
A new dawn in cancer immunotherapy: the promise of mutant KRAS-specific vaccines
Pancreatic ductal adenocarcinoma (PDA) is highly lethal, with a 5-year survival of 13% (1). Although early-stage PDA is potentially curable with surgery and adjuvant or perioperative multiagent chemotherapy, recurrence rates at 2 years are as high as 70% due to micrometastatic disease, chemoresistance, and lack of immune surveillance. Therefore, median disease-free or recurrence-free survival (DFS/RFS) and overall survival (OS) rates for potentially resectable PDA are only 10–15 and 24–36 months, respectively (2).
Colorectal cancer (CRC) is a major health burden globally and the second deadliest malignancy (3). Surgical resection of colon cancer and adjuvant or perioperative chemotherapy are the standard-of-care for patients with early-stage disease (stage II–III) and for oligometastatic stage IV disease. However, over 30% of patients with stage II or III CRC and 60% to 70% of patients with oligometastatic disease experience recurrence after surgery. Identifying patients at high risk of recurrence after completing standard adjuvant or perioperative treatment remains challenging, and improving outcomes in these patients is a high priority.
Postsurgical circulating tumor DNA (ctDNA) analysis can detect minimal residual disease (MRD). The presence of ctDNA (ctDNA+) compared to ctDNA negative (ctDNA−) status after surgery is associated with worse RFS in CRC (20 months vs. not reached) (4) and in PDA (3.7 vs. 14 months) (5). Additionally, failure to normalize tumor markers such as the carbohydrate antigen (CA) 19-9 after definitive therapy leads to early recurrence and worse survival in PDA [median RFS (mRFS) 10.4 vs. 29.6 months, and median OS (mOS) 24.7 vs. 91.2 months for patients with high vs. low postoperative CA19-9 levels, respectively] (6). Similarly, CRC patients with an elevated postoperative carcinoembryonic antigen (CEA) level have a significantly lower 3-year RFS compared to those with normal CEA (58.2% vs. 88.2%, respectively) (7).
Activating KRAS mutations, most commonly in codon 12, are oncogenic drivers present in over 90% of PDA and up to 50% of CRC and are highly conserved during disease progression. This mutation results in a single amino acid substitution of glycine by aspartic acid in G12D, valine in G12V, arginine in G12R, alanine in G12A, or cysteine in G12C mutations. In PDA, the KRAS G12D mutation is the most frequent (45%), followed by G12V (35%) and G12R (17%), whereas in CRC, KRAS G12D is frequently detected (33%), followed by G12V (21%), but G12R is uncommon (0.5%) (8).
Mutant KRAS is a promising tumor antigen target for cancer vaccines for multiple reasons: (I) KRAS mutations occur early and are expressed uniformly in all tumor cells; (II) mutant KRAS signaling is required for tumor growth and survival; (III) mutant KRAS is not centrally tolerized and the cognate T cell receptor (TCR) is present in the naïve repertoire (9); (IV) mutant KRAS has the potential for off-the-shelf vaccine production (10-12); (V) mutant KRAS-specific T cells are known to mediate anti-tumor efficacy (9); and (VI) the recognition of clonal and subclonal mutant KRAS variants helps to prevent immune escape. Harnessing the immune system to induce durable and functional T cell responses against KRAS mutated cancers may be effective, but several prior KRAS vaccines had mixed results due to poor immunogenicity (13). To date, autogene cevumeran, a personalized neoepitope cancer vaccine added to adjuvant folinic acid, 5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) and atezolizumab demonstrated promising efficacy in resected PDA patients able to mount a vaccine specific immune response, with mRFS not reached after a 3-year follow-up, in comparison to only 13.4 months for patients without an immune response (14,15). Nevertheless, personalized vaccines may be hindered by tumor heterogeneity, with genetic and epigenetic changes accumulating during disease progression.
ELI-002 2P is an off-the-shelf vaccine comprised of mutant KRAS G12D and G12R peptide antigens and a Toll-like receptor (TLR)-9 agonist CpG adjuvant. In comparison to conventional vaccines, the amphiphilic ELI-002 2P vaccine is thought to elicit superior and longer lasting innate and adaptive immune responses due to improved lymph node trafficking (16-18).
