=97,886 | P* |
|---|---|---|---|---|
| Age (years), mean (SD) | 63.9 (13.6) | 42.5 (6.0) | 67.7 (10.8) | <0.001 |
| Female, n (%) | 50,872 (44.1) | 7,862 (44.8) | 43,010 (43.9) | 0.02 |
| Race, n (%) | <0.001 | |||
| White | 90,512 (78.4) | 13,271 (75.7) | 77,241 (78.9) | |
| Black | 18,571 (16.1) | 2,942 (16.8) | 15,629 (16.0) | |
| Others | 5,423 (4.7) | 1,154 (6.6) | 4,269 (4.4) | |
| Missing | 916 (0.8) | 169 (1.0) | 747 (0.8) | |
| Charlson-Deyo score, n (%) | <0.001 | |||
| 0 | 85,626 (74.2) | 15,303 (87.3) | 70,323 (71.8) | |
| 1 | 19,994 (17.3) | 1,730 (9.9) | 18,264 (18.7) | |
| ≥2 | 9,802 (8.5) | 503 (2.9) | 9,299 (9.5) | |
| Rural-urban grouping, n (%) | <0.001 | |||
| Metro areas | 92,696 (80.3) | 14,263 (81.3) | 78,433 (80.1) | |
| Urban areas | 17,237 (14.9) | 2,306 (13.2) | 14,931 (15.3) | |
| Rural areas | 2,343 (2.0) | 269 (1.5) | 2,074 (2.1) | |
| Missing | 3,146 (2.7) | 698 (4.0) | 2,448 (2.5) | |
| Facility type, n (%) | <0.001 | |||
| Community cancer program | 9,933 (8.6) | 929 (5.3) | 9,004 (9.2) | |
| Comprehensive community cancer program | 43,519 (37.7) | 4,506 (25.7) | 39,013 (39.9) | |
| Academic/research program | 35,536 (30.8) | 5,207 (29.7) | 30,329 (31.0) | |
| Integrated network cancer program | 21,941 (19.0) | 2,401 (13.7) | 19,540 (20.0) | |
| Missing | 4,493 (3.9) | 4,493 (25.6) | 0 (0.0) | |
| Percent of no high school degree, n (%) | <0.001 | |||
| ≥21% | 20,422 (17.7) | 3,239 (18.5) | 17,183 (17.6) | |
| 13.0–20.9% | 27,589 (23.9) | 3,916 (22.3) | 23,673 (24.2) | |
| 7.0–12.9% | 32,171 (27.9) | 4,726 (27.0) | 27,445 (28.0) | |
| <7.0% | 22,081 (19.1) | 3,519 (20.1) | 18,562 (19.0) | |
| Missing | 13,159 (11.4) | 2,136 (12.2) | 11,023 (11.3) | |
| Median income, n (%) | <0.001 | |||
| <$38,000 | 20,444 (17.7) | 2,893 (16.5) | 17,551 (17.9) | |
| $38,000–$47,999 | 24,202 (21.0) | 3,505 (20.0) | 20,697 (21.1) | |
| $48,000–$62,999 | 26,895 (23.3) | 4,116 (23.5) | 22,779 (23.3) | |
| ≥$63,000 | 30,670 (26.6) | 4,879 (27.8) | 25,791 (26.3) | |
| Missing | 13,211 (11.4) | 2,143 (12.2) | 11,068 (11.3) | |
| Insurance status, n (%) | <0.001 | |||
| Not insured | 6,006 (5.2) | 1,395 (8.0) | 4,611 (4.7) | |
| Private insurance/Managed care | 42,464 (36.8) | 1,1612 (66.2) | 30,852 (31.5) | |
| Medicaid | 11,353 (9.8) | 3,186 (18.2) | 8,167 (8.3) | |
| Medicare | 52,487 (45.5) | 819 (4.7) | 51,668 (52.8) | |
| Other government | 1,376 (1.2) | 244 (1.4) | 1,132 (1.2) | |
| Missing | 1,736 (1.5) | 280 (1.6) | 1,456 (1.5) | |
| Diagnosis year, n (%) | <0.001 | |||
| 2010–2012 | 30,084 (26.1) | 4,317 (24.6) | 25,767 (26.3) | |
| 2013–2015 | 33,937 (29.4) | 5,034 (28.7) | 28,903 (29.5) | |
| 2016–2019 | 51,401 (44.5) | 8,185 (46.7) | 43,216 (44.1) | |
| Primary sites in rectum, n (%) | 23,534 (20.4) | 4,553 (26.0) | 18,981 (19.4) | <0.001 |
| CEA, mean (SD), ng/mL | 50.6 (41.1) | 50.1 (41.4) | 50.7 (41.0) | 0.01 |
| Grade, n (%) | <0.001 | |||
| Well differentiation | 5,426 (4.7) | 881 (5.0) | 4,545 (4.6) | |
| Moderate differentiation | 56,687 (49.1) | 9,344 (53.3) | 47,343 (48.4) | |
| Poor differentiation | 17,421 (15.1) | 2,600 (14.8) | 14,821 (15.1) | |
| Un-differentiation | 1,995 (1.7) | 289 (1.6) | 1,706 (1.7) | |
| Missing | 33,893 (29.4) | 4,422 (25.2) | 29,471 (30.1) | |
| Tumor size, mean (SD), cm | 5.7 (4.8) | 5.7 (4.6) | 5.7 (4.8) | 0.97 |
| Lymph invasion, n (%) | 0.31 | |||
| No | 22,654 (19.6) | 3,723 (21.2) | 18,931 (19.3) | |
| Yes | 28,782 (24.9) | 4,826 (27.5) | 23,956 (24.5) | |
| Missing | 63,986 (55.4) | 8,987 (51.2) | 54,999 (56.2) | |
