
Association between cholecystectomy and chronic kidney disease risk: a nationally retrospective cohort study
Highlight box
Key findings
• There may be an association between cholecystectomy and an increased risk of chronic kidney disease.
• The potential risk of chronic kidney disease might persist beyond 3 years post-cholecystectomy.
What is known and what is new?
• Previous studies have highlighted risks after cholecystectomy, such as cardiovascular disease and digestive disorders.
• This study reveals a previously unrecognized association between cholecystectomy and chronic kidney disease.
What is the implication, and what should change now?
• Physicians should consider monitoring kidney function in patients following cholecystectomy to better understand any potential association with chronic kidney disease.
Introduction
Cholecystectomy is an organ removal procedure performed primarily to treat gallstone disease (1). In 2021, 83,479 patients underwent cholecystectomy in South Korea, and the number has been steadily increasing since 2010. While cholecystectomy is a relatively simple procedure for patients, it can have health implications. The removal of the gallbladder can result in alterations to the composition of the gut microbiome (2), and post-surgery bile acid metabolism could change in ways that predispose to health problems (3). Therefore, it is important to identify the potential health implications of cholecystectomy. Cholecystectomy has been associated with an increased risk of digestive system cancers and non-alcoholic fatty liver disease (4,5). However, few studies have evaluated how cholecystectomy and the removal of the gallbladder from cholecystectomy are related to chronic kidney disease (CKD) in the Korean population.
CKD is characterized by renal impairment leading to diminished blood filtration capacity. Globally, it is estimated that approximately 13.4% of the population is affected by CKD (6). Patients with end-stage renal disease (ESRD) require dialysis, and the annual mortality rate for dialysis patients in the United States is up to 20% (7). Because CKD is a serious condition with a poor prognosis, relevant epidemiologic studies are needed. However, there is a paucity of research confirming the association between cholecystectomy and CKD. Previous study of increased risk of kidney cancer after cholecystectomy have interpreted the results through genetic susceptibility (8), but the underlying mechanisms remain unclear. Therefore, epidemiologic evidence of the risk of developing CKD after cholecystectomy is needed.
In recent previous studies on cholecystectomy, the effects on patients’ bodies were observed over both a short and a long period of time. It is possible that the patient’s body may be healthy after cholecystectomy within 2 years in terms of metabolic health. However, it is also possible that the patient’s body may be affected in a long period of time, such as more than 2 years after cholecystectomy (9). However, there may be side effects during the period within 2 years after cholecystectomy, and long-term effects after 2 years may also have both good and side effects. Therefore, the study also considered the risk of disease over time after surgery. Recognizing that CKD is most common in older adults (10), and thus, the association between cholecystectomy and the risk of CKD was analyzed using data from the National Health Insurance Service-Health Screening Cohort (NHIS-HEALS) in Korean adults aged 40 years and older. We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-98/rc).
Methods
Data source
The study population was acquired from the NHIS-HEALS cohort database of South Korea. About 98% of South Koreans are enrolled in the National Health Insurance Service (NHIS) (11). The NHIS-HEALS cohort data was constructed by selecting a 10% random sample of individuals aged 40–79 years who were eligible in 2002 and 2003 (12). These participants were followed up and included in the data until 2019. Health screenings conducted by the NHIS are conducted every 2 years for almost all Koreans enrolled in the NHIS.
The eligibility database included information about household income, demographic characteristics, and date and cause of mortality. Additionally, it comprised variables pertaining to various health indicators and risk factors, such as cigarette smoking status, amount of alcohol drinking, physical activity, and so on. Bio-clinical laboratory results include blood pressure, fasting glucose levels, lipid profile, creatinine, body mass index (BMI), and waist circumference. Notably, certain variables, such as smoking status, alcohol consumption, and physical activity, were updated during the follow-up period.
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB number: E-2311-054-1483). Informed consent was not required for participation in this study because the NHIS database used for data analysis underwent a rigorous anonymization process based on strict confidentiality rules before distribution. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study population and design
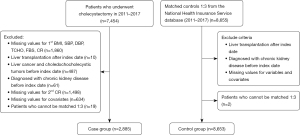
The NHIS-HEALS cohort data used in this study were constructed from 2002 to 2019. We extracted 7,454 patients who had a record of undergoing cholecystectomy between 2011 and 2017. Health screenings completed before the cholecystectomy were categorized as primary health screenings, and health screenings completed after the cholecystectomy were categorized as secondary health screenings. For example, if you had a cholecystectomy in 2011, your primary health screening would be in 2009 if you were born in an even year and in 2010 if you were born in an odd year. Your secondary health screening would be in 2011 if you were born in an even year and in 2012 if you were born in an odd year. We excluded a total of 1,860 patients with missing information on their first health screening. We also excluded 10 patients with a history of liver transplantation after the index date of cholecystectomy and 487 patients diagnosed with liver, gallbladder, or biliary tract cancer. We excluded 61 patients who were diagnosed with CKD before the index date. We excluded 1,498 patients with missing creatinine levels in the secondary health screening information and 634 patients with missing covariate values. Finally, we excluded 19 patients who were not matched by propensity score matching (PSM), which was performed at a ratio of 1:3. We included a total of 2,885 patients in the cholecystectomy group, and a total of 8,653 patients were followed up in the group without cholecystectomy (Figure 1).
