
Overlap syndromes in autoimmune liver disease: a review
Introduction
The term “overlap syndrome” (OS) is used to describe conditions that exhibit biochemical, serologic, immunologic, histologic, or cholangiographic features of more than one of the three autoimmune liver diseases (AILDs): autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) (1,2). Although the precise mechanism underlying the pathogenesis of these conditions is unknown, it is postulated that environmental triggers, genetic predisposition, and defects in immune tolerance mechanisms play a role in their development (3). In AIH, antibody and T-cell-mediated attack on liver-specific targets leads to injury primarily in the portal areas; however, some patients may exhibit weakly positive antimitochondrial antibodies and mild bile duct injury, leading to inflammation and fibrosis (4,5). AIH is predominantly associated with a hepatocellular pattern of injury, whereas PBC and PSC tend to have a cholestatic pattern of liver injury affecting the cholangiocytes, with a mild degree of parenchymal damage (5). Nevertheless, instances where AIH presents with a cholestatic pattern have been observed (6). Table 1 summarizes the general characteristic features of each of these autoimmune conditions.
Table 1
| Characteristics | Autoimmune hepatitis | Primary biliary cholangitis | Primary sclerosing cholangitis |
|---|---|---|---|
| Gender | Females > males | Females > males | Males > females |
| Type of liver injury | Hepatocellular | Cholestatic | Cholestatic |
| Disease-specific antibodies | ASMA | AMA | p-ANCA |
| Anti-sp100 antibody | |||
| Anti-gp120 antibody | |||
| Immunoglobulins | Increased IgG | Increased IgM | Increased IgG and IgM |
| Magnetic resonance cholangiopancreatography | Normal | Normal | Multifocal stricturing throughout the hepatobiliary tree |
| Inflammatory bowel disease | Low prevalence (3–10%) | Not present | High prevalence (80%) |
| Liver histology | Lymphoplasmacytic infiltrate in the portal area and interface hepatitis | Lymphoplasmacytic infiltrate in the portal region and interface hepatitis | Onion skin periductal fibrosis |
| Medical therapy | Steroids + immunosuppressive agents | Ursodiol | Ursodiol (off-label) |
| Obeticholic acid (second line) | |||
| Fibrates (off-label) | |||
| Elafibranor (second line) | |||
| Fibrates (second line, off label) |
AMA, anti-mitochondrial antibody; ASMA, anti-smooth muscle antibody; IgG, immunoglobulin G; IgM, immunoglobulin M; p-ANCA, perinuclear antineutrophil cytoplasmic antibody.
OS with predominant AIH characteristics can be further classified into three categories based on the concomitant cholestatic disorder (6). These include AIH-PBC, AIH-PSC and AIH-cholestatic syndrome (Figure 1). The term AIH-cholestatic syndrome is used to classify patients with AIH who also exhibit cholestatic patterns of liver injury yet lack the serologic, histologic, or cholangiographic features typical of PBC and PSC, for example, in cases of autoimmune cholangitis and anti-mitochondrial antibody (AMA)-negative PBC (7). An overlap between PBC and PSC is exceedingly rare, with only a limited number of cases documented in literature (Figure 2) (8). Additionally, some patients may transition from one autoimmune liver condition to another, suggesting that OS could represent an intermediate stage in disease progression; therefore, it is imperative to identify these patients at an earlier stage to prevent adverse outcomes (9,10). Currently, the diagnostic criteria for overlap remain undefined, but it is advisable to consider OS in patients with classical serologic, clinical, or biochemical findings of more than one AILD (6). This consideration is particularly important in patients who demonstrate an inadequate response to conventional therapy (7,11). The classification and guidelines for detecting OS are further discussed in Table 2. This review will discuss the various types of OS and the therapeutic approaches utilized in their management.
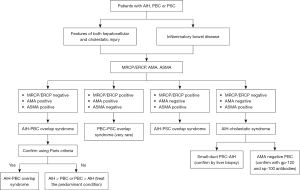
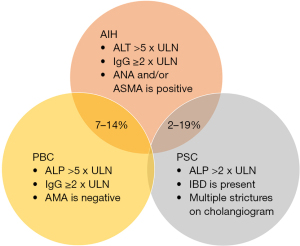
Table 2
| Paris criteria | IAIHG scoring system | Revised IAIHG scoring system | Simplified IAIHG scoring system |
|---|---|---|---|
| Recommended to be used for the diagnosis of PBC-AIH | Not recommended to be used for the diagnosis of PBC-AIH | Not recommended to be used for the diagnosis of PBC-AIH | Not recommended to be used for the diagnosis of PBC-AIH |
| At least 2 out of 3 criteria for PBC and AIH with AASLD and EASL guidelines recommending interface hepatitis to be present | Definite AIH (without steroid treatment) >15; definite AIH (after steroid treatment) >17 | Definite AIH >15; probable AIH 10–15 | Definite AIH ≥7; probable AIH ≥6 |
| AIH: (I) ALT >5 times ULN; (II) IgG >2 times ULN; (III) interface hepatitis (moderate/severe) on liver biopsy | Female gender +2 | Female gender +2 | |
| PBC: (I) presence of antimitochondrial antibody; (II) ALP >2 ULN or gamma glutamyl transaminase >5 ULN; (III) florid bile duct lesions on liver biopsy | ALP:AST (or ALT) ratio: (I) <1.5=2; (II) 1.5–3=0; (III) >3=−2 | ALP:AST ratio: (I) <1.5=2; (II) 1.5–3=0; (III) >3=−2 | ANA or SMA: (I) ≥1:40 =1; (II) ANA or SMA ≥1:80 or LKM1 ≥1:40 or SLA-positive =2 |
| Serum IgG levels: (I) >2=3; (II) 1.5–2=2; (III) 1–1.5=1; (IV) <1=0 | Serum IgG levels: (I) >2=3; (II) 1.5–2=2; (III) 1–1.5=1; (IV) <1=0 | Serum IgG: (I) > ULN =1; (II) >1.1 times ULN =2 | |
| ANA, SMA or LKM1: (I) >1:80=3; (II) 1:80=2; (III) 1:40=1; (IV) <1:40=0 | ANA, SMA or LKM1: (I) >1:80=3; (II) 1:80=2; (III) 1:40=1; (IV) <1:40=0 | Histologic findings: (I) compatible with AIH =1; (II) typical with AIH =2 | |
| Hepatotoxic drug history: (I) positive =−4; (II) negative =2 | Illicit drug use: (I) positive =−4; (II) negative =1 | Hepatitis viral markers: negative =+2 | |
| Daily alcohol intake: (I) <25 g/d =2; (II) >60 g/d=−2 | Daily alcohol intake: (I) <25 g/d =2; (II) >60 g/d=−2 | ||
| AMA positivity =−4 | AMA positivity =−4 | ||
| Other autoimmune diseases =2 | Other autoimmune diseases =2 | ||
| Hepatitis viral markers: (I) positive =−3; (II) negative =3 | Hepatitis viral markers: (I) positive =−3; (II) negative =3 | ||
| Histologic findings: (I) interface hepatitis =3; (II) lymphoplasmacytic infiltrate =1; (III) rosette formation =1; (IV) none of the above =−5; (V) biliary changes =−3; (VI) atypical changes =−2 | Histologic findings: (I) interface hepatitis =3; (II) lymphoplasmacytic infiltrate =1; (III) rosette formation =1; (IV) none of the above =−5; (V) biliary changes =−3; (VI) other changes =2 | ||
| Response to therapy: (I) remission =2; (II) relapse =3 | |||
| HLA-DR3 or DR4 =1 |
AASLD, American Association for Study of Liver Disease; AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine transaminase; AMA, anti-mitochondrial antibody; ANA, antinuclear antibody; AST, aspartate transaminase; EASL, European Association for Study of the Liver; HLA, human leucocyte antigen; IAIHG, International Autoimmune Hepatitis Group; IgG, immunoglobulin G; LKM, liver kidney microsome antibody; PBC, primary biliary cholangitis; SLA, soluble liver antigen; SMA, smooth muscle antibody; ULN, upper limit of normal.