| Perineural invasion, n (%) | <0.001 | |||
| No | 32,844 (28.5) | 5,026 (28.7) | 27,818 (28.4) | |
| Yes | 12,534 (10.9) | 2,337 (13.3) | 10,197 (10.4) | |
| Missing | 70,044 (60.7) | 10,173 (58.0) | 59,871 (61.2) | |
| T stage, n (%) | <0.001 | |||
| T1 | 5,527 (4.8) | 853 (4.9) | 4,674 (4.8) | |
| T2 | 2,920 (2.5) | 564 (3.2) | 2,356 (2.4) | |
| T3 | 35,407 (30.7) | 6,116 (34.9) | 29,291 (29.9) | |
| T4 | 26,009 (22.5) | 3,987 (22.7) | 22,022 (22.5) | |
| Missing | 45,559 (39.5) | 6,016 (34.3) | 39,543 (40.4) | |
| N stage, n (%) | <0.001 | |||
| N0 | 33,559 (29.1) | 4,292 (24.5) | 29,267 (29.9) | |
| N1 | 33,020 (28.6) | 5,422 (30.9) | 27,598 (28.2) | |
| N2 | 26,863 (23.3) | 4,913 (28.0) | 21,950 (22.4) | |
| Missing | 21,980 (19.0) | 2,909 (16.6) | 19,071 (19.5) | |
| M stage, n (%) | <0.001 | |||
| M1a | 54,630 (47.3) | 8,521 (48.6) | 46,109 (47.1) | |
| M1b | 35,328 (30.6) | 5,436 (31.0) | 29,892 (30.5) | |
| M1c | 3,378 (2.9) | 548 (3.1) | 2,830 (2.9) | |
| Missing | 22,086 (19.1) | 3,031 (17.3) | 19,055 (19.5) | |
| Bone metastases, n (%) | 0.01 | |||
| No | 107,965 (93.5) | 16,526 (94.2) | 91,439 (93.4) | |
| Yes | 6,071 (5.3) | 860 (4.9) | 5,211 (5.3) | |
| Missing | 1,386 (1.2) | 150 (0.9) | 1,236 (1.3) | |
| Brain metastases, n (%) | 0.40 | |||
| No | 112,444 (97.4) | 17,154 (97.8) | 95,290 (97.3) | |
| Yes | 1,284 (1.1) | 185 (1.1) | 1,099 (1.1) | |
| Missing | 1,694 (1.5) | 197 (1.1) | 1,497 (1.5) | |
| Lung metastases, n (%) | <0.001 | |||
| No | 86,427 (74.9) | 13,556 (77.3) | 72,871 (74.4) | |
| Yes | 27,025 (23.4) | 3,756 (21.4) | 23,269 (23.8) | |
| Missing | 1,970 (1.7) | 224 (1.3) | 1,746 (1.8) | |
| KRAS mutation, n (%) | <0.001 | |||
| No | 22,637 (19.6) | 4,542 (25.9) | 18,095 (18.5) | |
| Yes | 20,007 (17.3) | 3,649 (20.8) | 16,358 (16.7) | |
| Missing | 72,778 (63.1) | 9,345 (53.3) | 63,433 (64.8) | |
| MSI status, n (%) | 0.01 | |||
| MSS | 27,855 (24.1) | 5,754 (32.8) | 22,101 (22.6) | |
| MSI-high | 1,371 (1.2) | 240 (1.4) | 1,131 (1.2) | |
| MSI-low | 1,368 (1.2) | 269 (1.5) | 1,099 (1.1) | |
| Missing | 84,828 (73.5) | 11,273 (64.3) | 73,555 (75.1) | |
| Primary tumor site resection, n (%) | <0.001 | |||
| No | 60,623 (52.5) | 8,135 (46.4) | 52,488 (53.6) | |
| Yes | 54,465 (47.2) | 9,354 (53.3) | 45,111 (46.1) | |
| Missing | 334 (0.3) | 47 (0.3) | 287 (0.3) | |
| Metastases resection, n (%) | <0.001 | |||
| No | 99,397 (86.1) | 13,947 (79.5) | 85,450 (87.3) | |
| Yes | 15,739 (13.6) | 3,538 (20.2) | 12,201 (12.5) | |
| Missing | 286 (0.2) | 51 (0.3) | 235 (0.2) | |
| Radiation, n (%) | <0.001 | |||
| No | 100,269 (86.9) | 14,451 (82.4) | 85,818 (87.7) | |
| Yes | 12,314 (10.7) | 2,604 (14.8) | 9,710 (9.9) | |
| Missing | 2,839 (2.5) | 481 (2.7) | 2,358 (2.4) | |
| Chemotherapy, n (%) | <0.001 | |||
| No | 30,703 (26.6) | 1,704 (9.7) | 28,999 (29.6) | |
| Yes | 81,614 (70.7) | 15,458 (88.2) | 66,156 (67.6) | |
| Missing | 3,105 (2.7) | 374 (2.1) | 2,731 (2.8) | |
| Immunotherapy, n (%) | <0.001 | |||
| No | 90,089 (78.1) | 12,210 (69.6) | 77,879 (79.6) | |
| Yes | 24,894 (21.6) | 5,258 (30.0) | 19,636 (20.1) | |
| Missing | 439 (0.4) | 68 (0.4) | 371 (0.4) |
*, P value was calculated in the complete data excluding missing data. CEA, carcinoembryonic antigen; CRLM, colorectal liver metastases; EO-CRLM, early-onset colorectal liver metastases; LO-CRLM, late-onset colorectal liver metastases; MSI, microsatellite instability; MSS, microsatellite stability; KRAS, Kirsten rat sarcoma viral oncogene homolog; SD, standardized difference.