Key variables
We obtained the exposure variable for cholecystectomy using the procedure registry code Q7380. The dependent variable, CKD, was defined based on codes derived from the International Statistical Classification of Diseases, 10th Revision (ICD-10). The ICD-10 was created by the World Health Organization as a standardized code set for medical procedures and conditions (13). The occurrence of CKD was defined as a diagnosis of N18.0 to N18.9 according to ICD-10 criteria. We considered patients who were diagnosed with CKD during the follow-up period from immediately after cholecystectomy to 31 December 2019.
Covariates were included using data from the primary health screening before index date. These covariates comprised age (continuous variable), sex, BMI (continuous variable), household income (categorized into quintiles), smoking status (categorized as never, past, and current smoking), and alcohol consumption (categorized as never, 1–2 days/week, 3–4 days/week, and 5 or more days/week). Physical activity (categorical variable divided into never, 1–2 days/week, 3–4 days/week, and 5 or more days/week), creatinine, estimated glomerular filtration rate (eGFR), fasting serum glucose, total cholesterol, systolic blood pressure, triglyceride, high-density lipoprotein, diabetes (categorized as yes and no), hypertension (categorized as yes and no), and Charlson comorbidity index (CCI). The eGFR was estimated via the CKD-EPI equation (14). GFR estimates were calculated differently by gender using serum creatinine and age. Glomerular filtration rate (GFR) is considered a composite index of kidney function. GFR is not easily measured clinically; instead, it is estimated from an equation that uses serum creatinine, age, sex, etc. Diabetes was defined as ICD-10 code of E11 to E14 or a history of prescription for antidiabetic medications before the index date, or a fasting blood glucose level of 126 mg/dL or more. Similarly, hypertension was defined as I10 or a history of prescription for antihypertensive medications before the index date, or a systolic blood pressure of 140 mmHg or more (15).
Statistical analysis
Categorical variables are presented as frequencies and proportions and χ2 tests were used to determine the significance of differences between cholecystectomy groups. Continuous variables were presented as mean values and standard deviations, and t-tests and analysis of variance were used to determine the significance of differences between cholecystectomy groups.
PSM was performed to reduce potential confounding effects between the cholecystectomy and non-cholecystectomy groups. We used the greedy nearest neighbor matching algorithm as the propensity matching method. The different cholecystectomy groups were matched 1:3 based on a difference of ±0.2 caliper in the linearly transformed propensity score multiplied by the standard deviation, and sex was matched for exact matching. Participants who were not matched were excluded from the analysis. Standardized differences were calculated to assess the distribution of possible covariates after PSM. Variables selected as covariates in PSM were age, sex, BMI, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, triglycerides, fasting serum glucose, eGFR, household income, cigarette smoking status, alcohol consumption, physical activity, and CCI.
Time-to-event analyses involved the utilization of Kaplan-Meier analyses with unadjusted Cox proportional hazards models to examine the risks associated with the onset and progression of CKD by cholecystectomy status. Multivariable Cox proportional hazards model was applied to evaluate the adjusted hazard ratio (aHR) and corresponding 95% confidence intervals (CIs) of CKD according to whether cholecystectomy was performed. In sensitivity analyses, we created subgroups by sex, age, household income, BMI, smoking status, alcohol consumption, and physical activity within the 2011 to 2019 follow-up period. The risk estimation for incident CKD was adjusted for sex, age, household income, BMI, physical activity, smoking status, alcohol consumption, triglyceride, high density lipoprotein, total cholesterol, eGFR, diabetes, hypertension, and CCI. A significance threshold of P<0.05 was adopted. All data collection and subsequent statistical analyses were conducted using SAS Enterprise Guide 8.3 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of participants
From 2011 to 2017, a total of 2,887 individuals who had undergone cholecystectomy and 389,063 individuals who had not been matched at a 1:3 ratio based on their propensity scores and included in the analysis. After PSM, the different cholecystectomy groups were well balanced, with a standardized mean difference (SMD) less than 0.1 for all baseline characteristics (Table 1). Participants included in the analysis had a mean follow-up of 6.04 years. Among the 2,885 participants who had undergone cholecystectomy, 1,742 (60.4%) were males, with an average age of 63.4 years. The participants’ mean BMI was 24.6 kg/m2, mean creatinine level was 0.9 mg/dL, and mean eGFR value was 82.8 mL/min/1.73 m2. Most of the cholecystectomy group (60.4%) reported that they never smoked, while 42.2% reported not consuming any alcohol. Additionally, 26.4% of this group indicated that they engaged in regular physical exercise. A CCI score of 2 or higher was recorded in 1,416 (49.1%) of the participants. Of the 8,653 participants who did not undergo cholecystectomy, 5,223 (60.4%) were male. The average age was 63.4 years, and the mean BMI was 25.0 kg/m2. The average creatinine level was 0.9 mg/dL and the average eGFR was 82 mL/min/1.73 m2. Among the non-cholecystectomy group, 5,261 (60.8%) reported that they had never smoked and 3,642 (42.1%) reported consuming alcohol 1–2 times per week. Participants with a CCI of 2 or more were 4,172 (48.2%).
