
Comprehensive gut microbiota and metabolomics combined with network pharmacology reveal the effects of acupuncture treatment for chemotherapy-induced nausea and vomiting
Highlight box
Key findings
• Acupuncture significantly reduced kaolin consumption, mitigated anorexia, and decreased body weight loss in a chemotherapy-induced nausea and vomiting (CINV) model.
• It modulated gut microbiota composition, enhancing beneficial bacteria while reducing harmful ones.
• Serum metabolomic analysis showed alterations in metabolites related to fatty acid biosynthesis, the urea cycle, and amino acid metabolism.
• Key genes (MAPK1, STAT3, EGFR, AKT1, SRC) and pathways (PI3K/Akt, neuroactive ligand-receptor interaction) were identified as crucial for acupuncture’s anti-CINV effects.
What is known and what is new?
• CINV is a significant side effect of chemotherapy, often inadequately managed by traditional pharmacological treatments.
• This manuscript provides evidence that acupuncture can effectively ameliorate CINV by influencing gut microbiota, metabolic profiles, and molecular pathways, highlighting its potential as a complementary therapy.
What is the implication, and what should change now?
• The findings suggest that acupuncture should be considered as an adjunctive, non-pharmacological treatment for CINV.
• Clinical practices should incorporate acupuncture to improve patient outcomes, alongside traditional treatments, to address gut microbiota disruptions and metabolic abnormalities in cancer patients.
Introduction
Despite significant improvements in cancer treatment and supportive therapy over the past 40 years, chemotherapy-induced nausea and vomiting (CINV) remains a common gastrointestinal adverse reaction in cancer patients undergoing chemotherapy (1). If not intervened in a timely manner, CINV can lead to treatment discontinuation in 60% to 80% of patients due to electrolyte imbalances and decreased quality of life (2). Due to the complex mechanism of nausea and vomiting, the co-administration of multi-antiemetic drugs is very common to achieve efficacy in the hospital. While the American Society of Clinical Oncology (ASCO) advocates for the administration of 5-HT3 receptor antagonists and corticosteroids prior to chemotherapy in high-risk vomiting patients, a significant number still encounter nausea and vomiting. Furthermore, the substantial toxic side effects pose as significant constraints for the utilization of multiple antiemetic drugs (3). Consequently, there is a noteworthy focus on investigating complementary and non-pharmacological treatment modalities in conjunction with traditional antiemetic protocols.
Acupuncture has a long history in the treatment of vomiting. The Ling Shu Jing (the Miraculous Pivot) states: “For the patient who is frequent vomiting with bitter water, long sighs, feeling empty in the heart as if about to be captured by others... can needle the Sanli (ST 36) point to regulate the reversed gallbladder Qi, to balance deficiency and excess and eliminate pathogenic factors.” The Zhen Jiu Da Cheng (Great Compendium of Acupuncture and Moxibustion) records: “For cold deficiency of the spleen and stomach with persistent vomiting, an acupuncturist can needle Neiting (ST 44), Zhongwan (CV 12), Qihai (CV 6), Gongsun (SP 4).” The Zhen Jiu Feng Yuan (Origins of Acupuncture and Moxibustion) states: “Vomiting can be treated by needling Taiyuan (LU 9), Tailing (PC 7), Rugen (ST 18), Zhongwan (CV 12), Qihai (CV 6), Zusanli (ST 36), Tonggu (BL 66).” Currently, many clinical studies have shown the effectiveness of acupuncture in treating CINV. Acupuncture-point stimulation has long been studied as a complementary and alternative treatment for CINV and encompasses manual acupuncture, electroacupuncture (EA), moxibustion, acupoint injection, ear acupoint sticking, and acupressure.
The human gastrointestinal system harbors a vast and diverse community of microorganisms, collectively referred to as the intestinal microflora or gut microbiota. These microorganisms, including bacteria, viruses, fungi, and archaea, play a crucial role in various aspects of human health (4,5). They assist in digesting food, synthesizing essential nutrients, and maintaining a balanced immune system. Recent studies have highlighted the potential link between CINV and gut microbiota disorder (6), suggesting that alterations in the gut microbiota composition and function can influence the severity and duration of nausea and vomiting experienced by chemotherapy patients. Understanding the intricate relationship between CINV and the gut microbiota offers new avenues for research and the development of therapeutic strategies aimed at mitigating this challenging side effect of cancer treatment (7). Furthermore, network pharmacology has found extensive and successful applications in investigating the mechanisms of action of traditional Chinese medicine (TCM) formulae (8,9). Therefore, systems biology strategies are highly esteemed for their capability to uncover the mechanisms of TCM syndromes by integrating multi-omics approaches and network pharmacology.