Survival analyses
Patients without survival data (n=13,140) were excluded, resulting in 102,282 patients evaluable for the endpoint of OS. Within this cohort, 15,485 (15.1%) patients had EO-CRLM and 86,797 (84.9%) patients had LO-CRLM. After multiple imputation, the comparison of the unadjusted and adjusted SDs between EO-CRLM and LO-CRLM were presented in Table 3. After the IPTW adjustment, the weighted population between two groups was subsequently comparable.
Table 3
| Variables | CRLM, n=102,282 | Unweighted study population, n (%) | Weighted study population, % | |||||
|---|---|---|---|---|---|---|---|---|
| EO-CRLM, n=15,485 | LO-CRLM, n=86,797 | SD | EO-CRLM | LO-CRLM | SD | |||
| Female, n (%) | 45,319 (44.3) | 6,959 (44.9) | 38,360 (44.2) | 0.008 | 41.0 | 44.3 | 0.033 | |
| Race, n (%) | ||||||||
| White | 80,380 (78.6) | 11,743 (75.8) | 68,637 (79.1) | 0.031 | 76.4 | 79.2 | 0.027 | |
| Black | 16,421 (16.1) | 2,597 (16.8) | 13,824 (15.9) | 0.009 | 19.2 | 16.2 | 0.030 | |
| Others | 4,674 (4.6) | 995 (6.4) | 3,679 (4.2) | 0.022 | 4.4 | 4.6 | 0.003 | |
| Charlson-Deyo score, n (%) | ||||||||
| 0 | 75,985 (74.3) | 13,537 (87.4) | 62,448 (71.9) | 0.155 | 74.1 | 74.3 | 0.002 | |
| 1 | 17,943 (17.5) | 1,522 (9.8) | 16,421 (18.9) | 0.091 | 16.8 | 17.5 | 0.007 | |
| ≥2 | 8,354 (8.2) | 426 (2.8) | 7,928 (9.1) | 0.064 | 9.1 | 8.2 | 0.009 | |
| Rural-urban grouping, n (%) | ||||||||
| Metro areas | 82,161 (80.3) | 12,599 (81.4) | 69,562 (80.1) | 0.026 | 82.5 | 82.6 | 0.001 | |
| Urban areas | 15,228 (14.9) | 2,028 (13.1) | 13,200 (15.2) | 0.020 | 15.6 | 15.3 | 0.003 | |
| Rural areas | 2,073 (2.0) | 233 (1.5) | 1,840 (2.1) | 0.006 | 1.9 | 2.1 | 0.002 | |
| Facility type, n (%) | ||||||||
| Community cancer program | 8,762 (8.6) | 812 (5.2) | 7,950 (9.2) | 0.020 | 8.7 | 8.9 | 0.002 | |
| Comprehensive community cancer program | 38,604 (37.7) | 3,969 (25.6) | 34,635 (39.9) | 0.053 | 38.2 | 39.1 | 0.010 | |
| Academic/research program | 31,453 (30.8) | 4,611 (29.8) | 26,842 (30.9) | 0.087 | 34.2 | 32.2 | 0.020 | |
| Integrated network cancer program | 19,499 (19.1) | 2,129 (13.7) | 17,370 (20.0) | 0.015 | 18.9 | 19.8 | 0.009 | |
| Percent of no high school degree, n (%) | ||||||||
| ≥21% | 18,159 (17.8) | 28,59 (18.5) | 15,300 (17.6) | 0.011 | 23.8 | 20.0 | 0.039 | |
| 13.0–20.9% | 24,461 (23.9) | 3,434 (22.2) | 21,027 (24.2) | 0.019 | 28.4 | 26.9 | 0.015 | |
| 7.0–12.9% | 28,638 (28.0) | 4,199 (27.1) | 24,439 (28.2) | 0.007 | 29.2 | 31.5 | 0.023 | |
| <7.0% | 19,670 (19.2) | 3,138 (20.3) | 16,532 (19.0) | 0.015 | 18.5 | 21.7 | 0.031 | |
| Median income, n (%) | ||||||||
| <$38,000 | 18,225 (17.8) | 2,575 (16.6) | 15,650 (18.0) | 0.013 | 23.1 | 20.1 | 0.030 | |
| $38,000–$47,999 | 21,492 (21.0) | 3,095 (20.0) | 18,397 (21.2) | 0.012 | 26.2 | 23.7 | 0.026 | |
| $48,000–$62,999 | 23,919 (23.4) | 3,639 (23.5) | 20,280 (23.4) | 0.004 | 24.7 | 26.3 | 0.016 | |
| ≥$63,000 | 27,246 (26.6) | 4,314 (27.9) | 22,932 (26.4) | 0.020 | 26.0 | 30.0 | 0.040 | |
| Insurance status, n (%) | ||||||||
| Not insured | 5,403 (5.3) | 1,254 (8.1) | 4,149 (4.8) | 0.033 | 5.6 | 5.4 | 0.002 | |
| Private insurance/Managed care | 37,727 (36.9) | 10,256 (66.2) | 27,471 (31.6) | 0.348 | 38.2 | 37.4 | 0.008 | |
| Medicaid | 9,922 (9.7) | 2,780 (18.0) | 7,142 (8.2) | 0.098 | 10.2 | 9.8 | 0.003 | |
| Medicare | 46,498 (45.5) | 734 (4.7) | 45,764 (52.7) | 0.481 | 44.9 | 46.2 | 0.013 | |
| Other government | 1,158 (1.1) | 204 (1.3) | 954 (1.1) | 0.002 | 1.1 | 1.1 | 0.001 | |
| Diagnosis year, n (%) | ||||||||
| 2010–2012 | 30,036 (29.4) | 4,314 (27.9) | 25,722 (29.6) | 0.018 | 28.9 | 29.4 | 0.005 | |