Table 1
| Variables | Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No cholecystectomy (n=389,063) | Cholecystectomy (n=2,887) | SMD | P | No cholecystectomy (n=8,653) | Cholecystectomy (n=2,885) | SMD | P | ||
| Age (years) | 60.5±8.6 | 63.4±8.8 | 0.327 | <0.001 | 63.4±9.0 | 63.4±8.8 | 0.003 | 0.90 | |
| Sex: men | 207,342 (53.3) | 1,744 (60.4) | 0.144 | <0.001 | 5,223 (60.4) | 1,742 (60.4) | <0.001 | >0.99 | |
| Household income† | <0.001 | 0.87 | |||||||
| First (highest) | 137,775 (35.4) | 1,137 (39.4) | 0.083 | 3,435 (39.7) | 1,135 (39.3) | 0.008 | |||
| Second | 113,633 (29.2) | 791 (27.4) | 0.040 | 2,396 (27.7) | 791 (27.4) | 0.007 | |||
| Third | 80,384 (20.6) | 548 (19.0) | 0.040 | 1,522 (17.6) | 548 (18.9) | 0.009 | |||
| Fourth (lowest) | 57,271 (14.8) | 411 (14.2) | 0.017 | 1,300 (15.0) | 411 (14.4) | 0.008 | |||
| Body mass index (kg/m2) | 24.0±2.9 | 24.6±2.9 | 0.195 | <0.001 | 25.0±3.3 | 24.6±2.9 | 0.008 | 0.82 | |
| Systolic blood pressure (mmHg) | 125.2±15.1 | 126.0±14.4 | 0.054 | 0.003 | 125.9±14.9 | 126.0±14.4 | 0.010 | 0.65 | |
| Total cholesterol (mg/dL) | 199.7±37.6 | 194.1±39.5 | 0.144 | <0.001 | 193.8±37.1 | 194.2±39.5 | 0.034 | 0.64 | |
| High density lipoprotein cholesterol (mg/dL) | 54.6±23.3 | 51.4±13.5 | 0.168 | <0.001 | 51.4±12.5 | 51.4±13.5 | 0.028 | 0.90 | |
| Triglycerides (mg/dL) | 138.9±89.5 | 141.4±86.6 | 0.028 | 0.13 | 140.2±86.0 | 141.4±86.7 | 0.003 | 0.64 | |
| Fasting serum glucose (mg/dL) | 101.2±24.8 | 105.0±26.6 | 0.145 | <0.001 | 104.5±27.5 | 104.9±26.5 | 0.018 | 0.75 | |
| Serum creatinine (mg/dL) | 1.0±1.1 | 0.9±0.6 | 0.113 | <0.001 | 0.9±0.6 | 0.9±0.5 | <0.001 | 0.98 | |
| eGFR (mL/min/1.73 m2) | 82.9±18.9 | 82.8±18.6 | 0.005 | 0.93 | 82.0±18.8 | 82.8±18.4 | 0.011 | 0.74 | |
| Cigarette smoking | <0.001 | 0.67 | |||||||
| Never smoker | 250,818 (64.5) | 1,744 (60.4) | 0.085 | 5,261 (60.8) | 1,743 (60.4) | 0.008 | |||
| Past smoker | 73,766 (18.9) | 733 (25.4) | 0.157 | 2,128 (24.6) | 732 (25.4) | 0.018 | |||
| Current smoker | 64,479 (16.6) | 410 (14.2) | 0.067 | 1,264 (14.6) | 410 (14.2) | 0.011 | |||
| Alcohol consumption | <0.001 | 0.86 | |||||||
| None | 227,739 (58.5) | 1,216 (42.1) | 0.333 | 3,565 (41.2) | 1,216 (42.2) | 0.019 | |||
| 1–2 times/week | 106,573 (27.4) | 1,232 (42.7) | 0.325 | 3,642 (42.1) | 1,230 (42.6) | 0.011 | |||
| 3–4 times/week | 35,553 (9.2) | 312 (10.8) | 0.786 | 1,038 (12.0) | 312 (10.8) | 0.037 | |||
| ≥5 times/week | 19,198 (4.9) | 127 (4.4) | 0.100 | 408 (4.7) | 127 (4.4) | 0.010 | |||
| Physical activity | <0.001 | 0.79 | |||||||
| None | 178,660 (45.9) | 899 (31.1) | 0.308 | 2,669 (30.8) | 899 (31.2) | 0.007 | |||
| 1–2 times/week | 68,735 (17.7) | 788 (27.3) | 0.231 | 2,465 (28.5) | 786 (27.2) | 0.025 | |||
| 3–4 times/week | 54,183 (13.9) | 439 (15.2) | 0.037 | 1,304 (15.1) | 439 (15.2) | 0.002 | |||
| ≥5 times/week | 87,485 (22.5) | 761 (26.4) | 0.091 | 2,215 (25.6) | 761 (26.4) | 0.017 | |||
| Charlson comorbidity index | <0.001 | 0.55 | |||||||
| 0 | 138,518 (35.6) | 603 (20.9) | 0.331 | 1,886 (21.8) | 603 (20.9) | 0.021 | |||