AIH-PBC OS
Diagnostic criteria
The diagnosis of AIH-PBC OS is guided by several diagnostic criteria including the Paris Criteria, the International Autoimmune Hepatitis Group scoring system (IAIHG), the Revised IAIHG scoring system, and the simplified IAIHG scoring system (12-15). Notably, The Paris criteria is recognized for its excellent sensitivity and specificity in diagnosing OS and requires the presence of 2 out of 3 criteria from each disease to be diagnosed with OS (15) (Table 2). Additionally, the presence of moderate or severe interface hepatitis is necessary for the diagnosis of OS. Both the European Association for the Study of the Liver (EASL) and the American Association Society of Liver Diseases (AASLD) recommend using this criterion for the diagnosis of OS (16,17).
The remaining scoring systems were originally developed by experts to facilitate the comparison of AIH across multiple studies, however they have been used to diagnose OS in patients with established PBC (12,13,18). Given that these scoring systems were intended specifically to differentiate AIH from other diagnosis, their efficacy in diagnosing OS has not been substantiated. Therefore, the IAIHG recommends against the use of these criteria to diagnose OS. Despite these recommendations, multiple studies have compared Paris criteria to various IAIHG scores and have reported conflicting results (12,14). A Dutch study comparing 134 patients with PBC, AIH, and PBC-AIH patients reported Paris criteria to have sensitivity and specificity of 92% and 97% respectively (14). Another study of 368 patients with PBC conducted at the Mayo Clinic has reported a simplified IAIHG scoring system to have more specificity than the revised IAIHG scoring system (13).
New scores are currently being developed to differentiate OS from PBC and AIH. Recently Wang et al. reported that patients with OS exhibited higher immunoglobulin G (IgG) levels compared to patients with PBC, suggesting elevated IgG levels should prompt consideration for the diagnosis of OS (15). They also reported that the five strongest predictors to differentiate PBC from OS were alpha-fetoprotein, activated partial thromboplastin time (APTT), globulin, IgG, and immunoglobulin M (IgM). Another report by Zhang et al. developed a scoring classification based on biochemical, immunologic and histologic features of AIH and PBC to differentiate PBC from OS (16). While the Paris criteria demonstrate excellent sensitivity and specificity for diagnosing OS, it may not adequately capture patients with less severe forms of AIH-PBC OS. Therefore, further studies are needed to develop more refined tools that can help differentiate OS for AIH and PBC.
Epidemiology
Despite PBC-AIH being recognized as the most common type of overlap, accurately estimating its global prevalence remains challenging due to the variability in diagnostic criteria across different studies (17,19). Based on studies conducted at smaller medical centers, the prevalence of OS has been reported to range from 2.1% to 19.3% (20,21). Muratori et al. studied 235 consecutive patients with AILD and reported the prevalence of OS to be 2.1% using the IAIHG criteria (20). In contrast, Silveira et al. reviewed 135 patients with AILD using the revised IAIHG criteria and reported the prevalence of OS to be 19.3% (21). Yet when applying the Paris criteria, the prevalence has been estimated to be 4.8–9.2% (22,23). The variability in these findings can be explained by overdiagnosis of OS and underscores the need for further studies to accurately estimate the global prevalence of AIH-PBC OS, however, achieving this goal will require a consensus on the diagnostic criteria.
It is noteworthy that both PBC and AIH have a female predominance (17,19). A systematic review of 17 studies on PBC-AIH reported that women constituted between 87% and 100% of the cases (24). Additionally, disparities in complications from OS have been observed based on race. A study by Levy et al. reported that patients with PBC of Hispanic ethnicity were more likely to have additional autoimmune features and higher frequency of complications such as ascites, esophageal varices, and encephalopathy (25). The underlying mechanisms driving these findings remain unclear and further studies are needed to elucidate the reasons behind these disparities.
Clinical features and serology
Some patients with PBC-AIH OS can concurrently exhibit features of both autoimmune conditions at the time of diagnosis, while others may present with one condition and subsequently develop features of the other as the disease progresses (26). A study by Efe et al. collected data from 1,065 patients with PBC and AIH revealed that 1.8% of patients developed OS (26). Patients with PBC who develop OS had a higher prevalence of positive anti-smooth muscle antibody (ASMA) and moderate/severe interface hepatitis on their biopsy. Another study by Muratori et al. reported that patients with OS had higher rates of concomitant AMA and anti-double stranded DNA compared to patients with PBC or AIH alone (20). Reports also show that the presence of autoantibodies against soluble liver antigen (SLA)/liver pancreas (LP) and double stranded DNA is associated with the presence of AIH in patients with PBC (27). Therefore, testing for these autoantibodies should be considered in the workup of PBC patients with suspected AIH.
A recent study using the National Inpatient Sample database reported that patients with OS exhibit a markedly higher prevalence of complications such as ascites, hepatic encephalopathy, hepatorenal syndrome, and spontaneous bacterial peritonitis compared to patients with PBC and AIH alone (28). While a study by Chazouillères et al. reported that patients with PBC/AIH OS have higher rates of progression to cirrhosis-related complications (22). It has been suggested that overlapping immune-mediated processes in patients with OS can accelerate fibrogenesis in the liver (28). Patients with PBC/AIH OS have decreased 5-year adverse event-free survival than patients with PBC. In a study of 323 patients with PBC and OS reported that 58% of patients with OS had a 5-year adverse event-free survival compared to 81% of patients with PBC alone (29).