Pant et al. conducted a phase 1 multi-center multi-cohort study of adjuvant ELI-002 2P for patients with stages I–III and oligometastatic stage IV resected KRAS G12D or G12R mutated PDA or CRC with evidence of positive minimal residual disease (MRD+) after definitive resection and perioperative therapy (19). MRD+ was defined as either high CA19-9 (>90 U/mL) or CEA (>15 ng/mL), successively rising CA19-9/CEA, or positive ctDNA assessed by the Signatera assay. Treatment consisted of prime immunization with six doses of ELI-002 2P administered subcutaneously over 8 weeks, 3 months rest, followed by boost immunization with four weekly doses of ELI-002 2P (Figure 1). Primary objectives were to determine safety and the recommended phase 2 dose (RP2D), and secondary objectives were to assess pharmacodynamic biomarkers [tumor antigens, ctDNA, and blood immune biomarkers: functional interferon gamma (IFNγ) and granzyme B response to mKRAS peptides by FluoroSpot assay, and intracellular interleukin-2 (IL-2), IFNγ and tumor necrosis factor alpha (TNFα) cytokine staining by flow cytometry] and correlate with efficacy, immunogenicity, as well as RFS. A biomarker response was any decrease from baseline in CA19-9/CEA or in ctDNA. An immune response was defined as ≥2-fold increase in blood immune biomarkers compared to baseline. The RFS was calculated from the time of first immunization.
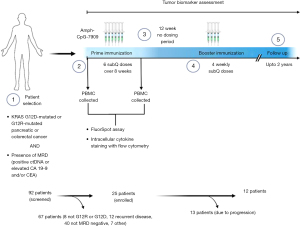
Ninety-two patients were screened for eligibility, and 25 patients (20 PDA and 5 CRC) were enrolled and treated with escalating doses of Amph-CpG-7909 (0.1, 0.5, 2.5, 5.0, and 10 mg) with a fixed dose of Amph-KRAS G12D/G12R peptides 2P (1.4 mg). This study enrolled a heterogenous population with early and late-stage PDA or CRC, but most had PDA (20/25, 80%), and stage III or oligometastatic stage IV disease (17/25, 68%). While most patients were likely treated with neoadjuvant and/or adjuvant therapy, it is unusual that a large proportion were enrolled more than 1 year from surgical resection. Less than half the patients (12/25, 48%) completed the entire 6 months of ELI-002 2P vaccine prime/rest/boost immunization, while the rest stopped early due to progression.
The study demonstrated that ELI-002 2P was overall safe, with no dose limiting toxicities (DLTs), including no grade ≥3 cytokine release syndrome (CRS), thus no maximum tolerated dose (MTD) was identified. Most common adverse events were fatigue (24%), injection site reactions (14%), and myalgias (12%), all grade 1 or 2. The RP2D was declared to be 10 mg Amph-CpG-7909/1.4 mg Amph-G12D/G12R based on safety and biomarker responses.
With respect to tumor biomarker efficacy, ELI-002 2P reduced CA19-9/CEA levels across all cohorts in 84% of patients and cleared ctDNA in 30% of PDA patients (3/9 with detectable ctDNA at baseline) and 60% of CRC patients (3/5 with detectable ctDNA at baseline). Furthermore, 84% of patients overall and 100% of patients treated at the highest dose level (5 and 10 mg Amph-CpG-7909) obtained strong KRAS-specific T cell responses. The immune responses entailed both CD4+ and CD8+ T cell expansion as well as IFNγ, TNFα and IL-2 cytokine production. Importantly, T cell responses specific to at least one other mutant KRAS antigen such as G12V, G12C and G12S but not to wild-type KRAS, were observed in 95% of patients, and 52% of patients mounted immune responses to all seven KRAS antigens, suggesting ELI-002 2P induced antigen spreading, a phenomenon which may help prevent antigen escape (18,20,21). Additionally, ELI-002 2P led to polyfunctional T cells and the expansion of the central memory T cell compartment, which is critical for sustained anti-cancer T cell responses.
With a median follow-up of only 8.5 months and many patients censored, the mOS and mRFS counted from the date of the first vaccination were both 16.3 months. To assess the association of ELI-002 2P-induced KRAS-specific immunity with RFS, patients were stratified into two groups with above-median and below-median T cell responses based on their fold change from baseline. Tumor biomarker and clinical responses were highly divergent based on T cell responses. No patients with below-median T cell responses achieved ctDNA clearance and 66.7% (8/12) showed biomarker reduction, whereas 46% (6/13) of above-median T cell responders achieved ctDNA clearance and 100% (13/13) showed biomarker reduction. RFS was 4.01 months vs. not reached for below-median vs. above-median T cell responders [hazard ratio (HR) =0.14; P=0.0167]. RFS was also associated with baseline absolute lymphocyte count, suggesting that lymphocyte recovery after cytotoxic chemotherapy is important for vaccine response.