| 1 | 123,508 (31.7) | 866 (30.0) | 0.037 | 2,595 (30.0) | 866 (30.0) | <0.001 | |||
| ≥2 | 127,037 (32.7) | 1,418 (49.1) | 0.338 | 4,172 (48.2) | 1,416 (49.1) | 0.017 | |||
Data are presented as n (%) or mean ± standard deviation. †, proxy for socioeconomic status based on the insurance premium of the National Health Insurance Service. eGFR, estimated glomerular filtration rate; SMD, standardized mean difference.
Association of cholecystectomy with CKD
The risk of CKD in the group that underwent cholecystectomy and the group that did not undergo cholecystectomy is presented in Table 2. During the follow-up period, a total of 50 CKD events occurred in the cholecystectomy group and 189 CKD events occurred in the non-cholecystectomy group. After adjusting for all covariates as potential confounding factors throughout the entire follow-up period, participants who underwent cholecystectomy were likely to have a higher risk of CKD compared to those who did not undergo cholecystectomy (aHR 1.89; 95% CI: 1.55–2.31). After adjusting for all covariates, participants who underwent cholecystectomy were likely to have an increased risk of CKD compared with those who did not undergo cholecystectomy, both from baseline to 3 years and from 3 years to 2019 (aHR 2.10, 95% CI: 1.72–2.56; aHR 1.68, 95% CI: 1.38–2.05).
Table 2
| Group by follow-up period | Event, n | Per 1,000 PYs | aHR (95% CI) | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||
| Whole period risk | ||||||
| No cholecystectomy (n=8,653) | 189 | 53.450 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| Cholecystectomy (n=2,885) | 50 | 10.124 | 2.10 (1.72–2.56)*** | 2.10 (1.72–2.56)*** | 2.08 (1.70–2.54)*** | 1.89 (1.55–2.31)*** |
| ≤3 years | ||||||
| No cholecystectomy (n=642) | 46 | 0.866 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| Cholecystectomy (n=1,078) | 33 | 0.048 | 2.31 (1.89–2.82)*** | 2.29 (1.89–2.82)*** | 2.27 (1.86–2.77)*** | 2.10 (1.72–2.56)*** |
| >3 years | ||||||
| No cholecystectomy (n=8,011) | 143 | 54.939 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| Cholecystectomy (n=1,807) | 17 | 10.124 | 1.89 (1.55–2.31)*** | 1.88 (1.54–2.30)*** | 1.87 (1.53–2.28)*** | 1.68 (1.38–2.05)*** |
Model 1: adjusted for age and sex; Model 2: adjusted for age, sex, and household income; Model 3: adjusted for age, sex, household income, smoking status, alcohol consumption and physical activity; Model 4: adjusted for age, sex, household income, smoking status, alcohol consumption, physical activity, body mass index, total cholesterol, triglyceride, high density lipoprotein, eGFR, diabetes, hypertension, and Charlson comorbidity index (diabetes was defined as ICD-10 codes E11-E14, use of antidiabetic medications, or fasting blood glucose ≥126 mg/dL. Hypertension was defined as ICD-10 code I10, use of antihypertensive medications, or systolic blood pressure ≥140 mmHg). ***, P<0.001. CI, confidence interval; eGFR, estimated glomerular filtration rate; aHR, adjusted hazard ratio; ICD-10, the International Statistical Classification of Diseases, 10th Revision; PYs, person years.