The present study aimed to assess the therapeutic effect of acupuncture on CINV and investigate its mechanisms. We established a CINV model to explore the impact of acupuncture and used gut microbiota, metabolomics, and network pharmacology to delve into the underlying mechanisms. This comprehensive approach addresses the limitations of experimental validation in network pharmacology and the lack of information on upstream molecular mechanisms and drug binding targets in metabolomics. Our study would contribute to a better understanding of how CINV alleviates CINV, offering rigorous scientific evidence for the potential application of acupuncture as a complementary therapy in managing CINV. We present this article in accordance with the ARRIVE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-35/rc).
Methods
Animals care and experiment details
During the experiment, sixty-four male Wistar rats (180–200 g, Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China) were housed with the conditions of 24±2 ℃, rotated through 12 hours of light and dark periods, and were fed with standard food and water. Each rat was housed in an individual cage for four days and given access to kaolin pellets. After the rats had adapted and had not consumed kaolin out of curiosity, they were divided into three groups, including control group, model group (cisplatin), and acupuncture group (acupuncture + cisplatin), sixteen rats per group. Acupuncture pre-treatment was administered daily to the acupuncture treatment group three days before modeling, while the two control groups were subjected to the same binding constraints. During the acupuncture treatment, rats were immobilized on a specially designed frame. Acupoints “Zhongwan” (CV 12) and bilateral “Neiguan” (PC 6) were selected. In the PC 6 location, the point is situated on the flexor side of the forearm, between the radius and ulna, approximately 3 mm proximal to the wrist joint, and was needled perpendicularly to a depth of 1 mm. Both left and right sides were used for EA. For the CV 12 point, it is found in the midline of the abdomen, 2 cm below the sternocostal angle and halfway to the umbilicus, needled perpendicularly to a depth of 3 mm. Additionally, a second needle was inserted perpendicularly to a depth of 1 mm at a non-acupuncture point, located 1 mm lateral to CV 12, to create a pair for EA. On the modeling day, both the model group and the acupuncture group received intraperitoneal injections of cisplatin at a dose of 6 mg/kg, with an injection volume of 10 mL/kg, following the protocols described in the previous study (10). In the blank control group, 10 mL/kg of physiological saline was injected intraperitoneally. Daily kaolin consumption, food intake, and body weight should be meticulously recorded every 24 hours. Experiments were performed under a project license (No. YS-JL-001) granted by ethics board of Shanghai YISHANG BIOTECH, in compliance with institutional guidelines for the care and use of animals. A protocol was prepared before the study without registration.
16S rRNA gene sequence analysis
16S rRNA gene sequence analysis was undertaken to extract DNA and analyze the hypervariable region V3–V4 of the 16S ribosomal RNA (rRNA) gene. Polymerase chain reaction (PCR) was employed with primers 357F (5’-ACT CCT ACG GRA GGC AGC AG-3’) and 806R (5’-GGA CTA CHVGGG TWT CTAAT-3’) to amplify the target region, subsequently facilitating the construction of the Miseq library. Sequences acquired from Miseq sequencing were initially merged based on overlapping relationships. Following this, rigorous sequence quality control and filtering procedures were implemented, succeeded by operational taxonomic unit (OTU) cluster analysis and taxonomic classification at the species level. Diversity index analysis was conducted using QIIME2 (https://qiime2.org/).
Network pharmacology construction
Aprepitant, Ondansetron, and dexamethasone are clinically recommended antiemetics for CINV. The molecular structures and SMILES of these three drugs were queried and downloaded from the PubChem database. The Swiss Target Prediction and STITCH online platforms were used to predict the targets of these drugs, resulting in the identification of antiemetics targets for CINV. The intersection between candidate CINV targets (Group 1) and the antiemetics targets for CINV was taken to obtain candidate antiemetics targets for CINV, designated as Group 2. The intersection between Group 1 and the targets for acupuncture antiemetic effect was taken to obtain the candidate targets of acupuncture for CINV, designated as Group 3. Using an online Venn diagram, the intersection of Group 2 and Group 3 was obtained, resulting in 57 common targets of acupuncture and drug for CINV, designated as Group 4. The candidate antiemetics targets for CINV with Ondansetron, Aprepitant, and Dexamethasone (Group 2) were imported into an Excel file named “Network File”. The candidate targets of acupuncture for CINV (Group 3) were also imported into the “Network File”. The “Network File” was then imported into Cytoscape 3.8.1 (https://cytoscape.org/).