| 2013–2015 | 33,897 (33.1) | 5,031 (32.5) | 28,866 (33.3) | 0.008 | 34.0 | 33.2 | 0.008 | |
| 2016–2019 | 38,349 (37.5) | 6,140 (39.7) | 32,209 (37.1) | 0.025 | 37.1 | 37.5 | 0.003 | |
| Primary sites in rectum, n (%) | 20,798 (20.3) | 3,994 (25.8) | 16,804 (19.4) | 0.064 | 22.3 | 20.3 | 0.019 | |
| CEA, mean (SD), ng/mL | 50.60 (41.08) | 50.12 (41.37) | 50.69 (41.03) | 0.028 | 50.7 (40.9) | 50.3 (41.0) | 0.013 | |
| Grade, n (%) | ||||||||
| Well differentiation | 4,903 (4.8) | 795 (5.1) | 4,108 (4.7) | 0.001 | 7.3 | 6.9 | 0.005 | |
| Moderate differentiation | 50,480 (49.4) | 8,297 (53.6) | 42,183 (48.6) | 0.019 | 70.5 | 69.6 | 0.009 | |
| Poor differentiation | 15,666 (15.3) | 2,349 (15.2) | 13,317 (15.3) | 0.018 | 19.9 | 21.3 | 0.014 | |
| Un-differentiation | 1,975 (1.9) | 285 (1.8) | 1,690 (1.9) | 0.002 | 2.2 | 2.2 | 0.001 | |
| Tumor size, mean (SD), cm | 5.66 (4.79) | 5.63 (4.46) | 5.66 (4.85) | 0.007 | 5.8 (4.9) | 5.8 (5.2) | 0.011 | |
| Lymph invasion, n (%) | 26,038 (25.5) | 4,317 (27.9) | 21,721 (25.0) | 0.034 | 35.1 | 35.8 | 0.007 | |
| Perineural invasion, n (%) | 12,526 (12.2) | 2,337 (15.1) | 10,189 (11.7) | 0.047 | 16.9 | 17.2 | 0.004 | |
| T stage, n (%) | ||||||||
| T1 | 5,176 (5.1) | 809 (5.2) | 4,367 (5.0) | 0.027 | 16.9 | 16.1 | 0.009 | |
| T2 | 2,680 (2.6) | 526 (3.4) | 2,154 (2.5) | 0.008 | 4.4 | 4.3 | 0.002 | |
| T3 | 32,310 (31.6) | 5,565 (35.9) | 26,745 (30.8) | 0.041 | 44.4 | 44.0 | 0.006 | |
| T4 | 23,249 (22.7) | 3,510 (22.7) | 19,739 (22.7) | 0.021 | 34.3 | 35.6 | 0.014 | |
| N stage, n (%) | ||||||||
| N0 | 29,755 (29.1) | 3,831 (24.7) | 25,924 (29.9) | 0.077 | 37.6 | 38.9 | 0.013 | |
| N1 | 29,657 (29.0) | 4,858 (31.4) | 24,799 (28.6) | 0.022 | 37.0 | 35.1 | 0.020 | |
| N2 | 24,216 (23.7) | 4,381 (28.3) | 19,835 (22.9) | 0.055 | 25.4 | 26.0 | 0.007 | |
| M stage, n (%) | ||||||||
| M1a | 47,621 (46.6) | 7,400 (47.8) | 40,221 (46.3) | 0.006 | 58.0 | 58.7 | 0.007 | |
| M1b | 32,083 (31.4) | 4,927 (31.8) | 27,156 (31.3) | 0.008 | 40.4 | 39.5 | 0.009 | |
| M1c | 1,578 (1.5) | 265 (1.7) | 1,313 (1.5) | 0.002 | 1.6 | 1.8 | 0.002 | |
| Bone metastases, n (%) | 5,335 (5.2) | 742 (4.8) | 4,593 (5.3) | 0.005 | 5.6 | 5.3 | 0.003 | |
| Brain metastases, n (%) | 1,147 (1.1) | 163 (1.1) | 984 (1.1) | 0.001 | 1.8 | 1.2 | 0.007 | |
| Lung metastases, n (%) | 23,736 (23.2) | 3,297 (21.3) | 20,439 (23.5) | 0.024 | 23.6 | 23.6 | 0.001 | |
| KRAS mutation, n (%) | 17,261 (16.9) | 3,142 (20.3) | 14,119 (16.3) | 0.023 | 46.1 | 46.6 | 0.007 | |
| MSI status, n (%) | ||||||||
| MSS | 2,0842 (20.4) | 4,491 (29.0) | 16,351 (18.8) | 0.011 | 90.7 | 90.8 | 0.003 | |
| MSI-high | 1,136 (1.1) | 207 (1.3) | 929 (1.1) | 0.006 | 5.1 | 5.2 | 0.004 | |
| MSI-low | 924 (0.9) | 195 (1.3) | 729 (0.8) | 0.005 | 4.2 | 4.0 | 0.003 | |
| Primary tumor site resection, n (%) | 49,128 (48.0) | 8,349 (53.9) | 40,779 (47.0) | 0.069 | 47.2 | 48.1 | 0.009 | |
| Metastases resection, n (%) | 14,050 (13.7) | 3,105 (20.1) | 10,945 (12.6) | 0.075 | 13.1 | 13.8 | 0.006 | |
| Radiation, n (%) | 10,916 (10.7) | 2,296 (14.8) | 8,620 (9.9) | 0.051 | 11.7 | 11.0 | 0.006 | |
| Chemotherapy, n (%) | 72,349 (70.7) | 13,666 (88.3) | 58,683 (67.6) | 0.205 | 71.4 | 72.4 | 0.010 | |
| Immunotherapy, n (%) | 20,526 (20.1) | 4,338 (28.0) | 16,188 (18.7) | 0.094 | 20.4 | 20.1 | 0.003 | |
CEA, carcinoembryonic antigen; CRLM, colorectal liver metastases; EO-CRLM, early-onset colorectal liver metastases; LO-CRLM, late-onset colorectal liver metastases; MSI, microsatellite instability; MSS, microsatellite stability; KRAS, Kirsten rat sarcoma viral oncogene homolog; SD, standardized difference.
