
Non-linear relationship between Dietary Inflammatory Index and constipation: threshold identification and insights from NHANES [2005–2010] and Mendelian randomization analysis
Highlight box
Key findings
• This study demonstrates a nonlinear relationship between the Dietary Inflammatory Index (DII) and constipation. The threshold of the DII can guide individual dietary adjustments and potentially improve constipation symptoms. This conclusion is based on data from the National Health and Nutrition Examination Survey (NHANES) and findings from Mendelian randomization analysis.
What is known and what is new?
• It is known that there exists a nonlinear relationship between DII and constipation.
• What is new is the identification of an appropriate threshold for the DII, along with the discovery of the disparate effects of the DII on constipation across various populations. Specifically, we found that females, individuals with low-income, and those with diabetes may not benefit from DII in terms of improving and preventing constipation.
What is the implication, and what should change now?
• Our findings establish a clear threshold for diet-induced DII in individuals, which can be utilized to alleviate symptoms in patients with constipation and serve as dietary guidance for hospitalized patients.
Introduction
Globally, functional gastrointestinal diseases have become increasingly common, with constipation emerging as a prevalent public health issue. It severely impacts patients’ quality of life and increases the risk of other diseases (1-3). Constipation is not only directly related to the digestive system but also potentially linked to cardiovascular diseases, obesity, and certain types of cancer (1). Currently, common treatments for constipation include pharmacotherapy, biofeedback therapy, and surgical interventions. However, these treatment modalities are not entirely satisfactory due to limitations in equipment, costs, treatment duration, and postoperative complications (4-7). Therefore, improving lifestyle and adjusting dietary structure are among the most economical, convenient, and feasible treatment approaches.
Although numerous studies have demonstrated associations among dietary fiber, saturated fats, trans fats, water intake, alcohol consumption, caffeine, and constipation, diet typically comprises a variety of food components that often interact, with their cumulative effects potentially altering the inflammatory response process and leading to disease onset and progression (1,8-10). The Dietary Inflammatory Index (DII) is a common index for assessing dietary structure and is closely related to inflammatory markers such as C-reactive protein (CRP), interleukin (IL)-4, IL-6, IL-10, IL-1β, and tumor necrosis factor-α (TNF-α) (11). Studies have shown the DII to be related to non-alcoholic fatty liver disease, chronic obstructive pulmonary disease, heart failure, and other diseases, but research about its impact on constipation remains limited (12-15). The National Health and Nutrition Examination Survey (NHANES) is conducted by the National Center for Health Statistics (NCHS) of the Communicable Disease Center (CDC) to assess the health and nutritional status of the American population (16). It collects demographics data, dietary data, examination data laboratory data and questionnaire data. The 24-hour dietary recall data from NHANES, combined with the Food Patterns Equivalents Database (FPED), can be used to calculate the DII.
To our knowledge, research on the pathophysiological mechanisms and etiological factors of constipation has mainly focused on colonic sensory and motor dysfunctions, genetic factors, neurotransmission, dysbiosis, medication, and emotional influences (1,17-19). However, the impact of dietary patterns and inflammation-induced states on constipation lacks extensive research. Therefore, we used large-scale cross-sectional study data combined with causal inference analysis to explore the relationship between the DII and constipation. Our findings offer more economical and effective ideas and strategies for the treatment and prevention of constipation. We present this article in accordance with the STROBE-MR reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-99/rc).
Methods
Study population in NHANES
NHANES is a cross-sectional survey study conducted in the United States to investigate the health, nutrition, and disease conditions of various types of populations. The study consists of a series of surveys conducted biennially, with data updates every 2 years. Based on data available on its official website regarding constipation, the DII, and covariates, we included 10,376 participants from three phases between 2005–2010 for our research. The study included all individuals aged 20 years and above, excluding those with missing variables. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Definition of constipation in NHANES
NHANES uses questionnaires regarding bowel movement frequency (BHD050: “How often do you usually have bowel movements?”) and stool types (BHQ060: “Please look at this card and tell me the number that corresponds to your usual or most common stool type.”) to determine participants’ constipation status individually (20). Participants with stool types classified as Type 1 or Type 2 are considered constipated, and those with a bowel movement frequency of less than three times per week are also considered constipated. Given the low correlation between stool characteristics and bowel movement frequency in NHANES (21), we primarily used stool characteristics to evaluate constipation levels, with bowel movement frequency serving as a criterion for the same analysis to increase result reliability.