Next, the common targets of acupuncture and drug for CINV (Group 4) were imported into an Excel file named “Format File” as a type file, labeled as “common gene”. The remaining targets of acupuncture and drug for CINV were labeled according to “Ondansetron”, “Aprepitant”, “Dexamethasone”, “β-Endorphin”, “Dopamine” and “Hydrocortisone”, and imported into the “Format File”. Finally, the “Format File” was imported into Cytoscape 3.8.1 to construct a network diagram of “treatment-compound-target” of acupuncture and drug for CINV.
Identification of hub common targets of acupuncture and drug for CINV
The STRING database (http://string-db.org/cgi/input.pl) was opened, and the CINV targets of acupuncture and drug (Group 4) were copied into the STRING database, with the species set as “Homo sapiens (human)”. The results were exported as a “TSV format” file. The file was then imported into Cytoscape 3.8.1 for network analysis. In the exported “CSV format” file, the median values of “degree”, “betweenness”, and “closeness” were calculated. Nodes with “degree” below twice the median values, “betweenness”, and “closeness” below the median values were removed, and the remaining nodes were identified as Hub targets, which are the key targets in the protein-protein interaction (PPI) network of acupuncture and drug for CINV. Finally, the Hub common targets of acupuncture and drug for CINV were entered into the type file of the “Format File” and highlighted in Cytoscape 3.8.1.
Analysis of functional processes and molecular pathways of acupuncture for CINV
We performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis using the DAVID database. We selected the top 10 pathways with the most enriched GO results, as well as the top 20 signaling pathways with significant relevance of the KEGG analysis, and created visual enrichment bubble plots.
Statistical analysis
The software R was used for data analysis and visualization, and the data were presented as the mean ± standard error of the mean (SEM). Independent sample t-test was used to compare the two groups of data, and P<0.05 was considered statistically significant.
Results
Acupuncture alleviated CINV in a cisplatin-induced Rat Pica model
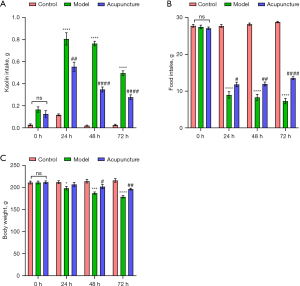
All rats exhibited pica behavior subsequent to cisplatin injection. Kaolin intake, food consumption, and body weight were measured at 24-hour intervals. To evaluate the anti-CINV efficacy of acupuncture, we monitored daily kaolin consumption from 0 to 72 hours post-cisplatin treatment in rats. Kaolin intake significantly increased at 24, 48, and 72 hours post-cisplatin administration, indicating the manifestation of acute (24 hours) and delayed (72 hours) phases of CINV. In comparison with the model group, acupuncture treatment markedly reduced kaolin consumption at 24 hours post-cisplatin injection. Notably, even at 72 hours after cisplatin treatment, acupuncture demonstrated a significant reduction in kaolin consumption (Figure 1A). Throughout the 0–72 hours experimental period, daily food intake and body weight in rats were significantly diminished following cisplatin treatment (Figure 1B,1C). Conversely, acupuncture administration effectively mitigated cisplatin-induced anorexia throughout 0–72 hours and attenuated body weight loss at 72 hours in rats (Figure 1B,1C). in addition, The H&E-stained tissue specimens of heart, liver, lung and colon from rats showed no toxic effects in treatment group compared with the control group (Figure S1). In summary, these findings suggest that acupuncture may alleviate both acute and delayed-phase emesis, anorexia, and body weight loss induced by cisplatin.
Acupuncture significantly improved the abnormality of gut microbiota of cisplatin-treated rats
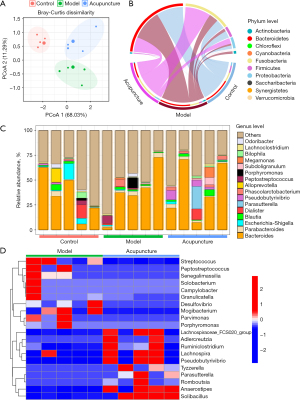
To determine whether the gut microbiota is associated with the anti-CINV effect of acupuncture, we conducted 16S rRNA gene sequencing of fecal samples from each group. Principal component analysis (PCoA), representing β-diversity, revealed that the composition and abundance of the microbiota in control, model, and acupuncture-treated rats were significantly different from each other (Figure 2A). Figure 2B revealed the distribution of gut microbiota at phylum level in each group. The top 5 abundant bacteria at phylum level were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria. We then assessed the general landscape of gut microbiome overtime at the genus level across three groups (Figure 2C). Gut microbiota in acupuncture-treated group featured with increase of Lachnospiraceae, Ruminiclostridium, Solibacillus, and Parasutterella. We also noticed the gut microbiota decreased in acupuncture-treated group, including Streptococcus, Peptostreptococcus, Senegalimassilia, Desulfovibrio, Mogibacterium, and Parvimonas (Figure 2D). Lactobacillaceae, Bifidobacteriaceae, Erysipelotrichaceae, and Ruminococcaceae, which are immunomodulatory SCFA-producing taxa, previously shown to be associated with healthy gut ecosystems (11), increased upon acupuncture treatment. These results underscore acupuncture’s potential in regulating gut microbiota composition and promoting microbial balance.