The median follow-up time were 57.0 (IQR, 56.4–57.6) months. The median OS for all patients were 16.8 (IQR, 16.6–16.9) months. At the time of analysis, 80,157 patients (78.4%) were deceased. The 1-, 3- and 5-year OS rates were 59.1%, 26.3% and 15.0%, respectively. Among sub-population stratified by demographics, tumor characteristics and treatment, the median OS and 1-, 3- and 5-year OS rates in CRLM patients, EO-CRLM patients and LO-CRLM patients were shown in Table 4 and Table S1. Compared with LO-CRLM patients, EO-CRLM patients had a significantly favorable OS before [median: 27.2 (IQR, 13.3–57.1) vs. 14.9 (IQR, 4.0–34.8) months, P<0.001 in non IPTW-adjusted log-rank test] and after [median: 19.6 (IQR, 6.6–42.0) vs. 16.3 (IQR, 4.7–36.9) months, P<0.001 in IPTW-adjusted log-rank test] the IPTW adjustment (Figure 6). EO-CRLM was associated with a superior OS in IPTW-adjusted Cox proportional hazards regression analysis (HR: 0.876, 95% CI: 0.838–0.915; P<0.001).
Table 4
| Variables | Median OS (95% CI), months | ||
|---|---|---|---|
| CRLM | EO-CRLM | LO-CRLM | |
| None stratifying | 16.8 (16.6–16.9) | 27.2 (13.3–57.1) | 14.9 (4.0–34.8) |
| Charlson-Deyo score | |||
| 0 | 18.5 (18.2–18.7) | 27.7 (27.1–28.3) | 16.5 (16.3–16.8) |
| 1 | 14.3 (13.9–14.7) | 26.6 (25.1–28.1) | 13.2 (12.8–13.6) |
| ≥2 | 7.7 (7.2–8.1) | 16.4 (13.5–19.3) | 7.4 (6.9–7.8) |
| Sex | |||
| Female | 17.6 (17.4–17.9) | 27.1 (26.3–27.8) | 16.0 (15.7–16.2) |
| Male | 15.7 (15.5–16.0) | 27.4 (26.6–28.3) | 13.6 (13.3–13.8) |
| Race | |||
| White | 16.8 (16.6–17.0) | 28.8 (28.1–29.5) | 14.9 (14.7–15.1) |
| Black | 15.4 (15.0–15.8) | 21.3 (20.3–22.3) | 14.0 (13.6–14.4) |
| Others | 20.9 (20.0–21.7) | 29.5 (27.1–32.0) | 18.8 (17.7–19.8) |
| Rural-urban grouping | |||
| Metro areas | 16.7 (16.5–16.9) | 27.3 (26.6–27.9) | 14.9 (14.6–15.1) |
| Urban areas | 16.0 (15.6–16.5) | 25.6 (24.3–26.9) | 14.6 (14.1–15.0) |
| Rural areas | 14.7 (13.5–15.9) | 26.8 (23.8–29.8) | 13.2 (12.1–14.4) |
| Facility type | |||
| Community cancer program | 13.7 (13.1–14.2) | 24.7 (22.6–26.8) | 12.7 (12.1–13.2) |
| Comprehensive community cancer program | 14.6 (14.4–14.9) | 25.0 (24.0–26.0) | 13.5 (13.2–13.7) |
| Academic/research program | 20.0 (19.7–20.4) | 29.8 (28.8–30.9) | 18.4 (18.0–18.8) |
| Integrated network cancer program | 15.5 (15.1–15.9) | 28.1 (26.8–29.5) | 14.0 (13.6–14.4) |
| Percent of no high school degree | |||
| ≥21% | 16.1 (15.7–16.5) | 25.2 (24.1–26.4) | 14.5 (14.0–14.9) |
| 13.0–20.9% | 15.9 (15.5–16.2) | 25.2 (24.1–26.3) | 14.2 (13.9–14.6) |
| 7.0–12.9% | 16.7 (16.3–17.0) | 28.0 (26.8–29.1) | 14.7 (14.3–15.0) |
| <7.0% | 17.8 (17.4–18.2) | 30.1 (28.6–31.6) | 15.5 (15.1–15.9) |
| Median income | |||
| <$38,000 | 14.6 (14.2–15.0) | 24.4 (23.1–25.7) | 13.2 (12.8–13.6) |
| $38,000–$47,999 | 15.8 (15.5–16.2) | 25.1 (24.0–26.2) | 14.1 (13.7–14.5) |
| $48,000–$62,999 | 16.5 (16.2–16.9) | 26.7 (25.5–28.0) | 14.7 (14.3–15.0) |
| ≥$63,000 | 18.6 (18.2–19.0) | 30.9 (29.7–32.2) | 16.4 (16.0–16.7) |
| Insurance status | |||
| Not insured | 16.7 (15.9–17.4) | 20.4 (19.0–21.9) | 15.2 (14.3–16.4) |
| Private insurance/Managed care | 24.8 (24.5–25.1) | 30.3 (29.5–31.0) | 22.9 (22.5–23.3) |
| Medicaid | 18.4 (17.8–19.0) | 23.1 (21.9–24.3) | 16.7 (16.0–17.4) |
| Medicare | 10.7 (10.5–10.9) | 18.6 (16.5–20.7) | 10.6 (10.3–10.8) |
| Other government | 18.9 (16.9–20.9) | 30.6 (25.6–35.6) | 16.6 (14.7–18.5) |
| Diagnosis year | |||
| 2010–2012 | 15.5 (15.2–15.8) | 25.4 (24.5–26.3) | 13.8 (13.5–14.1) |
| 2013–2015 | 16.6 (16.3–16.9) | 26.0 (25.1–26.8) | 14.8 (14.5–15.1) |
| 2016–2019 | 18.1 (17.8–18.4) | 30.9 (29.7–32.0) | 16.0 (15.7–16.3) |
| Primary tumor site | |||