Management
Due to the low prevalence of PBC/AIH OS, randomized controlled trials are difficult to design and pose significant challenges, particularly with patient enrollment. Therefore, most evidence regarding the treatment of OS is derived from retrospective case series or clinical anecdotes and observations. For PBC, standard first-line treatment begins with ursodeoxycholic acid (UDCA), or ursodiol, at a dose of 13–15 mg/kg/day. Treatment for AIH encompasses immunosuppressive agents such as corticosteroids, azathioprine, mycophenolate mofetil (MMF) and tacrolimus (30,31). In some patients, UDCA therapy alone can induce biochemical remission of OS, although, most patients require a combination therapy with immunosuppressive agents (12,23). In the study by Chazouillères et al. of 17 patients with OS, the use of combination therapy was associated with a reduction in fibrosis progression compared to patients on UDCA alone (31). Azathioprine and MMF are accepted anti-proliferative agents for patients who require long-term immunosuppression (12). The EASL guidelines recommend combination therapy with UDCA and immunosuppression for the management of OS, particularly for patients who meet the Paris Criteria and exhibit severe interface hepatitis (12). Patients with moderate interface hepatitis should be considered for treatment, whereas the potential benefit of immunosuppressive therapy in patients with mild interface hepatitis remains uncertain, especially given the risk of osteoporosis. For patients who have features suggestive of OS but fail to meet the Paris criteria, initial treatment should prioritize the management of the predominant condition. However, given the low risk of adverse effects and potential benefit of UDCA, it may be advisable to include it in the initial treatment in patients with predominant AIH who fail to meet the Paris criteria. For patients with predominant PBC features who fail to meet the Paris criteria, the EASL guidelines suggests initiating UDCA monotherapy with consideration of adding immunosuppressants if the patient fails to show any improvement (12).
AIH-PSC OS
Epidemiology
Among patients diagnosed with PSC, the occurrence of AIH-PSC has been reported to range between 7–14% (12). Similar to PSC, this OS has been reported to be more common among males as evidenced by a meta-analysis of 109 patients with PSC-AIH, which also reported a higher prevalence of men in the study population (32,33). Additionally, just like PSC, PSC-AIH has also been reported to be a disease of younger populations with studies reporting mean age of 25.2 years in the study population (34,35).
The prevalence of abnormal cholangiography suggestive of PSC in patients with AIH varies across studies and is influenced by factors such as patient age and the presence of inflammatory bowel disease (IBD) (36,37). In younger patients with AIH, the prevalence has been reported to be as high as 50%, while the prevalence in adults has been reported to be between 2–10% (34,36,38-41). Up to 41% of patients with AIH and ulcerative colitis may have cholangiographic findings suggestive of PSC (37). Given the strong association between PSC and IBD, a cholangiogram should be performed in any IBD patient presenting with a cholestatic pattern liver enzyme elevation (42). Additionally, a cholangiogram should also be considered in patients with IBD and a hepatocellular injury pattern , once additional causes of liver disease have been excluded (36). Furthermore, some studies have also suggested that patients with an original diagnosis of AIH but who are subsequently noted to have PSC on cholangiography should be reclassified as PSC, if PSC was not excluded initially (12).
Diagnostic criteria
The OS are relatively well defined compared to AIH-PBC OS (43). The diagnosis of AIH-PSC OS requires:
- A probable or definite diagnosis of AIH in accordance with the IAIHG criteria (interface hepatitis should be present);
- Cholangiographic evidence of multifocal bile duct strictures;
- Commonly they have concurrent IBD, but its presence is not necessary for diagnosis;
- Negative AMA.
Clinical features and natural history
Like AIH-PBC, the development of AIH-PSC OS can occur sequentially with AIH often being the initial presentation and PSC emerging later in the disease course. However, it is rare for PSC to precede AIH, although simultaneous presentation of both conditions is possible (4).
The data regarding the natural history of AIH-PSC OS has been primarily elucidated through small retrospective studies. In a study by Deneau et al. involving 781 patients, it was observed that 33% of children with PSC can have overlapping features of AIH (39). Here, patients with OS experienced the same incidence of adverse outcomes as PSC patients with overlap (39). Similarly, liver transplantation (LT) rates have also been reported to be similar in adult patients with PSC and AIH-PSC OS (44,45).
A meta-analysis involving 109 patients identified diarrhea, fatigue, jaundice and pruritus as the most commonly reported symptoms at presentation (32). It is important to note that these findings are derived from case series. However, it is posited that most patients are diagnosed at an earlier stage, when they are still asymptomatic but have laboratory abnormalities such as an elevated ALP or abnormal cholangiogram findings in patients with history of AIH or through concomitant elevation of ALP, AST and ALT with biopsy findings suggestive of both PSC and AIH (46,47).
Patients with AIH/PSC have been reported to have poorer long-term survival compared to patients with AIH alone (48). A study by Al-Chalabi et al. noted that patients with AIH-PSC had higher rates of liver related mortality and LT compared to patients with AIH alone (33% vs. 8%, P=0.05) (48). Nevertheless, studies have reported better survival outcomes in patients with AIH-PSC than those with PSC alone (49,50).
Management
The data regarding the role of immunosuppression in patients with AIH/PSC OS is variable (51). Corticosteroids have been reported to be beneficial in improving ALT and bilirubin levels in patients with PSC who have histological features of AIH (52-54). It has been reported that the rates of remission in patients with AIH/PSC is lower than those with classical AIH (49). Additionally, a small case series documented the emergence of PSC in patients previously treated for AIH despite the administration of adequate immunosuppression (2,55).
Azathioprine and MMF have been utilized as “steroid sparing” agents in a manner analogous to their application in isolated AIH (24,56). A small case series involving 4 patients who were either non responders to or intolerant of AZA demonstrated that treatment with MMF resulted in a biochemical response in all patients, with three out of four patients achieving complete remission (57). However, there remains a paucity of data regarding the efficacy of tacrolimus in the treatment of PSC/AIH OS.
UDCA is not approved for the treatment of PSC but is commonly used off-label at a dosage of 13–15 mg/kg/day (58). The efficacy of UDCA in patients with OS has been reported to have conflicting results (58,59). EASL guidelines recommend a combination of UDCA and immunosuppressive agents for managing patients with AIH/PSC (58). This combination is theoretically advantageous as it targets both the hepatocellular and cholestatic injury (24).
In patients with advanced liver disease, LT remains the only available treatment option (60,61). A small comparative study of patients with AIH-PBC, AIH-PSC and AIH-small duct PSC reported no significant difference in survival rates among these three groups. However, it was noted that patients with AIH-PSC were more likely to require LT compared to those with AIH-PBC (62). Another study reported that while the graft and patient survival rates are similar among patients with AIH/PSC, AIH and PSC, patients with AIH/PSC are at higher risk of disease recurrence following transplantation (63).
Immunoglobulin G subclass 4 (IgG4) levels in PSC
A distinct subset of patients with PSC and elevated IgG4 levels have been defined recently (64). These patients must be carefully distinguished from patients with IgG4-related autoimmune cholangitis (IgG4-AIC) which is a subset of IgG4-related disease (Table 3).