Pant et al. were able to demonstrate a significant tumor biomarker response from ELI-002 2P in many patients at high risk of recurrence, but high patient discontinuation rates (13/25, 52%), the short follow-up (median 8.5 months), and high rates of censoring (approximately 50%) preclude a robust assessment of RFS and OS. Moreover the 2-year RFS of approximately 40% in this study is numerically comparable to the RFS with standard adjuvant chemotherapy for unselected patients. Nevertheless, the RFS duration generally starts from the time of resection or start of adjuvant therapy, while in this study the RFS reflects time from first vaccination, which often occurred more than 1 year from definitive locoregional therapy (range, 2.9 to 31.9 months, median 5.7 months). Given that all patients had MRD+ high risk status it is not surprising that RFS rates in the AMPLIFY-201 trial are not higher. The patient population in this study was also highly heterogenous, and although patients with MRD+ disease appeared to have similar outcomes irrespective of stage (I–II vs. III–IV), this study’s results are hence difficult to compare side by side with other adjuvant trials using chemo or immunotherapies initiated after curative intent resection. Similar to other recent vaccine trials though (mRNA, dendritic cells), clinical outcomes between patients with below- and above-median T cell responses were highly divergent, underscoring the importance of vaccine specific immunogenicity (14,15,22). Recent data from Lee et al. in the DYNAMIC-pancreas trial noted that early-stage, resected MRD+ PDA patients treated with adjuvant chemotherapy had mRFS of only 13 months, whereas the RFS was 22 months for MRD− patients (HR =0.28; P=0.003) (23). Meanwhile, the GALAXY study in patients with resected stage II–IV colon cancer showed a median DFS of only 6 months vs. not reached in MRD+ vs. MRD− patients (24).
Several ongoing studies will help address more definitively the role of adjuvant vaccine immunotherapy in PDA and CRC. The ongoing randomized phase 2 trial NCT05968326 in PDA evaluates the safety and efficacy of adjuvant autogene cevumeran plus atezolizumab and modified FOLFIRINOX vs. modified FOLFIRINOX alone in 260 patients after surgery (25). The open-label phase 2 trial NCT04486378 in colon cancer evaluates autogene cevumeran vs. watchful waiting following adjuvant chemotherapy in high-risk stage II/III patients who are ctDNA+ following surgical resection (26).
Although AMPLIFY-201 was a small, first in human phase 1 trial, it was conducted in multiple centers and initial results provide a proof-of-concept for Amph KRAS targeting vaccines. Long-term safety and efficacy must still be assessed. The phase 1/randomized phase 2 AMPLIFY-7P trial (NCT05726864) is currently evaluating a seven-valent (KRAS/NRAS G12D, G12R, G12V, G12S, G12A, G12C, G13D) formulation of ELI-002 in patients with resected PDA. Patients with stages I–III PDA must have undergone surgical resection and adjuvant/neoadjuvant therapy and start immunization within 6 months from completing locoregional treatment. Further study of the pharmacodynamic effects on host and tumor immune microenvironment in blood and tumor samples will be paramount to fully understand the breadth and depth of immune cells infiltration, activation, and persistence elicited with ELI-002, and correlate with efficacy. In AMPLIFY-201 among six patients who underwent tumor biopsies at the time of progression after immunization, all had evidence of T cells infiltration, and two had MRD clearance or radiological response after treatment with immune checkpoint inhibitors. This suggests that combination strategies of KRAS vaccines with other immune therapies may induce additional efficacy. Moreover, determining the best timing and sequence of vaccination in relation to locoregional therapy, including its use in the perioperative space may be key to prevent relapses. The off-the-shelf ELI-002 vaccine has demonstrated safety and preliminary efficacy in MRD+ pancreatic cancer and CRC which makes it an attractive vaccine candidate for cancers harboring KRAS mutations and adds to a new armamentarium of promising immunotherapies in high-risk malignancies.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-121/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-121/coif). V.G.P. reports lab research funding from NGM, OncoResponse and Merck and payment from Sensei, Umoja and TriSalus. R.A.S. reports research funding (paid to the institution) by Verastem, Exelixis, Replimune as well as the V Foundation, and advisory board member for Ipsen, Guardant Health, and Agenus. E.G.C. reports advisory board role for Ipsen, Merus, Pfizer, BMS, Takeda, Regeneron, BPGBio, IGM Biosciences, G1 Therapeutics, Merck, Novartis, Astellas, and Foundation Medicine; and research funding, paid to the institution, by Lonza, AffiniT, Erasca, BMS, Novartis, Gilead, Merck, Roche, AADi, Boehringer-Ingelheim, Fibrogen, Cornerstone, Biosplice. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Sohal DPS, Duong M, Ahmad SA, et al. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2021;7:421-7. [Crossref] [PubMed]