Calculation of the DII in NHANES
The DII is an index designed to assess the impact of an individual’s dietary pattern on the body’s inflammatory level. We collected participants’ dietary recalls for the first and second days to obtain the types and quantities of food consumed. Based on FPED, we converted food and beverages into components of the United States Department of Agriculture (USDA) food patterns to calculate the DII. We used the dietaryindex package for calculation and averaged the two days’ values as the final DII for participants, dividing the DII into four ordered categories based on quartiles.
Covariates in NHANES
Age was divided into two groups: <60 and ≥60 years. Ethnicity was categorized into five groups: Mexican American, other Hispanic, White, Black, and other races. Income status was calculated using the INDFMPIR indicator, with INDFMPIR <2 indicating low income, INDFMPIR >3 indicating high income, and the middle range indicating a normal level. Education level was defined as a binary variable, including below high school and above high school. Body mass index (BMI) was divided into three groups based on standards of 18.5 and 25 kg/m2: thin, normal, and fat. Smoking status was divided into smokers and non-smokers based on serum cotinine concentration, with a level above 14 ng/mL indicating a long-term smoking history and defined as smokers. Alcohol consumption status was defined through the question “Had at least 12 alcohol drinks/1 year?” with participants answering “Yes” considered drinkers. Physical activity level was assessed based on four questions (PAQ605, PAQ620, PAQ650, PAQ665), categorized into mild intensity activity, moderate intensity activity, and vigorous activity. Hypertension was defined according to the 2017 American Heart Association (AHA) guidelines and the use of antihypertensive medication and self-reported hypertension. The diagnostic criteria for diabetes followed the American Diabetes Association standards, including one of the following four conditions: glycated hemoglobin >5.7% or 6.5%, fasting blood glucose >100/126 mg/dL, doctor-diagnosed diabetes, or impaired glucose tolerance in the questionnaire (22).
Selection of Mendelian randomization instrumental variables
We searched for genome-wide association study (GWAS) summary data for the 45 components constituting the DII in the UK Biobank (UKBB) GWAS summary data (http://www.nealelab.is/uk-biobank) and OPEN GWAS (https://gwas.mrcieu.ac.uk/) databases (23), finding summary data for 11 components. We selected the largest dataset from a European background as the exposure data for selecting instrumental variables. To select strong instrumental variables related to food components, we used a standard of P<5.0×10−8 and an F-statistic >10 for screening (24). To ensure the independence of instrumental variables, we used a window of 10,000 kb and an r2 of 0.001 as the criteria for linkage disequilibrium test.
Selection of GWAS summary data for constipation
The GWAS summary data for constipation was obtained from the latest version of the FINNGEN database (https://www.finngen.fi/en, version 10) (25), which consolidates data from nine databases, including 9 Biobanks, comprising 41,124 cases of constipation and 371,057 controls, all of European background.
Statistical analysis
The analysis of NHANES data adhered to the NHANES analysis and reporting guidelines, with all participants’ data processed and weighted using the survey package. We constructed three multivariable-adjusted logistic regression models to assess the relationship between the DII and constipation: Model 1 with no adjustments, Model 2 adjusted for age, sex, race, education, income, BMI, physical activity, and alcohol consumption, and Model 3 adjusted for all covariates. additionally, we employed the inverse probability of treatment weighting (IPTW) method to balance differences in covariates between the two groups and reanalyzed the data. Restricted cubic spline (RCS) analysis was used to explore the nonlinear relationship between the DII and constipation. To further investigate the relationship between the DII and constipation occurrence in different subgroups, we used the jstable package for subgroup analysis and assessed the interactions between the DII and other covariates. We also examined the relationship between different components of the DII and constipation.
In the Mendelian randomization analysis, we employed five methods [MR Egger, weighted median, inverse variance weighted (IVW), simple mode, weighted mode] to assess the relationship between food components and constipation. MR Egger and IVW were used as the primary basis. We also utilized the Steiger Test for directional verification. The main process is shown in Figure 1.