Table 3
| Characteristics | IgG4-AIC | PSC with elevated IgG4 |
|---|---|---|
| Age | Greater than 60 years old | Less than 50 years old |
| Association with IBD | Rare | 80% of cases |
| Pancreatic involvement | Seen in 90–95% | Rare |
| Serology | Elevated IgG4 levels >2 times upper normal limit | Serum IgG4 <2 times upper normal limit |
| Histology | Lymphoplasmacytic infiltrate with predominant IgG4-positive plasma cells | Periportal sclerosis and “onion ring fibrosis” without predominant IgG4 positive plasma cell infiltrate |
| Treatment | Corticosteroids (first line) | High dose corticosteroid trial |
| Immunomodulators such as azathioprine (second line) | UDCA use controversial | |
| Rituximab for refractory or relapsing disease | Low dose UDCA (13–15 mg/kg) | |
| High dose UDCA (28–30 mg/kg) is toxic in PSC |
AIC, autoimmune cholangiopathy; AIH, autoimmune hepatitis; IBD, inflammatory bowel disease; IgG, immunoglobulin G; PSC, primary sclerosing cholangitis; UDCA, ursodeoxycholic acid.
IgG4-AIC
IgG4-AIC is characterized by chronic inflammation and fibrosis of the bile ducts (64). Clinically, IgG4-AIC can present with features that overlap with PBC and PBC (65). While elevated IgG4 levels are indicative, not all patients present with elevated levels. For this reason, a definitive diagnosis often requires histological confirmation of lymphoplasmacytic infiltration with IgG4-positive plasma cells and associated fibrosis (66).
Management
Corticosteroids have been shown to be highly effective in alleviating inflammation and curbing disease progression (67). In patients with an inadequate response to corticosteroids, immunosuppressive agents can be used as an adjunctive therapy (67).
PSC with elevated IgG4
Several retrospective studies report that approximately 10–27% of PSC patients exhibit elevated IgG4 levels (68-71). PSC is diagnosed using liver biochemistries and classic bile duct changes on cholangiography (70,72,73). EASL guidelines recommend measuring serum IgG4 levels in all patients with large duct-PSC at diagnosis (74). Liver biopsy is typically not warranted unless there is a need to confirm small duct PSC based on a high clinical suspicion and normal cholangiogram and to distinguish PSC with elevated IgG4 from IgG4-AIC (64).
Management
The role of corticosteroids has shown some benefit in these patients. However, this data is derived from retrospective studies and no clinical trials have been conducted to test their efficacy (69,75). Currently LT is the only definitive treatment.
AIH-cholestatic syndrome
Epidemiology
AIH-cholestatic syndromes can be loosely defined as AIH-PBC or AIH-PSC but without a positive AMA or obvious cholangiographic abnormalities. The prevalence of AIH-cholestatic syndrome remains understudied but its prevalence has been reported to be between 5% to 20% (11,40).
Diagnostic criteria
The diagnosis of AIH-cholestatic syndrome requires the absence of serological and histological features suggestive of PBC and the absence of cholangiogram features suggestive of PSC (11,76,77). This includes patients AMA-negative PBC and small duct PSC (11,76,77). Historically, these patients have been described as having autoimmune cholangitis (78). Liver biopsy is essential for a definitive diagnosis, and the histological findings may vary depending on the underlying cholestatic liver disease. Histologic features can include portal edema, portal fibrosis, ductopenia or lymphoplasmacytic infiltrate with bile duct lesion suggestive of PBC (7,79,80). As testing for other autoantibodies such as gp210, sp100 that meet PBC criteria becomes more widespread, we will be able to identify more patients with AMA-negative PBC (81-86).
Management
Management options for AIH-cholestatic syndrome include treatment with UDCA alone, corticosteroid alone, or combination of these therapies (87). However, these patients often exhibit poor response to conventional corticosteroid therapy, with 88–100% failing to achieve biochemical remission with steroids alone. Combination therapy of corticosteroids with UDCA has also been attempted in this patient subset with variable results (Figure 3). We believe that a complete clinical picture as well as the intensity of cholestatic features must be taken into account prior to initiating treatment with ursodiol (88).
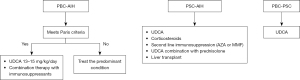
PBC-PSC OS
The concurrent diagnosis of PBC and PSC is rare compared to cholestatic variants of AIH. The first case of PBC-PSC overlap was reported by Rubel et al. in 1984 (89). In this patient, the diagnosis of PBC was made based on positive AMA, elevated ALP and liver biopsy demonstrating portal fibrosis. At that time, MRCP was not available for diagnostic purposes and multiple endoscopic attempts via ERCP to visualize the bile ducts was unsuccessful until a definitive diagnosis was achieved through a percutaneous transhepatic cholangiogram which showed stricturing and dilatations in the bile ducts. There have been a handful of case reports that have reported this OS, with most patients receiving, UDCA as the sole treatment (90-93).
OS in IBD
Patients with IBD are at increased risk of all AILDs such as PBC, PSC and AIH (94,95). The shared autoimmune mechanisms between IBD and various AILDs have been hypothesized to be responsible for this association (96). It has been estimated that 60–80% of patients with PSC have UC and 2–14% of patients with IBD have concomitant PSC (97). A recent meta-analysis by Barberio et al. reported that PSC is present in 2.16% of patients with IBD. 2.47% of patients with UC were noted to have PSC, while 0.96% of the patients with CD were noted to have PSC (98). AIH has also been reported to be associated with IBD, but has a lower prevalence, compared to PSC (94). Zhu et al. have also reported that IBD may also lead to increased risk of developing PBC (99). The incidence of PBC among patients with IBD is not well established. The current data is from single-center studies. In a study by Li et al., out of 890 patients with ulcerative colitis, only 4 individuals suffered from UC and PBC (100). Currently, there is lack of literature on the epidemiology of OS, especially among patients with IBD. However, it has been reported that patients with IBD are at increased risk of developing OS, compared to patients without IBD. This is seen more common in patients with Crohn’s disease (101). It has been reported that incidence of IBD is 40–50% among patients with PSC-AIH OS patients, however PSC-AIH OS is rare in IBD patients (102). Thus it is very important to screen patients with OS with IBD and patients with IBD should have their liver enzymes checked periodically to identify the disease at earlier stages to prevent the progression of the disease.