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Tie J, Cohen JD, Wang Y, et al. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol 2019;5:1710-7. [Crossref] [PubMed]
- Gong Z, Esmail A, Abdelrahim M. Circulating tumor DNA (ctDNA) and disease recurrence in early stage pancreatic cancer. J Clin Oncol 2023;41:e16313. [Crossref]
- Imaoka H, Shimizu Y, Senda Y, et al. Post-adjuvant chemotherapy CA19-9 levels predict prognosis in patients with pancreatic ductal adenocarcinoma: A retrospective cohort study. Pancreatology 2016;16:658-64. [Crossref] [PubMed]
- Pu H, Yang W, Liu M, et al. Elevated postoperative carcinoembryonic antigen guides adjuvant chemotherapy for stage II colon cancer: a multicentre cohort retrospective study. Sci Rep 2024;14:6889. [Crossref] [PubMed]
- Koulouridi A, Karagianni M, Messaritakis I, et al. Prognostic Value of KRAS Mutations in Colorectal Cancer Patients. Cancers (Basel) 2022;14:3320. [Crossref] [PubMed]
- Tran E, Robbins PF, Lu YC, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med 2016;375:2255-62. [Crossref] [PubMed]
- Palmer DH, Valle JW, Ma YT, et al. TG01/GM-CSF and adjuvant gemcitabine in patients with resected RAS-mutant adenocarcinoma of the pancreas (CT TG01-01): a single-arm, phase 1/2 trial. Br J Cancer 2020;122:971-7. [Crossref] [PubMed]
- Carbone DP, Ciernik IF, Kelley MJ, et al. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol 2005;23:5099-107. [Crossref] [PubMed]
- Bear AS, Blanchard T, Cesare J, et al. Biochemical and functional characterization of mutant KRAS epitopes validates this oncoprotein for immunological targeting. Nat Commun 2021;12:4365. [Crossref] [PubMed]
- Abou-Alfa GK, Chapman PB, Feilchenfeldt J, et al. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol 2011;34:321-5. [Crossref] [PubMed]
- Guasp P, Sethna Z, Reiche C, et al. Personalized RNA neoantigen vaccines induce long-lived CD8+ T effector cells in pancreatic cancer. Cancer Immunol Res 2024;12:PR-06. [Crossref]
- Rojas LA, Sethna Z, Soares KC, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023;618:144-50. [Crossref] [PubMed]
- Yousefpour P, Ni K, Irvine DJ. Targeted modulation of immune cells and tissues using engineered biomaterials. Nat Rev Bioeng 2023;1:107-24. [Crossref] [PubMed]
- Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov 2007;6:231-48. [Crossref] [PubMed]
- Moynihan KD, Opel CF, Szeto GL, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med 2016;22:1402-10. [Crossref] [PubMed]
- Pant S, Wainberg ZA, Weekes CD, et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial. Nat Med 2024;30:531-42. [Crossref] [PubMed]
- Ma L, Dichwalkar T, Chang JYH, et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 2019;365:162-8. [Crossref] [PubMed]
- Drakes DJ, Abbas AM, Shields J, et al. Lymph Node-Targeted Vaccine Boosting of TCR T-cell Therapy Enhances Antitumor Function and Eradicates Solid Tumors. Cancer Immunol Res 2024;12:214-31. [Crossref] [PubMed]
- van 't Land FR, Willemsen M, Bezemer K, et al. Dendritic Cell-Based Immunotherapy in Patients With Resected Pancreatic Cancer. J Clin Oncol 2024;42:3083-93. [Crossref] [PubMed]
- Lee B, Tie J, Wang Y, et al. The potential role of serial circulating tumor DNA (ctDNA) testing after upfront surgery to guide adjuvant chemotherapy for early stage pancreatic cancer: The AGITG DYNAMIC-Pancreas trial. J Clin Oncol 2024;42:107. [Crossref]
- Kotani D, Oki E, Nakamura Y, et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med 2023;29:127-34. [Crossref] [PubMed]
- A Phase II, Open-Label, Multicenter, Randomized Study of the Efficacy and Safety of Adjuvant Autogene Cevumeran Plus Atezolizumab and mFOLFIRINOX Versus mFOLFIRINOX Alone in Patients With Resected Pancreatic Ductal Adenocarcinoma. ClinicalTrials.gov identifier: NCT05968326. Updated December 05, 2024. Accessed December 27, 2024. Available online: https://www.clinicaltrials.gov/study/NCT05968326
- A Multi-site, Open-label, Phase II, Randomized, Controlled Trial to Compare the Efficacy of RO7198457 Versus Watchful Waiting in Resected, Stage II (High Risk) and Stage III Colorectal Cancer Patients Who Are ctDNA Positive Following Resection. ClinicalTrials.gov identifier: NCT04486378. Updated December 10, 2024. Accessed December 27, 2024. Available online: https://www.clinicaltrials.gov/study/NCT04486378
Cite this article as: Damle SR, Pillarisetty VG, Safyan RA, Chiorean EG. A new dawn in cancer immunotherapy: the promise of mutant KRAS-specific vaccines. Transl Gastroenterol Hepatol 2025;10:20.