| Primary sites in colon | 15.4 (15.2–15.6) | 26.0 (25.4–26.7) | 13.7 (13.5–13.9) |
| Primary sites in rectum | 22.2 (21.8–22.6) | 30.9 (29.6–32.1) | 20.3 (19.9–20.8) |
| CEA level | |||
| <5 ng/mL | 24.9 (24.2–25.7) | 38.1 (35.5–40.8) | 22.8 (22.0–23.5) |
| ≥5 ng/mL | 15.4 (15.2–15.7) | 24.6 (23.9–25.2) | 13.8 (13.5–14.0) |
| Grade | |||
| Well differentiation | 23.2 (22.2–24.1) | 35.9 (32.7–39.1) | 20.8 (19.8–21.8) |
| Moderate differentiation | 22.6 (22.4–22.9) | 32.8 (32.0–33.7) | 20.5 (20.2–20.8) |
| Poor differentiation | 11.8 (11.4–12.1) | 18.5 (17.5–19.5) | 10.5 (10.2–10.9) |
| Un-differentiation | 11.7 (10.8–12.5) | 18.9 (15.9–21.9) | 10.6 (9.7–11.4) |
| Tumor size | |||
| <5 cm | 22.7 (22.3–23.0) | 33.6 (32.5–34.7) | 20.6 (20.3–22.0) |
| ≥5 cm | 18.3 (18.0–18.7) | 27.8 (26.7–28.9) | 16.6 (16.2–17.0) |
| Lymph invasion | |||
| No | 27.4 (26.8–27.9) | 39.8 (37.8–41.8) | 25.2 (24.6–25.7) |
| Yes | 20.4 (20.0–20.8) | 31.2 (30.0–32.3) | 18.4 (18.1–18.8) |
| Perineural invasion | |||
| No | 22.5 (22.1–22.9) | 34.1 (32.8–35.4) | 20.5 (20.1–20.9) |
| Yes | 21.2 (20.7–21.7) | 31.4 (29.9–33.0) | 19.1 (18.6–19.7) |
| T stage | |||
| T1 | 13.3 (12.5–14.0) | 23.7 (21.5–25.9) | 11.3 (10.6–12.0) |
| T2 | 32.9 (31.0–34.7) | 49.1 (41.3–56.9) | 30.5 (28.6–32.4) |
| T3 | 27.1 (26.7–27.5) | 38.7 (37.4–40.0) | 24.7 (24.3–25.1) |
| T4 | 16.2 (15.8–16.5) | 24.4 (23.4–25.4) | 14.6 (14.3–15.0) |
| N stage | |||
| N0 | 16.7 (16.3–17.0) | 28.9 (27.6–30.2) | 14.9 (14.5–15.2) |
| N1 | 21.4 (21.0–21.8) | 31.8 (30.6–32.9) | 19.4 (19.0–19.8) |
| N2 | 17.9 (17.6–18.3) | 27.5 (26.4–28.5) | 16.1 (15.8–16.4) |
| M stage | |||
| M1a | 22.3 (22.0–22.6) | 36.0 (35.0–37.1) | 19.9 (19.6–20.2) |
| M1b | 12.0 (11.8–12.2) | 19.8 (19.2–20.5) | 10.5 (10.2–10.7) |
| M1c | 11.9 (10.9–12.8) | 20.0 (16.6–23.4) | 10.6 (9.4–11.9) |
| Bone metastases | |||
| No | 17.6 (17.5–17.8) | 28.4 (27.8–29.0) | 15.8 (15.6–16.0) |
| Yes | 6.8 (6.4–7.2) | 12.5 (11.1–13.7) | 5.9 (5.5–6.3) |
| Brain metastases | |||
| No | 17.1 (16.9–17.3) | 27.6 (27.0–28.1) | 15.2 (15.0–15.4) |
| Yes | 4.5 (3.9–5.1) | 9.6 (5.6–13.5) | 3.9 (3.4–4.5) |
| Lung metastases | |||
| No | 19.0 (18.7–19.2) | 30.7 (30.0–31.4) | 17.0 (16.8–17.3) |
| Yes | 11.6 (11.3–11.9) | 19.8 (19.0–20.5) | 10.3 (10.0–10.6) |
| KRAS mutation | |||
| No | 23.9 (23.5–24.4) | 33.1 (31.8–34.3) | 21.7 (21.2–22.2) |
| Yes | 19.4 (19.0–19.7) | 23.7 (22.8–24.7) | 18.4 (18.1–18.8) |
| MSI status | |||
| MSS | 24.7 (24.2–25.2) | 32.9 (31.7–34.0) | 22.5 (22.0–23.0) |
| MSI-high | 25.4 (22.8–28.0) | 35.5 (28.7–42.3) | 24.1 (21.6–26.7) |
| MSI-low | 21.4 (19.0–23.7) | 36.3 (30.4–42.2) | 18.3 (16.1–20.6) |
| Primary tumor site resection | |||
| No | 10.4 (10.2–10.6) | 18.5 (17.9–19.0) | 9.0 (8.8–9.2) |
| Yes | 25.9 (25.5–26.2) | 37.8 (36.7–38.9) | 23.7 (23.3–24.0) |
| Metastases resection | |||
| No | 14.5 (14.3–14.7) | 23.6 (23.0–24.1) | 13.0 (12.9–13.2) |
| Yes | 39.4 (38.4–40.3) | 50.0 (47.6–52.5) | 36.2 (35.2–37.2) |
| Radiation | |||
| No | 15.8 (15.6–16.0) | 26.5 (25.8–27.1) | 14.0 (13.8–14.2) |
| Yes | 24.0 (23.3–24.6) | 33.0 (31.3–34.8) | 21.7 (21.0–22.3) |
| Chemotherapy | |||
| No | 2.5 (2.4–2.6) | 5.7 (4.5–6.8) | 2.4 (2.4–2.5) |
| Yes | 23.2 (23.1–23.5) | 29.2 (28.6–30.0) | 22.0 (21.7–22.2) |
| Immunotherapy | |||
| No | 14.0 (13.8–14.2) | 25.6 (25.0–26.3) | 12.3 (12.1–12.5) |
| Yes | 25.9 (25.5–26.3) | 31.1 (30.1–32.2) | 24.7 (24.2–25.1) |
CEA, carcinoembryonic antigen; CI, confidence interval; CRLM, colorectal liver metastases; EO-CRLM, early-onset colorectal liver metastases; LO-CRLM, late-onset colorectal liver metastases; MSI, microsatellite instability; MSS, microsatellite stability; KRAS, Kirsten rat sarcoma viral oncogene homolog; OS, overall survival.