Acupuncture significantly regulated the serum metabolic profiles in cisplatin-treated rats
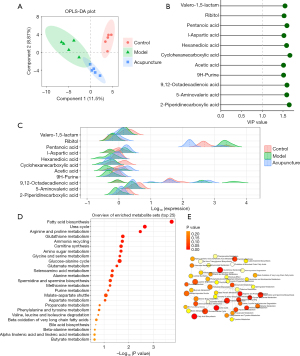
Given the complex interplay between gut microbiota and host through metabolic pathways, we investigated the serum metabolome using untargeted high performance liquid chromatography-mass spectrometry (LCMS) analysis. Orthogonal projections to latent structures discriminant analysis (OPLS-DA) models were utilized to illustrate the metabolomic distributions among the control, model, and acupuncture-treated groups. The OPLS-DA model was based on one predictive component and one orthogonal component. OPLS-DA revealed that control, model, and acupuncture-treated groups were clearly separated (Figure 3A). This result provides a theoretical basis for further analysis of differential expression of metabolites. Therefore, we continued to screen differential metabolites involving in the pathology of CINV, as well as the effects of acupuncture by computing variable importance in projection (VIP) scores of each metabolite. According to the VIP score plot, metabolites with VIP score >1.5 were further analyzed. Figure 3B showed the top 11 metabolites in the rankings, according to their VIP scores. The top 11 metabolites included cyclohexanecarboxylic acid, 2-piperidinecarboxylic acid, 9,12-octadecadienoic acid, pentanoic acid, 5-aminovaleric acid, hexanedioic acid, valero-1,5-lactam, ribitol, l-aspartic acid, 9H-purine. Among the metabolites, cyclohexanecarboxylic acid,2-piperidinecarboxylic acid, and ribitol tended to be upregulated, while the remaining metabolites tended to be downregulated (Figure 3C). KEGG pathway enrichment analysis showed that six metabolic pathways were involved in the mechanisms of the effects of acupuncture on CINV, including fatty acid biosynthesis, urea cycle, arginine and proline metabolism, glutathione metabolism, ammonia recycling, carnitine synthesis, amino sugar metabolism, glycine and serine metabolism, glucose-alanine cycle, and glutamate metabolism (Figure 3D,3E).
Interaction between the gut microbiota and metabolites
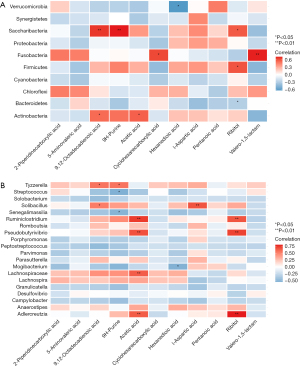
We then conducted Spearman correlation analysis between statin-associated microbial taxa and serum metabolites to further investigate acupuncture’s anti-CINV effects on gut microbiota and metabolism. Our results showed a significant increase in the F/B ratio in the acupuncture-treated group compared to the model group, with notable correlations between the abundances of Firmicutes and Bacteroidetes and Ribitol levels in the serum samples (Figure 4A). Furthermore, at the genus level, a few genera, including Solobacterium, Romboutsia, Porphyromonas, Peptostreptococcus, Parvimonas, Parasutterella, Lachnospira, Anaerostipes, Campylobacter, and Desulfovibrio, were not significantly correlated with any metabolites, while Adlercreutzia, Pseudobutyrivibrio, Ruminiclostridium, Solibacillus, and Tyzzerella were significantly correlated with at least two metabolites in the serum samples (Figure 4B). Moreover, there was an obvious clustering phenomenon among the bacteria and metabolites. Altogether, these results suggested that the changes in gut microbes and metabolites were significantly involved the pathogenesis of CINV as well as the effects of acupuncture.