Long-term prognosis and transplantation of patients with OS
The goal of various therapies among patients with OS is to prevent the progression of liver disease to decompensated cirrhosis. Progression to cirrhosis among patients with PBC-AIH at 10 years was noted to be 44–48% and transplant-free survival was noted to be 52–92% (14,21,103,104). Once these patients develop cirrhosis, LT becomes necessary. Thus, it is essential to identify patients at higher risk of worse outcomes and patients at risk of requiring LT. A study by Al-Chalabi et al. reported patients with PSC/AIH to have higher severe disease severity and worse prognosis than patients with PBC/AIH or AIH. In their study, a significant reduction in survival was noted in patients with PSC/AIH than those without (48). In a separate study by Jayabalan et al., patients with PBC-AIH were noted to have no significant differences in survival compared to patients with PSC-AIH (105). It has been reported that patients with OS undergoing LT have higher rates of liver-transplant related complications. A single center study by Bhanji et al. noted that patients with OS have a higher risk of disease recurrence after LT, however the survival rates were comparable between the two groups (44). A recent study by Lee et al. using UNOS database reported that patients with AIH-PBC overlap have higher risk of mortality secondary to risk of disease recurrence and pulmonary complications, while patients with AIH-PSC overlap have higher risk of mortality due to graft infection (106). These findings highlight the importance of early identification and initiation of therapy to prevent adverse outcomes among these patients.
Upcoming therapies for OS
Multiple therapies are currently under development for patients with various AILDs. Recently, two drugs elafibranor and seladelpar were approved for the treatment of PBC, and two other drugs are currently under investigation (107-110). Drugs being evaluated for PSC include nor-UDCA, berberine with ursodeoxycholic acid and simvastatin (111). For AIH, B-cell depleting therapies such as ianalumab, zetomipzomib and JKB-122 are currently under investigation (112). Information regarding the new and upcoming therapies is beyond the scope of this article and has been described elsewhere (111,113,114). Many of these clinical trials exclude patients with OS. This poses a challenge for patients with OS as they are not candidates for clinical trials. Furthermore, once the drug gets approved, it is up to the clinician regarding the decision of starting these therapies on patients with OS. We believe that there is an unmet need for therapies for patients with OS and further studies evaluating the role of these investigational drugs in patients with OS are needed.
Conclusions
In summary, AILD involves self-directed immune-mediated damage to hepatocytes and cholangiocytes, defined into three types: AIH, primary biliary cirrhosis (PBC), and PSC, each with distinct features. AIH is primarily hepatocyte-focused, while PBC and PSC affect bile ducts. Treatment involves immunosuppressive agents for AIH and ursodiol for PBC. OS can occur, with some patients exhibiting features of more than one AILD. Diagnosis criteria are not yet clear, and treatment is often extrapolated primarily from AILD data. AIH-PBC, AIH-PSC, and AIH-cholestatic syndrome are categories of OS, with different diagnostic criteria and treatment approaches. Treatment options include ursodiol, corticosteroids, azathioprine, MMF, and tacrolimus, depending on the specific OS and its characteristics. The choice of therapy should be based on individual patient features. Studies indicate poorer long-term outcomes for OS patients compared to those with single AILDs. Thus, further studies must be performed to define and revise guidelines in the diagnosis process.
Acknowledgments
None.
Footnote
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-140/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-140/coif). A.S. serves as an unpaid editorial board member of Translational Gastroenterology and Hepatology from August 2023 to July 2025. A.S., N.N. and K.V.K. are employees of Liver Institute Northwest. K.V.K. declares research support from CymaBay Therapeutics; grants and/or contracts from 89Bio, Genfit, Gilead, GlaxoSmithKline, Hanmi, HighTide, Intercept, Madrigal, Mirum, NGM, Pfizer, Pliant, and Viking; royalties/licenses from UpToDate; consulting fees from 89Bio, Calliditas Therapeutics, CymaBay Therapeutics, Genfit, Gilead, Inipharm, Intercept, Madrigal, Mirum, NGM, and Pliant; payment/honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Gilead, and Intercept; payment for expert testimony from Court; participation in a data safety monitoring board or advisory board for CTI, Medpace and Labcorp; stock or stock options for Inipharm; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Sonic Insight. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beuers U, Rust C. Overlap syndromes. Semin Liver Dis 2005;25:311-20. [Crossref] [PubMed]
- Rust C, Beuers U. Overlap syndromes among autoimmune liver diseases. World J Gastroenterol 2008;14:3368-73. [Crossref] [PubMed]
- Arndtz K, Hirschfield GM. The Pathogenesis of Autoimmune Liver Disease. Dig Dis 2016;34:327-33. [Crossref] [PubMed]
- Abdo AA, Bain VG, Kichian K, et al. Evolution of autoimmune hepatitis to primary sclerosing cholangitis: A sequential syndrome. Hepatology 2002;36:1393-9. [Crossref] [PubMed]
- Washington MK. Autoimmune liver disease: overlap and outliers. Mod Pathol 2007;20:S15-30. [Crossref] [PubMed]
- Fallatah HI, Akbar HO. Autoimmune liver disease - are there spectra that we do not know? Comp Hepatol 2011;10:9. [Crossref] [PubMed]
- Czaja AJ. Diagnosis and management of the overlap syndromes of autoimmune hepatitis. Can J Gastroenterol 2013;27:417-23. [Crossref] [PubMed]
- Yacoub H, Ben Azouz S, Hassine H, et al. Overlap syndrome of primary biliary cholangitis and primary sclerosing cholangitis: two case reports. J Med Case Rep 2023;17:169. [Crossref] [PubMed]
- Carpenter HA, Czaja AJ. The role of histologic evaluation in the diagnosis and management of autoimmune hepatitis and its variants. Clin Liver Dis 2002;6:685-705. [Crossref] [PubMed]
- Dienes HP, Erberich H, Dries V, et al. Autoimmune hepatitis and overlap syndromes. Clin Liver Dis 2002;6:349-62. vi. [Crossref] [PubMed]
- Czaja AJ. Frequency and nature of the variant syndromes of autoimmune liver disease. Hepatology 1998;28:360-5. [Crossref] [PubMed]
- Boberg KM, Chapman RW, Hirschfield GM, et al. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol 2011;54:374-85. [Crossref] [PubMed]
- Neuhauser M, Bjornsson E, Treeprasertsuk S, et al. Autoimmune hepatitis-PBC overlap syndrome: a simplified scoring system may assist in the diagnosis. Am J Gastroenterol 2010;105:345-53. [Crossref] [PubMed]
- Kuiper EM, Zondervan PE, van Buuren HR. Paris criteria are effective in diagnosis of primary biliary cirrhosis and autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol 2010;8:530-4. [Crossref] [PubMed]