Discussion
Early-onset CRC is becoming increasingly common, particularly those with synchronous liver metastasis. This study is the first, to our knowledge, to present a comprehensive insight into the epidemiology, clinicopathological characteristics and survival outcome of synchronous EO-CRLM patients. We identify socioeconomic and clinicopathologic patterns among EO-CRLM to suggest it as a distinct disease from LO-CRLM. Altogether, our study provides crucial context to support further research on the prevention and treatment of this disease.
Over the past decade, the annual growth rate of EO-CRLM exceeded that of LO-CRLM. This aligned with the relative growth patterns observed between EO-CRC and LO-CRC (14). When considering geographical distribution, we observed that the annual growth rate of EO-CRLM in metro/urban areas is significantly higher than that in rural areas. This phenomenon could be due higher screening rates in metro/urban areas than rural areas. Additionally, differences in socioeconomic and lifestyle factors between urban and rural may account for higher EO-CRLM rates. For example, the high incidence of CRC is correlated with the high Human Development Index (HDI) and thus better socioeconomic conditions in metro/urban areas may account for the increase in EO-CRLM (15,16). Similarly, the incidence of EO-CRC has been consistently rising in several developing countries across Andean Latin America, East Asia, and the Middle East (17-19). The heightened occurrence of EO-CRC in these previously low-risk and low-HDI countries can be attributed to lifestyle factors such higher meat consumption, a more sedentary lifestyle, and a higher prevalence of overweight individuals. These factors are independently associated with an increased risk of EO-CRLM (20). Our study supports the pattern that there is a higher incidence of EO-CRLM in urban and well-developed communities than rural.
Sub-population analysis revealed that the annual growth rate of EO-CRLM in high-incoming patients (median incoming ≥$63,000) significantly exceeded the growth rate in lower income patients. Additionally, the growth rate of EO-CRLM decreased among the uninsured population. This could be attributed to the economic affluence that enables individuals to access better healthcare coverage and undergo more comprehensive medical examinations, resulting in earlier detection of EO-CRLM. Our study highlights the need for equitable medical care and screening in order to detect and treat EO-CLRM across all socioeconomic populations.
Furthermore, we investigated the growth trends of different genetic sub-types in the population of EO-CRLM. This study found a significant increase in the annual growth rate of EO-CRLM with MSI-high or KRAS-mutation. MSI-high and KRAS-mutation are important targets for immunotherapy and targeted therapy in EO-CRLM, respectively (5,21). The treatment effects for these sub-types often outperform those of other genetic sub-types. Therefore, the aforementioned growth trends are expected to benefit more EO-CRLM patients, enabling them to receive better treatment and consequently achieve improved prognoses. The proportional shift in treatment strategies over the past decade demonstrated a significant increase in the use of immunotherapy (from 0.62% in 2010 to 33.82% in 2019), aligning with the growth pattern observed in MSI-high EO-CRLM. This further emphasized the groundbreaking significance of immunotherapy in the treatment of EO-CRLM over the past decade [comparisons for MSI (n=137) vs. MSS (n=1,715) among EO-CRLM patients receiving immunotherapy, median OS: 42.9 (95% CI: 35.9–49.9) vs. 32.9 (95% CI: 31.4–34.3) months, P=0.02].