Prediction of potential drug-target pathways associated with the anti‑CINV activity of acupuncture through network pharmacology analysis
Network pharmacology was used to further explore the mechanism of acupuncture in treating CINV. Firstly, 4,177 CINV targets, 372 candidate CINV targets, 117 candidate antiemetic targets, 121 targets for acupuncture treatment, 57 common targets for acupuncture and drug treatment, and 64 non-drug treatment targets for acupuncture were identified through PubChem, Pharm Mapper, and Swiss Target Prediction databases. Among them, MAPK1, STAT3, EGFR, AKT1, and SRC are hug common targets for acupuncture and drug treatment (Figure 5A,5B).
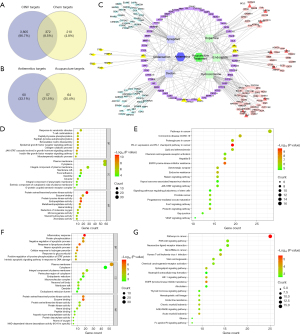
To pinpoint the pivotal genes involved in acupuncture treatment of CINV, we effectively established a PPI network by incorporating the shared target genes into the STRING database. Subsequently, we utilized Cytoscape 3.7.2 software for visualization and data processing (Figure 5C). The top 10 hub genes in the PPI network, including ABL1, CDK4, MTOR, TNF, GAPDH, BCL2, BCL2L1, REN, POMC, and NFKB1, were determined based on the node degree and these hub genes might be the crucial target genes of non-drug acupuncture treatment for CINV. Furthermore, we have conducted PCR verification of the top hub genes, including ABL1, CDK4, MTOR, TNF, and BCL2 (Figure S2). The observed data suggest that these targets have a bridge role in treating CINV with acupuncture.
In order to determine the anti-CINV function of candidate targets, we performed GO and KEGG pathway enrichment analyses by David. As a result, 398 GO terms and 118 KEGG signaling pathways were enriched in the analysis of common targets for acupuncture and drug treatment (Figure 5D,5E). Two hundred and eighty-six GO terms and 84 KEGG signaling pathways were enriched in the analysis of non-drug treatment targets for acupuncture treatment (Figure 5F,5G). Further analysis revealed that the genes, including BCL2, BCL2L1, POMC, and MTOR as well as the signaling pathways, including PI3K/Akt signaling pathway, neuroactive ligand-receptor interaction, and thyroid hormone signaling pathway, played different therapeutic roles in acupuncture treatment for CINV compared to drug therapy.
Discussion
Exploring the role of gut microbiota in CINV has emerged as a focal point in medical research. Nausea and vomiting are common side effects experienced by cancer patients undergoing chemotherapy, significantly impacting their quality of life and treatment outcomes (12). Despite some advancements, there remain numerous questions regarding the precise mechanisms and functions of gut microbiota in CINV. The existing knowledge gap in this research area provides an opportunity to delve deeper into the relationship between gut microbiota and CINV. Under normal circumstances, the gut microbiota in the human body is closely associated with various physiological functions, including food digestion and immune system regulation (13). However, during chemotherapy in cancer patients, drugs not only target cancer cells but may also influence the structure and function of the gut microbiota (14). These influences can occur through direct drug actions, alterations in the immune system, and changes in the overall physiological state of the patient. Such changes may disrupt the balance of the gut microbiota, thereby affecting CINV (15). Studies on the role of gut microbiota in CINV encompass both laboratory research and clinical observations. Yet, the specific mechanisms underlying this relationship remain unclear. Some studies suggest that alterations in the gut microbiota may participate in CINV through effects on the enteric nervous system, release of intestinal hormones, and modulation of immune responses (16,17). Nevertheless, numerous puzzles persist, and a comprehensive understanding of the role of gut microbiota in CINV is a pressing research question.
The 16S rRNA gene has been a mainstay of sequence-based bacterial analysis for decades. Recent advancements in this technology have revolutionized our ability to characterize the gut microbiota with unprecedented accuracy and detail (18). This technology has been crucial in identifying specific microbial communities that may contribute to the severity and frequency of CINV. In this investigation, we successfully instituted a rat model of cisplatin-induced pica. Our findings unveil the profound impact of acupuncture on the gut microbiota composition. Notably, at the phylum level, acupuncture mitigates gut microbiota dysbiosis by modulating the Firmicutes to Bacteroidetes (F/B) ratio. Moreover, at the genus level, the heightened abundance of Alistipes [linked to a high-fat diet (19)] and Mucispirillum [associated with pro-inflammatory effects (20)] observed in the cisplatin-induced pica rat model diminishes following acupuncture intervention. Conversely, the prevalence of Akkermansia [known for its anti-inflammatory effects and metabolic influence (21)] markedly rises post-acupuncture. Akkermansia comprises two species, namely Akkermansia muciniphila and Akkermansia glycaniphila, the latter being an anaerobic gut symbiotic bacterium representing 1–4% of the human fecal microbiota (22). A. glycaniphila actively promotes intestinal barrier function, intimating that acupuncture might ameliorate CINV by enhancing this vital aspect of gut physiology. These outcomes collectively propose that acupuncture assumes a pharmacological role in the cisplatin-induced pica rat model by finely modulating the composition and functional aspects of metabolism and inflammation-related bacterial communities.