- Wang K, Li Y, Pan J, et al. Noninvasive diagnosis of AIH/PBC overlap syndrome based on prediction models. Open Med (Wars) 2022;17:1550-8. [Crossref] [PubMed]
- Zhang W, De D, Mohammed KA, et al. New scoring classification for primary biliary cholangitis-autoimmune hepatitis overlap syndrome. Hepatol Commun 2018;2:245-53. [Crossref] [PubMed]
- Trivella J, John BV, Levy C. Primary biliary cholangitis: Epidemiology, prognosis, and treatment. Hepatol Commun 2023;7:e0179. [Crossref] [PubMed]
- Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929-38. [Crossref] [PubMed]
- Tunio NA, Mansoor E, Sheriff MZ, et al. Epidemiology of Autoimmune Hepatitis (AIH) in the United States Between 2014 and 2019: A Population-based National Study. J Clin Gastroenterol 2021;55:903-10. [Crossref] [PubMed]
- Muratori L, Cassani F, Pappas G, et al. The hepatitic/cholestatic "overlap" syndrome: an Italian experience. Autoimmunity 2002;35:565-8. [Crossref] [PubMed]
- Silveira MG, Talwalkar JA, Angulo P, et al. Overlap of autoimmune hepatitis and primary biliary cirrhosis: long-term outcomes. Am J Gastroenterol 2007;102:1244-50. [Crossref] [PubMed]
- Chazouillères O, Wendum D, Serfaty L, et al. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology 1998;28:296-301. [Crossref] [PubMed]
- Joshi S, Cauch-Dudek K, Wanless IR, et al. Primary biliary cirrhosis with additional features of autoimmune hepatitis: response to therapy with ursodeoxycholic acid. Hepatology 2002;35:409-13. [Crossref] [PubMed]
- Freedman BL, Danford CJ, Patwardhan V, et al. Treatment of Overlap Syndromes in Autoimmune Liver Disease: A Systematic Review and Meta-Analysis. J Clin Med 2020;9:1449. [Crossref] [PubMed]
- Levy C, Naik J, Giordano C, et al. Hispanics with primary biliary cirrhosis are more likely to have features of autoimmune hepatitis and reduced response to ursodeoxycholic acid than non-Hispanics. Clin Gastroenterol Hepatol 2014;12:1398-405. [Crossref] [PubMed]
- Efe C, Ozaslan E, Heurgué-Berlot A, et al. Sequential presentation of primary biliary cirrhosis and autoimmune hepatitis. Eur J Gastroenterol Hepatol 2014;26:532-7. [Crossref] [PubMed]
- Zhang WC, Zhao FR, Chen J, et al. Meta-analysis: diagnostic accuracy of antinuclear antibodies, smooth muscle antibodies and antibodies to a soluble liver antigen/liver pancreas in autoimmune hepatitis. PLoS One 2014;9:e92267. [Crossref] [PubMed]
- Jiang Y, Xu BH, Rodgers B, et al. Characteristics and Inpatient Outcomes of Primary Biliary Cholangitis and Autoimmune Hepatitis Overlap Syndrome. J Clin Transl Hepatol 2021;9:392-8. [Crossref] [PubMed]
- Yang F, Wang Q, Wang Z, et al. The Natural History and Prognosis of Primary Biliary Cirrhosis with Clinical Features of Autoimmune Hepatitis. Clin Rev Allergy Immunol 2016;50:114-23. [Crossref] [PubMed]
- Younossi ZM, Bernstein D, Shiffman ML, et al. Diagnosis and Management of Primary Biliary Cholangitis. Am J Gastroenterol 2019;114:48-63. [Crossref] [PubMed]
- Chazouillères O, Wendum D, Serfaty L, et al. Long term outcome and response to therapy of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. J Hepatol 2006;44:400-6. [Crossref] [PubMed]
- Ballotin VR, Bigarella LG, Riva F, et al. Primary sclerosing cholangitis and autoimmune hepatitis overlap syndrome associated with inflammatory bowel disease: A case report and systematic review. World J Clin Cases 2020;8:4075-93. [Crossref] [PubMed]
- Invernizzi P, Carbone M, Jones D, et al. Setanaxib, a first-in-class selective NADPH oxidase 1/4 inhibitor for primary biliary cholangitis: A randomized, placebo-controlled, phase 2 trial. Liver Int 2023;43:1507-22. [Crossref] [PubMed]
- Abdalian R, Dhar P, Jhaveri K, et al. Prevalence of sclerosing cholangitis in adults with autoimmune hepatitis: evaluating the role of routine magnetic resonance imaging. Hepatology 2008;47:949-57. [Crossref] [PubMed]
- Gheorghe L, Iacob S, Gheorghe C, et al. Frequency and predictive factors for overlap syndrome between autoimmune hepatitis and primary cholestatic liver disease. Eur J Gastroenterol Hepatol 2004;16:585-92. [Crossref] [PubMed]
- Gregorio GV, Portmann B, Karani J, et al. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology 2001;33:544-53. [Crossref] [PubMed]
- Perdigoto R, Carpenter HA, Czaja AJ. Frequency and significance of chronic ulcerative colitis in severe corticosteroid-treated autoimmune hepatitis. J Hepatol 1992;14:325-31. [Crossref] [PubMed]
- Deneau MR, Mack C, Mogul D, et al. Oral Vancomycin, Ursodeoxycholic Acid, or No Therapy for Pediatric Primary Sclerosing Cholangitis: A Matched Analysis. Hepatology 2021;73:1061-73. [Crossref] [PubMed]
- Deneau MR, El-Matary W, Valentino PL, et al. The natural history of primary sclerosing cholangitis in 781 children: A multicenter, international collaboration. Hepatology 2017;66:518-27. [Crossref] [PubMed]
- Lewin M, Vilgrain V, Ozenne V, et al. Prevalence of sclerosing cholangitis in adults with autoimmune hepatitis: a prospective magnetic resonance imaging and histological study. Hepatology 2009;50:528-37. [Crossref] [PubMed]
- Xi D, Lin H, Shah AA. Overview of autoimmune liver disease: Prevalence, risk factors, and role of autoantibodies. Clin Liver Dis (Hoboken) 2022;20:111-5. [Crossref] [PubMed]
- Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology 2006;44:1063-74. [Crossref] [PubMed]
- Floreani A, Rizzotto ER, Ferrara F, et al. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol 2005;100:1516-22. [Crossref] [PubMed]
- Bhanji RA, Mason AL, Girgis S, et al. Liver transplantation for overlap syndromes of autoimmune liver diseases. Liver Int 2013;33:210-9. [Crossref] [PubMed]
- Perez FM, Adekunle F, Mayne T, et al. P09 Long-term obeticholic acid (OCA) for Primary Biliary Cholangitis (PBC) in a clinical trial improved event free survival (death, liver transplant and hepatic decompensation) compared to external controls from the GLOBAL PBC real-world database. In: BMJ Publishing Group Ltd and British Society of Gastroenterology; 2022:A37. Available online: https://gut.bmj.com/lookup/doi/10.1136/gutjnl-2022-BASL.60
- Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ 2005;172:367-79. [Crossref] [PubMed]
- Malakouti M, Kataria A, Ali SK, et al. Elevated Liver Enzymes in Asymptomatic Patients - What Should I Do? J Clin Transl Hepatol 2017;5:394-403. [Crossref] [PubMed]
- Al-Chalabi T, Portmann BC, Bernal W, et al. Autoimmune hepatitis overlap syndromes: an evaluation of treatment response, long-term outcome and survival. Aliment Pharmacol Ther 2008;28:209-20. [Crossref] [PubMed]
- Lian M, Li B, Xiao X, et al. Comparative clinical characteristics and natural history of three variants of sclerosing cholangitis: IgG4-related SC, PSC/AIH and PSC alone. Autoimmun Rev 2017;16:875-82. [Crossref] [PubMed]
- Graziadei IW, Wiesner RH, Batts KP, et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology 1999;29:1050-6. [Crossref] [PubMed]
- Nayagam JS, Miquel R, Joshi D. Overlap Syndrome with Autoimmune Hepatitis and Primary Sclerosing Cholangitis. EMJ Hepatol 2019;95-104.