Stratified analysis
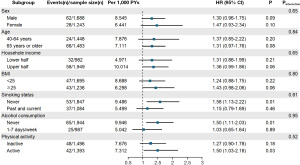
Figure 2 shows the results of stratified analyses by sex, age group, household income, BMI, physical activity, smoking status, and alcohol consumption. Among these subgroups, females had a higher risk of CKD associated with cholecystectomy compared to males (aHR 1.70; 95% CI: 1.19–2.42). For participants aged 65 years or older, those who underwent cholecystectomy had a 1.94 times higher risk of CKD compared to those who did not undergo cholecystectomy (aHR 1.94; 95% CI: 1.19–3.17). Among participants who underwent cholecystectomy, the risk of CKD was higher in those with lower household income (aHR 1.90; 95% CI: 1.21–2.98). In the subgroup on alcohol consumption, the risk of developing CKD was higher in those who had undergone cholecystectomy in the group that consumed more alcohol (aHR 1.98; 95% CI: 1.19–3.30). The increased risk of CKD associated with cholecystectomy was consistently observed across various subgroup analyses, including female participants, older participants, those with lower income, and those with higher alcohol consumption.
Discussion
In summary, our data suggest a potential association between cholecystectomy and an increased risk of CKD. Furthermore, the risk of CKD beyond 3 years after cholecystectomy was still likely to be increased. To our knowledge, this is the first Korean longitudinal cohort study to evaluate NHIS data to assess the association between cholecystectomy and the development of CKD. We also designed the study to ensure that preoperative patient characteristics were not significantly associated with post-cholecystectomy outcomes by utilizing PSM.
There are varying opinions about the health outcomes after cholecystectomy. Previous studies have associated cholecystectomy with an increased risk of developing Parkinson’s disease, colorectal cancer, and nonalcoholic fatty liver disease (5,16,17). However, one study has linked cholecystectomy to improved metabolic health (9). Among these conflicting results, no association between cholecystectomy and CKD has been found. In a previous study that examined the relationship between cholecystectomy and kidney cancer, reported the risk of kidney cancer increased by 1.17 times after cholecystectomy, and the increased risk of kidney cancer persisted for 3 years after surgery (8). Kidney cancer has also been found to increase, even after excluding cancers that occurred within 2 years of cholecystectomy (18). In a study of Italians and Swiss, the risk of kidney cancer increased by 1.57 times after a cholelithiasis diagnosis, and this increased risk was observed for up to 10 years or more (19). In our data, we observed a possible 1.89 times higher association between cholecystectomy and the risk of CKD. These results can be understood as postoperative physiologic changes.
The mechanisms for the relationship between cholecystectomy and CKD aren’t well understood. There are studies that explain the increased risk of CKD after cholecystectomy with changes in the biliary system after cholecystectomy. Biliary system changes associated with cholecystectomy could include bile duct stricture, biliary obstruction, cholestasis, recurrent stones in the common bile duct, biliary dyskinesia, and bile duct dilatation (3,20-22). After cholecystectomy, cholestasis, a condition in which the flow of bile might be reduced or stopped, could occur. This can potentially lead to recurring stones. If stones detected 2 years after cholecystectomy can be defined as recurrent stones, they might be observed up to 15 years later (23,24). Kidney damage due to overproduction of bile acids could also be a possible mechanism. After cholecystectomy, bile acids overproduction might occur (8,25), leading to increased secondary bile acid synthesis, which could alter bile acid composition and potentially causes oxidative stress, farnesoid X receptor (FXR) inhibition, and changes in metabolic hormone levels. Thes factors, in turn, might contribute to inflammation and potential renal injury (8,26). Furthermore, renal tubular oxidative stress due to elevated bile acid levels could result in bile cast nephropathy by depositing bile casts in the renal tubules, possibly contributing to acute kidney injury (25,27,28). Finally, bile duct obstruction might be associated with the kidney to act as an alternative excretory pathway for bile acids, which could contribute to renal tubular cell necrosis and be linked to CKD. CKD might prevent bile acids from being excreted through the urine, contributing to a cycle of worsening kidney damage (27).
The gut microbiome is believed to play various roles in the normal functioning of the human body (29). Among these are the synthesis of vitamins as well as the breakdown of indigestible plant polysaccharides and oxalates. Unlike simple sugars, animals have a limited capacity to utilize complex polysaccharides. The symbiotic relationship between humans and gut bacteria has led to the predominance of Bacteroides species in the distal gut, capable of metabolizing diverse plant polysaccharides, thus providing essential nutrients to the human host. Furthermore, microbial fermentation of polysaccharides in the anaerobic environment of the distal gastrointestinal (GI) tract yields short-chain fatty acids (SCFAs) like acetate, propionate, and butyrate. Colonic epithelial cells utilize as a source of nourishment, contributing to their health (30,31).