Additionally, this study delineated the OS of both EO-CRLM and LO-CRLM, as well as the OS within various sub-populations. The findings have direct clinical implications. By identifying the OS within various sub-populations, clinicians can better assess the prognosis of individual patients and tailor treatment plans accordingly. This personalized approach could potentially improve patient outcomes and guide decision-making in the management of EO-CRLM. This study indicated that the prognosis of EO-CRLM was better than that of LO-CRLM. Importantly, this is consistent with the prognosis of EO-CRC being better than that of LO-CRC in a population-based analyses (22). A comparison of the clinical pathological features between EO-CRLM and LO-CRLM revealed that, compared with LO-CRLM, EO-CRLM patients were more likely to reside in urban areas, have higher rates of high school completion, higher income, and covered by private insurance/managed care. As previously mentioned, patients with EO-CRLM are more commonly receiving primary tumor resection, liver resection, chemotherapy, and immunotherapy which may also contribute to their better outcomes.
This study has several limitations. First, the NCDB data used in our study were from the patient population in the United States, and the epidemiology, clinicopathological characteristics and survival outcome in EO-CRLM patients in other countries still need to be further explored. Second, several established prognostic indicators (e.g., size of liver metastases, number of liver metastases, KRAS status, and microsatellite status) were not captured by the NCDB either partially or completely, preventing inclusion of these modern-day prognostic variables in the analyses. Further, the NCDB does not include important oncologic outcomes of disease-specific and disease-free survival. Such drawbacks are inherent to any retrospective, population-based study and may raise concerns about the generalizability of the findings. However, the size of the present study, which we believe to be the largest to date, and the long duration of follow-up provide a comprehensive and contemporary epidemiological framework for EO-CRLM.
Conclusions
There is a rapid increase in the incidence and prevalence of patients with EO-CRLM, with notable variations in the disease burden across different socioeconomic strata and with distinct clinicopathological characteristics. These disparities reflect health care inequities within the overall population. Interestingly, the prognosis for EO-CRLM was found to be better than LO-CRLM. These data may provide context for patients with EO-CRLM including their disease biology which could help tailor future studies and management of this distinct disease.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-66/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-66/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-66/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Wagle NS, Cercek A, et al. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73:233-54. [Crossref] [PubMed]
- Sinicrope FA. Increasing Incidence of Early-Onset Colorectal Cancer. N Engl J Med 2022;386:1547-58. [Crossref] [PubMed]
- Review A. JAMA Surg 2021;156:865-74. REACCT Collaborative; Zaborowski AM, Abdile A, et al Characteristics of Early-Onset vs Late-Onset Colorectal Cancer:. [Crossref] [PubMed]
- Araghi M, Soerjomataram I, Bardot A, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol 2019;4:511-8. [Crossref] [PubMed]
- Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021;325:669-85. [Crossref] [PubMed]
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335-51. [Crossref] [PubMed]
- Wu D, Wong P, Guo C, et al. Pattern and trend of five major musculoskeletal disorders in China from 1990 to 2017: findings from the Global Burden of Disease Study 2017. BMC Med 2021;19:34. [Crossref] [PubMed]
- Schuster-Bruce J, Jani C, Goodall R, et al. A Comparison of the Burden of Thyroid Cancer Among the European Union 15+ Countries, 1990-2019: Estimates From the Global Burden of Disease Study. JAMA Otolaryngol Head Neck Surg 2022;148:350-9. [Crossref] [PubMed]
- Zhang J, Ma B, Han X, et al. Global, regional, and national burdens of HIV and other sexually transmitted infections in adolescents and young adults aged 10-24 years from 1990 to 2019: a trend analysis based on the Global Burden of Disease Study 2019. Lancet Child Adolesc Health 2022;6:763-76. [Crossref] [PubMed]
- Resche-Rigon M, White IR. Multiple imputation by chained equations for systematically and sporadically missing multilevel data. Stat Methods Med Res 2018;27:1634-49. [Crossref] [PubMed]
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661-79. [Crossref] [PubMed]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J Wiley & Sons 1989;45.
- Patel SG, Karlitz JJ, Yen T, et al. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol 2022;7:262-74. [Crossref] [PubMed]
- Bray F. Transition in human development and the global cancer burden. World Cancer Report 2014:54-68.
- Fidler MM, Soerjomataram I, Bray F. A global view on cancer incidence and national levels of the human development index. Int J Cancer 2016;139:2436-46. [Crossref] [PubMed]
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683-91. [Crossref] [PubMed]
- Arnold M, Abnet CC, Neale RE, et al. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020;159:335-349.e15. [Crossref] [PubMed]
- Pan H, Zhao Z, Deng Y, et al. The global, regional, and national early-onset colorectal cancer burden and trends from 1990 to 2019: results from the Global Burden of Disease Study 2019. BMC Public Health 2022;22:1896. [Crossref] [PubMed]
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70:145-64. [Crossref] [PubMed]
- Boukouris AE, Theochari M, Stefanou D, et al. Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: A 2022 update. Crit Rev Oncol Hematol 2022;173:103663. [Crossref] [PubMed]
- Saraste D, Järås J, Martling A. Population-based analysis of outcomes with early-age colorectal cancer. Br J Surg 2020;107:301-9. [Crossref] [PubMed]
Cite this article as: Chen Q, Zhao S, Deng Y, Lidsky ME, Rhodin KE, Eckhoff A, Chen S, Ding P. Trends in epidemiology, clinicopathological characteristics and survival outcomes among patients with synchronous early-onset colorectal liver metastases in the United States from 2010 to 2019. Transl Gastroenterol Hepatol 2025;10:30.