- Aizawa Y, Hokari A. Autoimmune hepatitis: current challenges and future prospects. Clin Exp Gastroenterol 2017;10:9-18. [Crossref] [PubMed]
- Boberg KM, Egeland T, Schrumpf E. Long-term effect of corticosteroid treatment in primary sclerosing cholangitis patients. Scand J Gastroenterol 2003;38:991-5. [Crossref] [PubMed]
- Zhao B, Zhang HY, Xie GJ, et al. Evaluation of the efficacy of steroid therapy on acute liver failure. Exp Ther Med 2016;12:3121-9. [Crossref] [PubMed]
- Ricciuto A, Kamath BM, Hirschfield GM, et al. Primary sclerosing cholangitis and overlap features of autoimmune hepatitis: A coming of age or an age-ist problem? J Hepatol 2023;79:567-75. [Crossref] [PubMed]
- Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J Gastroenterol 2017;23:6030-48. [Crossref] [PubMed]
- Ferrari F, Ranucci G, Aloi M, et al. A promising medium-term follow-up of pediatric sclerosing cholangitis: Mild phenotype or early diagnosis? Hepatol Res 2018;48:556-65. [Crossref] [PubMed]
- Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 2009;50:808-14. [Crossref] [PubMed]
- Mitchell SA, Bansi DS, Hunt N, et al. A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology 2001;121:900-7. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol 2016;64:433-85. [Crossref]
- Mahmud N. Selection for Liver Transplantation: Indications and Evaluation. Curr Hepatol Rep 2020;19:203-12. [Crossref] [PubMed]
- Chayanupatkul M, Fiel MI, Schiano TD. The clinical characteristics, pre- and post-liver transplantation outcomes in patients having autoimmune overlap syndromes. Clin Transplant 2020;34:e13841. [Crossref] [PubMed]
- Cholongitas E, Burroughs AK. Recurrence of autoimmune liver diseases after liver transplantation: clinical aspects. Auto Immun Highlights 2012;3:113-8. [Crossref] [PubMed]
- Manganis CD, Chapman RW, Culver EL. Review of primary sclerosing cholangitis with increased IgG4 levels. World J Gastroenterol 2020;26:3126-44. [Crossref] [PubMed]
- Zhu K, Yang J, Chen YZ, et al. Differences in Clinical Features and Diagnostic Strategies Between IgG4-Related Autoimmune Cholangitis and Cholangiocarcinoma. Front Oncol 2021;11:540904. [Crossref] [PubMed]
- Zen Y, Quaglia A, Portmann B. Immunoglobulin G4-positive plasma cell infiltration in explanted livers for primary sclerosing cholangitis. Histopathology 2011;58:414-22. [Crossref] [PubMed]
- Wallace ZS, Katz G, Hernandez-Barco YG, et al. Current and future advances in practice: IgG4-related disease. Rheumatol Adv Pract 2024;8:rkae020. [Crossref] [PubMed]
- Zhang L, Lewis JT, Abraham SC, et al. IgG4+ plasma cell infiltrates in liver explants with primary sclerosing cholangitis. Am J Surg Pathol 2010;34:88-94. [Crossref] [PubMed]
- Björnsson E, Chari S, Silveira M, et al. Primary sclerosing cholangitis associated with elevated immunoglobulin G4: clinical characteristics and response to therapy. Am J Ther 2011;18:198-205. [Crossref] [PubMed]
- Alswat K, Al-Harthy N, Mazrani W, et al. The spectrum of sclerosing cholangitis and the relevance of IgG4 elevations in routine practice. Am J Gastroenterol 2012;107:56-63. [Crossref] [PubMed]
- Parhizkar B, Mohammad Alizadeh AH, Asadzadeh Aghdaee H, et al. Primary sclerosing cholangitis associated with elevated immunoglobulin-g4: a preliminary study. ISRN Gastroenterol 2012;2012:325743. [Crossref] [PubMed]
- Nakazawa T, Ohara H, Sano H, et al. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest Endosc 2004;60:937-44. [Crossref] [PubMed]
- Kim JH, Byun JH, Kim SY, et al. Sclerosing cholangitis with autoimmune pancreatitis versus primary sclerosing cholangitis: comparison on endoscopic retrograde cholangiography, MR cholangiography, CT, and MRI. Acta Radiol 2013;54:601-7. [Crossref] [PubMed]
- EASL Clinical Practice Guidelines. management of cholestatic liver diseases. J Hepatol 2009;51:237-67. [Crossref] [PubMed]
- Mendes FD, Jorgensen R, Keach J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol 2006;101:2070-5. [Crossref] [PubMed]
- Olsson R, Glaumann H, Almer S, et al. High prevalence of small duct primary sclerosing cholangitis among patients with overlapping autoimmune hepatitis and primary sclerosing cholangitis. Eur J Intern Med 2009;20:190-6. [Crossref] [PubMed]
- Kim WR, Ludwig J, Lindor KD. Variant forms of cholestatic diseases involving small bile ducts in adults. Am J Gastroenterol 2000;95:1130-8. [Crossref] [PubMed]
- Czaja AJ. The overlap syndromes of autoimmune hepatitis. Dig Dis Sci 2013;58:326-43. [PubMed]
- Bunchorntavakul C, Reddy KR. Diagnosis and management of overlap syndromes. Clin Liver Dis 2015;19:81-97. [Crossref] [PubMed]
- Czaja AJ, Carpenter HA, Santrach PJ, et al. Autoimmune cholangitis within the spectrum of autoimmune liver disease. Hepatology 2000;31:1231-8. [Crossref] [PubMed]
- Hu CJ, Zhang FC, Li YZ, et al. Primary biliary cirrhosis: what do autoantibodies tell us? World J Gastroenterol 2010;16:3616-29. [Crossref] [PubMed]
- Manuel Lucena J, Montes Cano M, Luis Caro J, et al. Comparison of two ELISA assays for anti-Sp100 determination. Ann N Y Acad Sci 2007;1109:203-11. [Crossref] [PubMed]
- Milkiewicz P, Buwaneswaran H, Coltescu C, et al. Value of autoantibody analysis in the differential diagnosis of chronic cholestatic liver disease. Clin Gastroenterol Hepatol 2009;7:1355-60. [Crossref] [PubMed]
- Miyachi K, Hankins RW, Matsushima H, et al. Profile and clinical significance of anti-nuclear envelope antibodies found in patients with primary biliary cirrhosis: a multicenter study. J Autoimmun 2003;20:247-54. [Crossref] [PubMed]