In cases of progressive renal failure, elevated levels of urea in the bloodstream occur. Intestinal bacteria exposed to urea convert it into ammonia via bacterial urease, leading to an overgrowth of urease-containing bacterial families. Patients with ESRD demonstrate an increased prevalence of bacterial families that produce uricase and enzymes involved in the formation of indole and p-cresyl, as opposed to their counterparts who are in good health. Changes in appetite accompanying renal failure may be related to changes in the intake of foods that could affect the microbiome, which might be associated with the progression of CKD (32,33).
Severe renal failure results in the colon becoming the primary route for uric acid and oxalate excretion, promoting the proliferation of uricase-producing bacterial species. A study shows differences in microbial flora between rats and humans with renal impairment compared to healthy controls, with Actinobacteria, Firmicutes, and Proteobacteria being notably increased (29). Similar differences were observed in patients on peritoneal dialysis, with certain beneficial bacterial species being less prevalent. Various factors might contribute to these changes, including reduced fiber intake, altered colonic transit time, and comorbidities like diabetes. Notably, constipation rates tend to be elevated in patients undergoing hemodialysis and peritoneal dialysis. In summary, alterations in the biliary system and gut microbiota post-cholecystectomy might be associated with renal function (29).
In our stratified analysis, we observed that 1.89 times increased risk of CKD after cholecystectomy was present in female. The epidemiology of CKD is gender-specific and can affect females more than males (34). In addition, the female gender is a significant risk factor for gallstone formation. This is believed to be attributed to the naturally higher levels of estrogen in females (35-37). Previous studies suggested that CKD risk may increase with older age and lower income. Increased age is known for a major risk factor for gallstone disease, such as cholelithiasis (35,38,39). Depending on hepatic cholesterol and bile acid metabolism, gallstone formation may be more prevalent in increased age (35,40,41). An association was observed between cholecystectomy patients with low income and CKD. A study in Koreans found that the risk of CKD increases with lower income (42). Income level was associated with the incidence of cholecystectomy, and a higher incidence of cholelithiasis was observed in lower income groups (43).
First, the limitation of this study is that we used a claims database, so there may be inconsistencies between CKD diagnosis and surgical history. Second, to address potential confounding factors of patients who underwent cholecystectomy, we excluded patients who had a liver transplant or had a history of liver cancer, gallbladder cancer, or biliary tract cancer before cholecystectomy. We also adjusted for chronic diseases such as diabetes and hypertension. However, unclaimed factors could not be included in the analysis, and residual confounding may remain. In addition to the postoperative physiologic changes, we could not determine from the data the surgical characteristics of the cholecystectomy, such as urgency or whether it was laparoscopic or not, that might be associated with the development of CKD. Third, there may be uncertainty in the definition of CKD found in the NHIS database. In addition, GFR, a measure of kidney function, is often estimated, so the full impact on eGFR outcomes cannot be accurately assessed (44). Fourth, the average follow-up time in our data was approximately 6 years, and the low number of CKD events may have overfitted the results. The limited number of CKD events might be due to the relatively short 6-year follow-up period [2011–2017]. Although the dataset started in 2002, key indicators of kidney function, such as creatinine and eGFR, were only available from 2009. Because health screenings in Korea are conducted biennially, we began selecting participants in 2011, 2 years after 2009, to ensure sufficient and consistent data. Therefore, future studies with longer follow-up periods are needed to validate these findings. Fifth, the study’s findings may not be generalizable beyond the specific cohort examined, particularly given that the study population was drawn from a single national database in Korea. This limits the applicability of the results to other populations with different baseline characteristics or healthcare systems. It is important to note that this study simply implies an association, and future studies should be conducted to determine the exact causal relationship between cholecystectomy and CKD. Due to the limitations of our study, these findings should not be interpreted as definitive clinical guidelines, but rather as preliminary results that require further investigation to be fully substantiated.
Conclusions
This study may provide insight into the potential association between cholecystectomy and the increased risk of CKD. Using a longitudinal cohort and applying PSM to reduce confounding, we observed a consistent association between cholecystectomy and the development of CKD, which persisted for more than 3 years post-surgery. While these findings suggest a possible association, the underlying mechanisms remain unclear. Physiological changes in bile metabolism and alterations in the gut microbiome associated with cholecystectomy may contribute to renal dysfunction, warranting further investigation. These results emphasize the need for more detailed studies to clarify the exact pathways involved and to evaluate the renal implications of cholecystectomy. Nevertheless, the observational nature of this study underlines the importance of cautious interpretation, and further research is needed to establish causality and inform clinical guidelines.