Acupuncture has been demonstrated to induce notable alterations in the composition of the gut microbiota, and these changes may extend to the modulation of gut metabolism (23). Previous research has underscored the significance of Bacteroidetes as a predominant phylum in the gut microbiota, with a discernible correlation between its abundance and fatty acid biosynthesis, urea cycle, arginine and proline metabolism (24). This suggests that acupuncture may exert an influence on gut metabolism by orchestrating the abundance of Bacteroidetes. Building upon these insights, our study delved into the functional analysis of gut microbiota and conducted a metabolomic analysis of serum samples, revealing substantial disparities in metabolic profiles, particularly within fatty acid biosynthesis metabolism, between the model group and the acupuncture group. The well-established impact of gut microbiota on host metabolism cannot be overstated (25). For instance, Akkermansia, renowned for its role in maintaining intestinal mucosal barriers and regulating lipid metabolism, wields regulatory effects on gastrointestinal function, overall metabolism, and the brain-gut axis (26). In our investigation, acupuncture treatment resulted in a discernible increase in levels of cyclohexanecarboxylic acid, 2-piperidinecarboxylic acid, 9,12-octadecadienoic acid, pentanoic acid, signifying a positive influence on the composition and metabolic status of the gut microbiota. Moreover, Akkermansia and Alistipes exhibited correlations with at least five metabolites in serum l samples, underscoring the substantial role these two gut microbiota play in interacting with host metabolic products.
Integrating gut microbiota and metabolomic information has provided novel insights into the molecular underpinnings of multiple diseases (27). By examining the metabolic products of the microbiota and their interaction with the host’s gene expression during chemotherapy, we aimed to outline potential new molecular signatures that can mitigate CINV. In this study, we constructed interlayer and intralayer networks to visually display the correlations between gut microbiota, and differentially regulated metabolites involved in CINV, as well as the impact of acupuncture. The observed non-significant correlations of certain genera, such as Solobacterium, Romboutsia, Porphyromonas, Peptostreptococcus, Parvimonas, Parasutterella, Lachnospira, Anaerostipes, Campylobacter, and Desulfovibrio, with any metabolites at the genus level suggest potential functional independence or specific metabolic roles (28). In contrast, the significant correlations of Adlercreutzia, Pseudobutyrivibrio, Ruminiclostridium, Solibacillus, and Tyzzerella with at least two metabolites in samples highlight their potential metabolic significance (29). The observed clustering phenomenon among bacteria and metabolites indicates potential co-occurrence patterns or functional relationships in the gut microbiota-metabolome interplay (30).
To gain deeper insights into the molecular mechanisms underlying the treatment of CINV with acupuncture, a comprehensive network was constructed, linking acupuncture, their corresponding targets, and genes associated with CINV. A total of 121 targets germane to acupuncture’s therapeutic intervention for CINV were discerned. Within this cohort, 57 targets exhibited shared relevance to both acupuncture and pharmaceutical treatment modalities, while 64 targets were exclusive to the non-pharmacological sphere of acupuncture’s influence on CINV. Via PPI network analysis, pivotal shared targets such as MAPK1, STAT3, EGFR, AKT1 and SRC emerged. Concurrently, ABL1, CDK4, MTOR, TNF, GAPDH, BCL2, BCL2L1, REN and POMC were identified as central non-pharmacological targets of acupuncture in the context of CINV. The BCL2 gene encodes a comprehensive mitochondrial outer membrane protein, demonstrating apoptotic inhibition in specific cellular contexts, notably lymphocytes (31). Scientific investigations have illuminated BCL2’s role in mitigating inflammation through the downregulation of NLRP1 inflammasomes (32). The protein encoded by the BCL2L1 gene, belonging to the BCL-2 protein family, is situated in the mitochondrial outer membrane. Its regulatory prowess encompasses control over mitochondrial reactive oxygen species generation and the release of cytochrome C, achieved through the modulation of the voltage-dependent anion channel, thus impeding apoptosis (33). Positioned as a pivotal downstream protein in the PI3K/Akt signaling cascade, the BCL2 protein interfaces with diverse biological functions (34). The Akt signaling pathway, a crucial regulator in numerous cell survival cascades, functions predominantly as an apoptosis inhibitor (35). A plethora of investigations substantiate acupuncture’s reparative impact through the orchestration of the PI3K/Akt signaling pathway (36). This inquiry postulates that acupuncture may intricately regulate the treatment landscape of CINV through its modulatory influence on this signaling pathway. The POMC gene encodes the precursor polypeptide of proopiomelanocortin, synthesized predominantly in the anterior pituitary corticotrophs. Adrenocorticotropic hormone (ACTH) and β-endorphin constitute the principal end products of the POMC gene, with ACTH pivotal in corticosteroid production and regulation within the human physiological milieu (37). By elucidating the intricate connections between acupuncture’s active components, target pathways, and gut microbial influences, this study enhances our comprehension of how acupuncture orchestrates its therapeutic effects at the molecular level, paving the way for more targeted and effective interventions in the management of CINV.