- Ruffatti A, Arslan P, Floreani A, et al. Nuclear membrane-staining antinuclear antibody in patients with primary biliary cirrhosis. J Clin Immunol 1985;5:357-61. [Crossref] [PubMed]
- Wesierska-Gadek J, Hohenuer H, Hitchman E, et al. Autoantibodies against nucleoporin p62 constitute a novel marker of primary biliary cirrhosis. Gastroenterology 1996;110:840-7. [Crossref] [PubMed]
- Hasegawa S, Yoneda M, Kurita Y, et al. Cholestatic Liver Disease: Current Treatment Strategies and New Therapeutic Agents. Drugs 2021;81:1181-92. [Crossref] [PubMed]
- Lindor KD, Bowlus CL, Boyer J, et al. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2019;69:394-419. [Crossref] [PubMed]
- Rubel LR, Seeff LB, Patel V. Primary biliary cirrhosis-primary sclerosing cholangitis overlap syndrome. Arch Pathol Lab Med 1984;108:360-1. [PubMed]
- Burak KW, Urbanski SJ, Swain MG. A case of coexisting primary biliary cirrhosis and primary sclerosing cholangitis: a new overlap of autoimmune liver diseases. Dig Dis Sci 2001;46:2043-7. [Crossref] [PubMed]
- Kingham JG, Abbasi A. Co-existence of primary biliary cirrhosis and primary sclerosing cholangitis: a rare overlap syndrome put in perspective. Eur J Gastroenterol Hepatol 2005;17:1077-80. [Crossref] [PubMed]
- Rust C, Beuers U. Medical treatment of primary biliary cirrhosis and primary sclerosing cholangitis. Clin Rev Allergy Immunol 2005;28:135-45. [Crossref] [PubMed]
- Holtmeier J, Leuschner U. Medical treatment of primary biliary cirrhosis and primary sclerosing cholangitis. Digestion 2001;64:137-50. [Crossref] [PubMed]
- Zhang MY, Xu TM, Sun YH, et al. Risk of comorbidity of autoimmune liver disease in patients with inflammatory bowel disease: A single-center case-control study in China. J Dig Dis 2024;25:587-93. [Crossref] [PubMed]
- Zhao J, Li K, Liao X, et al. Causal associations between inflammatory bowel disease and primary biliary cholangitis: a two-sample bidirectional Mendelian randomization study. Sci Rep 2023;13:10950. [Crossref] [PubMed]
- Restellini S, Chazouillères O, Frossard JL. Hepatic manifestations of inflammatory bowel diseases. Liver Int 2017;37:475-89. [Crossref] [PubMed]
- van Munster KN, Bergquist A, Ponsioen CY. Inflammatory bowel disease and primary sclerosing cholangitis: One disease or two? J Hepatol 2024;80:155-68. [Crossref] [PubMed]
- Barberio B, Massimi D, Cazzagon N, et al. Prevalence of Primary Sclerosing Cholangitis in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Gastroenterology 2021;161:1865-77. [Crossref] [PubMed]
- Zhu Q, Fu Y, Qiu J, et al. Causal relationship between primary biliary cholangitis and inflammatory bowel disease: a Mendelian randomization study. Gastroenterol Rep (Oxf) 2024;12:goae049. [Crossref] [PubMed]
- Li Q, Zong Y. Primary Biliary Cholangitis Associated with Ulcerative Colitis: Case Series and Literature Review. Medicina (Kaunas) 2024;61:44. [Crossref] [PubMed]
- Woodward J, Neuberger J. Autoimmune overlap syndromes. Hepatology 2001;33:994-1002. [Crossref] [PubMed]
- Voss J, Schneider CV, Kleinjans M, et al. Hepatobiliary phenotype of individuals with chronic intestinal disorders. Sci Rep 2021;11:19954. [Crossref] [PubMed]
- Martínez Casas OY, Díaz Ramírez GS, Marín Zuluaga JI, et al. Autoimmune hepatitis - primary biliary cholangitis overlap syndrome. Long-term outcomes of a retrospective cohort in a university hospital. Gastroenterol Hepatol 2018;41:544-52. [Crossref] [PubMed]
- Lindgren S, Glaumann H, Almer S, et al. Transitions between variant forms of primary biliary cirrhosis during long-term follow-up. Eur J Intern Med 2009;20:398-402. [Crossref] [PubMed]
- Jayabalan D, Huang Y, Calzadilla-Bertot L, et al. Predictors of survival in autoimmune liver disease overlap syndromes. World J Hepatol 2024;16:1269-77. [Crossref] [PubMed]
- Lee DU, Ponder R, Lee K, et al. The differences in post-liver transplant outcomes of patients with autoimmune hepatitis who present with overlapping autoimmune liver diseases. Hepatol Int 2023;17:720-34. [Crossref] [PubMed]
- Kowdley KV, Bowlus CL, Levy C, et al. Efficacy and Safety of Elafibranor in Primary Biliary Cholangitis. N Engl J Med 2024;390:795-805. [Crossref] [PubMed]
- Hirschfield GM, Bowlus CL, Mayo MJ, et al. A Phase 3 Trial of Seladelpar in Primary Biliary Cholangitis. N Engl J Med 2024;390:783-94. [Crossref] [PubMed]
- Vuppalanchi R, González-Huezo MS, Payan-Olivas R, et al. A Multicenter, Open-Label, Single-Arm Study to Evaluate the Efficacy and Safety of Saroglitazar in Patients With Primary Biliary Cholangitis. Clin Transl Gastroenterol 2021;12:e00327. [Crossref] [PubMed]
- Yamaguchi M, Asano T, Arisaka T, et al. Effects of pemafibrate on primary biliary cholangitis with dyslipidemia. Hepatol Res 2022;52:522-31. [Crossref] [PubMed]
- Sohal A, Kowdley KV. Novel preclinical developments of the primary sclerosing cholangitis treatment landscape. Expert Opin Investig Drugs 2024;33:335-45. [Crossref] [PubMed]
- Pedersen MR, Mayo MJ. Advances in the evaluation and treatment of autoimmune hepatitis. Curr Opin Gastroenterol 2024;40:126-33. [Crossref] [PubMed]
- Sohal A, Kowdley KV. Primary Biliary Cholangitis: Promising Emerging Innovative Therapies and Their Impact on GLOBE Scores. Hepat Med 2023;15:63-77. [Crossref] [PubMed]
- Bhushan S, Sohal A, Kowdley KV. Primary Biliary Cholangitis and Primary Sclerosing Cholangitis Therapy Landscape. Am J Gastroenterol 2025;120:151-8. [Crossref] [PubMed]
Cite this article as: Sohal A, Nikzad N, Kowdley KV. Overlap syndromes in autoimmune liver disease: a review. Transl Gastroenterol Hepatol 2025;10:33.