Acknowledgments
S.P., S.J.P., J.S., and H.J.K. received a scholarship from the BK21 FOUR education program. It was provided by the National Research Foundation of Korea.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-98/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-98/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-98/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was carefully considered and approved by the Institutional Review Board of Seoul National University Hospital (IRB number: E-2311-054-1483). Informed consent was not required for participation in this study because the NHIS database used for data analysis underwent a rigorous anonymization process based on strict confidentiality guidelines before distribution. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fan Y, Hu J, Feng B, et al. Increased Risk of Pancreatic Cancer Related to Gallstones and Cholecystectomy: A Systematic Review and Meta-Analysis. Pancreas 2016;45:503-9. [Crossref] [PubMed]
- Yoon WJ, Kim HN, Park E, et al. The Impact of Cholecystectomy on the Gut Microbiota: A Case-Control Study. J Clin Med 2019;8:79. [Crossref] [PubMed]
- Jiang X, Jiang Z, Cheng Q, et al. Cholecystectomy promotes the development of colorectal cancer by the alternation of bile acid metabolism and the gut microbiota. Front Med (Lausanne) 2022;9:1000563. [Crossref] [PubMed]
- Nogueira L, Freedman ND, Engels EA, et al. Gallstones, cholecystectomy, and risk of digestive system cancers. Am J Epidemiol 2014;179:731-9. [Crossref] [PubMed]
- Latenstein CSS, Alferink LJM, Darwish Murad S, et al. The Association Between Cholecystectomy, Metabolic Syndrome, and Nonalcoholic Fatty Liver Disease: A Population-Based Study. Clin Transl Gastroenterol 2020;11:e00170. [Crossref] [PubMed]
- Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 2022;12:7-11. [Crossref] [PubMed]
- Ren Z, Fan Y, Li A, et al. Alterations of the Human Gut Microbiome in Chronic Kidney Disease. Adv Sci (Weinh) 2020;7:2001936. [Crossref] [PubMed]
- Kharazmi E, Scherer D, Boekstegers F, et al. Gallstones, Cholecystectomy, and Kidney Cancer: Observational and Mendelian Randomization Results Based on Large Cohorts. Gastroenterology 2023;165:218-227.e8. [Crossref] [PubMed]
- Park S, Jeong S, Park SJ, et al. Associations of cholecystectomy with metabolic health changes and incident cardiovascular disease: a retrospective cohort study. Sci Rep 2024;14:3195. [Crossref] [PubMed]
- Liu P, Quinn RR, Lam NN, et al. Progression and Regression of Chronic Kidney Disease by Age Among Adults in a Population-Based Cohort in Alberta, Canada. JAMA Netw Open 2021;4:e2112828. [Crossref] [PubMed]
- Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan 2009;24:63-71. [Crossref] [PubMed]
- Cheol Seong S, Kim YY, Khang YH, et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol 2017;46:799-800. [PubMed]
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. [Crossref] [PubMed]
- Gutiérrez OM, Sang Y, Grams ME, et al. Association of Estimated GFR Calculated Using Race-Free Equations With Kidney Failure and Mortality by Black vs Non-Black Race. JAMA 2022;327:2306-16. [Crossref] [PubMed]
- Ryou IS, Chang J, Son JS, et al. Association between CVDs and initiation and adherence to statin treatment in patients with newly diagnosed hypercholesterolaemia: a retrospective cohort study. BMJ Open 2021;11:e045375. [Crossref] [PubMed]
- Kim R, Lee JY, Park S, et al. Cholecystectomy and subsequent risk of Parkinson's disease: a nationwide retrospective cohort study. NPJ Parkinsons Dis 2021;7:100. [Crossref] [PubMed]
- Kim SB, Kim KO, Kim TN. Prevalence and Risk Factors of Gastric and Colorectal Cancer after Cholecystectomy. J Korean Med Sci 2020;35:e354. [Crossref] [PubMed]
- Goldacre MJ, Abisgold JD, Seagroatt V, et al. Cancer after cholecystectomy: record-linkage cohort study. Br J Cancer 2005;92:1307-9. [Crossref] [PubMed]
- Tavani A, Rosato V, Di Palma F, et al. History of cholelithiasis and cancer risk in a network of case-control studies. Ann Oncol 2012;23:2173-8. [Crossref] [PubMed]
- Sah DN, Bhandari RS. Iatrogenic bile duct injury during cholecystectomy presenting after 11 years as a biliary stricture: a case report. J Med Case Rep 2020;14:16. [Crossref] [PubMed]
- Díaz-Martínez J, Chapa-Azuela O, Roldan-García JA, et al. Bile duct injuries after cholecystectomy, analysis of constant risk. Ann Hepatobiliary Pancreat Surg 2020;24:150-5. [Crossref] [PubMed]