It is crucial to acknowledge several limitations inherent in this study, as they impact the interpretation and generalizability of our findings. Firstly, our reliance on a single analytical method for the detection of serum metabolites may impose constraints on the comprehensiveness of our research. To overcome this limitation, future investigations should incorporate diverse analytical methods to uncover additional metabolic pathways associated with acupuncture-mediated CINV treatment. Secondly, our examination of metabolic changes was confined to serum samples obtained from cisplatin-induced pica rat models. A more thorough understanding of the regulatory networks linked to CINV could be achieved through multi-site. Such an approach would provide a more accurate depiction of the intricate metabolic alterations associated with acupuncture in treating CINV. Lastly, it is important to note that our study utilized a single animal model and a specific drug dosage. To enhance the robustness and applicability of our findings, future research endeavors should encompass different animal models and explore varying drug dosages. This diversified approach would contribute to the validation and extension of our results, fostering a more comprehensive understanding of the efficacy and mechanisms of acupuncture in the context of CINV treatment.
Conclusions
This study provides a theoretical framework for elucidating the mechanisms underlying acupuncture’s efficacy in treating CINV. The comprehensive application of diverse methodologies, including serum metabolomics, gut microbiota analysis, and network pharmacology, contributes to a holistic understanding. Through muti-omics, this study illuminates potential acupuncture mechanisms in the treatment of CINV, spanning both macroscopic and microscopic dimensions of classical behavior. These findings not only augment our comprehension of CINV pathogenesis but also deepen our insight into the nuanced ways acupuncture impacts CINV.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-35/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-35/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-35/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-35/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. YS-JL-001) granted by ethics board of Shanghai YISHANG BIOTECH, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008;358:2482-94. [Crossref] [PubMed]
- Sommariva S, Pongiglione B, Tarricone R. Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: A systematic review. Crit Rev Oncol Hematol 2016;99:13-36. [Crossref] [PubMed]
- Widgren Y, Silén M, Wåhlin I, et al. Chemotherapy-induced Emesis: Experienced Burden in Life, and Significance of Treatment Expectations and Communication in Chemotherapy Care. Integr Cancer Ther 2023;22:15347354231217296. [Crossref] [PubMed]
- Meng C, Bai C, Brown TD, et al. Human Gut Microbiota and Gastrointestinal Cancer. Genomics Proteomics Bioinformatics 2018;16:33-49. [Crossref] [PubMed]
- Yang N, Zhan Y, Wan J, et al. Effects of Lacidophilin Tablets, Yogurt, and Bifid Triple Viable Capsules on the Gut Microbiota of Mice with Antibiotic-Associated Diarrhea. Can J Infect Dis Med Microbiol 2022;2022:6521793. [Crossref] [PubMed]
- Zhao X, Wu H, Zhu R, et al. Combination of thalidomide and Clostridium butyricum relieves chemotherapy-induced nausea and vomiting via gut microbiota and vagus nerve activity modulation. Front Immunol 2023;14:1220165. [Crossref] [PubMed]
- Rapoport BL. Delayed Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Incidence, and Current Management. Front Pharmacol 2017;8:19. [Crossref] [PubMed]
- Zhao J, Tian S, Lu D, et al. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine 2021;85:153315. [Crossref] [PubMed]
- Ye M, Luo G, Ye D, et al. Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of Toujie Quwen Granules against coronavirus disease 2019 pneumonia. Phytomedicine 2021;85:153401. [Crossref] [PubMed]
- Raghavendran HR, Rekha S, Shin JW, et al. Effects of Korean ginseng root extract on cisplatin-induced emesis in a rat-pica model. Food Chem Toxicol 2011;49:215-21. [Crossref] [PubMed]