- Wang H, He YQ, Dong SY, et al. Recurrence of common bile duct stones after choledocholithotomy in elderly patients: risk factor analysis and clinical prediction model development. Front Med (Lausanne) 2023;10:1239902. [Crossref] [PubMed]
- Aniort J, Poyet A, Kemeny JL, et al. Bile Cast Nephropathy Caused by Obstructive Cholestasis. Am J Kidney Dis 2017;69:143-6. [Crossref] [PubMed]
- Gupta V, Sharma AK, Kumar P, et al. Residual gall bladder: An emerging disease after safe cholecystectomy. Ann Hepatobiliary Pancreat Surg 2019;23:353-8. [Crossref] [PubMed]
- Yusuf F, Weissman S, Qureshi N, et al. Bile Cast Nephropathy an Important Biliary Culprit of Kidney Injury. J Community Hosp Intern Med Perspect 2021;11:253-5. [Crossref] [PubMed]
- Barrera F, Azócar L, Molina H, et al. Effect of cholecystectomy on bile acid synthesis and circulating levels of fibroblast growth factor 19. Ann Hepatol 2015;14:710-21. [Crossref] [PubMed]
- Liu J, Qu J, Chen H, et al. The pathogenesis of renal injury in obstructive jaundice: A review of underlying mechanisms, inducible agents and therapeutic strategies. Pharmacol Res 2021;163:105311. [Crossref] [PubMed]
- Somagutta MR, Jain MS, Pormento MKL, et al. Bile Cast Nephropathy: A Comprehensive Review. Cureus 2022;14:e23606. [PubMed]
- Hobby GP, Karaduta O, Dusio GF, et al. Chronic kidney disease and the gut microbiome. Am J Physiol Renal Physiol 2019;316:F1211-7. [Crossref] [PubMed]
- Pérez-Reytor D, Puebla C, Karahanian E, et al. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front Physiol 2021;12:650313. [Crossref] [PubMed]
- Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol 2019;10:277. [Crossref] [PubMed]
- Wong J, Piceno YM, DeSantis TZ, et al. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 2014;39:230-7. [Crossref] [PubMed]
- Sung CC, Liao MT, Chao CT. Independent Determinants of Appetite Impairment among Patients with Stage 3 or Higher Chronic Kidney Disease: A Prospective Study. Nutrients 2021;13:2863. [Crossref] [PubMed]
- Kao HY, Chang CC, Chang CF, et al. Associations between Sex and Risk Factors for Predicting Chronic Kidney Disease. Int J Environ Res Public Health 2022;19:1219. [Crossref] [PubMed]
- Pak M, Lindseth G. Risk Factors for Cholelithiasis. Gastroenterol Nurs 2016;39:297-309. [Crossref] [PubMed]
- Pérez-Moreno P, Riquelme I, García P, et al. Environmental and Lifestyle Risk Factors in the Carcinogenesis of Gallbladder Cancer. J Pers Med 2022;12:234. [Crossref] [PubMed]
- Jackson SS, Graubard BI, Gabbi C, et al. Association with menopausal hormone therapy and asymptomatic gallstones in US women in the third National Health and Nutrition Examination Study. Sci Rep 2024;14:191. [Crossref] [PubMed]
- Völzke H, Baumeister SE, Alte D, et al. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion 2005;71:97-105. [Crossref] [PubMed]
- Festi D, Dormi A, Capodicasa S, et al. Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project). World J Gastroenterol 2008;14:5282-9. [Crossref] [PubMed]
- Wang DQ. Aging per se is an independent risk factor for cholesterol gallstone formation in gallstone susceptible mice. J Lipid Res 2002;43:1950-9. [Crossref] [PubMed]
- Sun H, Warren J, Yip J, et al. Factors Influencing Gallstone Formation: A Review of the Literature. Biomolecules 2022;12:550. [Crossref] [PubMed]
- Chang TI, Lim H, Park CH, et al. Association Between Income Disparities and Risk of Chronic Kidney Disease: A Nationwide Cohort Study of Seven Million Adults in Korea. Mayo Clin Proc 2020;95:231-42. [Crossref] [PubMed]
- Dupont B, Dejardin O, Bouvier V, et al. Systematic Review: Impact of Social Determinants of Health on the Management and Prognosis of Gallstone Disease. Health Equity 2022;6:819-35. [Crossref] [PubMed]
- Farrance I, Badrick T, Frenkel R. Uncertainty in measurement: A review of the procedures for determining uncertainty in measurement and its use in deriving the biological variation of the estimated glomerular filtration rate. Pract Lab Med 2018;12:e00097. [Crossref] [PubMed]
Cite this article as: Yu J, Park S, Jeong S, Park SJ, Song J, Kim HJ, Lee J, Ko A, Oh SN, Park SM. Association between cholecystectomy and chronic kidney disease risk: a nationally retrospective cohort study. Transl Gastroenterol Hepatol 2025;10:27.