- Burrello C, Garavaglia F, Cribiù FM, et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun 2018;9:5184. [Crossref] [PubMed]
- Rao KV, Faso A. Chemotherapy-induced nausea and vomiting: optimizing prevention and management. Am Health Drug Benefits 2012;5:232-40. [PubMed]
- Chen HM, Yu YN, Wang JL, et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr 2013;97:1044-52. [Crossref] [PubMed]
- Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351-71. [Crossref] [PubMed]
- Rapoport B, van Eeden R, Smit T. Rolapitant for the prevention of delayed nausea and vomiting over initial and repeat courses of emetogenic chemotherapy. Expert Rev Clin Pharmacol 2017;10:17-29. [Crossref] [PubMed]
- Chen D, Guo Y, Yang Y. Liujunanwei decoction attenuates cisplatin-induced nausea and vomiting in a Rat-Pica model partially mediated by modulating the gut micsrobiome. Front Cell Infect Microbiol 2022;12:876781. [Crossref] [PubMed]
- Yixia Y, Sripetchwandee J, Chattipakorn N, et al. The alterations of microbiota and pathological conditions in the gut of patients with colorectal cancer undergoing chemotherapy. Anaerobe 2021;68:102361. [Crossref] [PubMed]
- Johnson JS, Spakowicz DJ, Hong BY, et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat Commun 2019;10:5029. [Crossref] [PubMed]
- Wan Y, Wang F, Yuan J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 2019;68:1417-29. [Crossref] [PubMed]
- Eicher TP, Mohajeri MH. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022;14:2661. [Crossref] [PubMed]
- Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016;65:426-36. [Crossref] [PubMed]
- Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 2019;25:1096-103. [Crossref] [PubMed]
- Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242-9. [Crossref] [PubMed]
- Akinsuyi OS, Roesch LFW. Meta-Analysis Reveals Compositional and Functional Microbial Changes Associated with Osteoporosis. Microbiol Spectr 2023;11:e0032223. [Crossref] [PubMed]
- Krishnamurthy HK, Pereira M, Bosco J, et al. Gut commensals and their metabolites in health and disease. Front Microbiol 2023;14:1244293. [Crossref] [PubMed]
- Derrien M, Vaughan EE, Plugge CM, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 2004;54:1469-76. [Crossref] [PubMed]
- Wang J, Dong P, Zheng S, et al. Advances in gut microbiome in metabonomics perspective: based on bibliometrics methods and visualization analysis. Front Cell Infect Microbiol 2023;13:1196967. [Crossref] [PubMed]
- Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12:R60. [Crossref] [PubMed]
- Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105-8. [Crossref] [PubMed]
- Hou Y, Li J, Ying S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023;13:1166. [Crossref] [PubMed]
- Huang L, Ye Q, Lan C, et al. AZD6738 Inhibits fibrotic response of conjunctival fibroblasts by regulating checkpoint kinase 1/P53 and PI3K/AKT pathways. Front Pharmacol 2022;13:990401. [Crossref] [PubMed]
- Bruey JM, Bruey-Sedano N, Luciano F, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell 2007;129:45-56. [Crossref] [PubMed]
- Inoue-Yamauchi A, Jeng PS, Kim K, et al. Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nat Commun 2017;8:16078. [Crossref] [PubMed]
- Wen R, Hu S, Xiao Q, et al. Leptin exerts proliferative and anti-apoptotic effects on goose granulosa cells through the PI3K/Akt/mTOR signaling pathway. J Steroid Biochem Mol Biol 2015;149:70-9. [Crossref] [PubMed]
- Liang K, Ye Y, Wang Y, et al. Formononetin mediates neuroprotection against cerebral ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2 ratio and upregulation PI3K/Akt signaling pathway. J Neurol Sci 2014;344:100-4. [Crossref] [PubMed]
- Wang HL, Liu FL, Li RQ, et al. Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation. Neural Regen Res 2021;16:1011-6. [Crossref] [PubMed]
- Schiöth HB, Raudsepp T, Ringholm A, et al. Remarkable synteny conservation of melanocortin receptors in chicken, human, and other vertebrates. Genomics 2003;81:504-9. [Crossref] [PubMed]
Cite this article as: Wang X, Fan Y, Xiang Y, Zhang S, Yang Y. Comprehensive gut microbiota and metabolomics combined with network pharmacology reveal the effects of acupuncture treatment for chemotherapy-induced nausea and vomiting. Transl Gastroenterol Hepatol 2025;10:26.