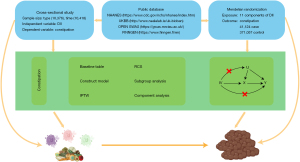
For the statistical analysis, we used R statistical software (version 4.2.2) together with several packages, including tidyverse, data.table, TwoSampleMR, haven, survey, gtsummary, rms, WeightIt. The default significance P value level was set at 0.05.
Results
Baseline characteristics of participants in NHANES
Based on stool characteristics as the diagnostic criterion, this study included 10,376 participants. At baseline, 19% of participants were aged 60 years or older, 52% were female, 79% were non-Hispanic Whites, 77% had a high school education or higher, 64% belonged to the high-income group, 66% were overweight or obese, 49% engaged in moderate-intensity physical activity, 79% consumed alcohol, 21% were smokers, 26% had hypertension, and 7.8% had diabetes. Gender, race, education level, income, BMI, physical activity level, alcohol consumption, and the DII showed differences between the constipated and normal groups (Table 1).
Table 1
| Characteristic | Constipation | P value | ||
|---|---|---|---|---|
| Overall (N=10,376) | No (N=9,680) | Yes (N=696) | ||
| Age (years) | 0.9 | |||
| <60 | 7,603 (81%) | 7,070 (81%) | 533 (82%) | |
| ≥60 | 2,773 (19%) | 2,610 (19%) | 163 (18%) | |
| Gender | <0.001 | |||
| Female | 5,346 (52%) | 4,809 (50%) | 537 (80%) | |
| Male | 5,030 (48%) | 4,871 (50%) | 159 (20%) | |
| Race | 0.02 | |||
| Mexican American | 1,069 (4%) | 996 (4%) | 73 (5%) | |
| Non-Hispanic Black | 1,953 (9%) | 1,784 (8%) | 169 (13%) | |
| Non-Hispanic White | 6,289 (79%) | 5,903 (80%) | 386 (74%) | |
| Other Hispanic | 590 (3%) | 546 (3%) | 44 (3%) | |
| Other races | 475 (5%) | 451 (5%) | 24 (5%) | |
| Education | 0.02 | |||
| High school and above | 7,560 (77%) | 7,094 (77%) | 466 (71%) | |
| Below high school | 2,816 (23%) | 2,586 (23%) | 230 (29%) | |
| Income | 0.008 | |||
| Low | 3,188 (21%) | 2,938 (21%) | 250 (26%) | |
| Normal | 1,613 (15%) | 1,479 (15%) | 134 (20%) | |
| High | 5,575 (64%) | 5,263 (65%) | 312 (54%) | |
| BMI | <0.001 | |||
| Thin | 153 (1%) | 134 (1%) | 19 (2%) | |
| Normal | 3,010 (33%) | 2,739 (32%) | 271 (44%) | |
| Fat | 7,213 (66%) | 6,807 (67%) | 406 (54%) | |
| Physical activity | 0.02 | |||
| Mild | 1,639 (11%) | 1,498 (11%) | 141 (15%) | |
| Moderate | 4,932 (49%) | 4,596 (49%) | 336 (50%) | |
| Vigorous | 3,805 (39%) | 3,586 (40%) | 219 (35%) | |
| Drinker | <0.001 | |||
| No | 2,523 (21%) | 2,288 (20%) | 235 (29%) | |
| Yes | 7,853 (79%) | 7,392 (80%) | 461 (71%) | |
| Smoker | 0.7 | |||
| No | 8,236 (79%) | 7,667 (79%) | 569 (81%) | |
| Yes | 2,140 (21%) | 2,013 (21%) | 127 (19%) | |
| Hypertension | 0.2 | |||
| No | 7,225 (74%) | 6,698 (73%) | 527 (78%) | |
| Yes | 3,151 (26%) | 2,982 (27%) | 169 (22%) | |
| Diabetes | 0.09 | |||
| Normal | 6,660 (70%) | 6,177 (70%) | 483 (71%) | |
| Prediabetes | 2,633 (23%) | 2,468 (22%) | 165 (24%) | |
| Diabetes | 1,083 (7%) | 1,035 (8%) | 48 (5%) | |
| DII | 0.001 | |||
| Q1 | 2,590 (27%) | 2,493 (28%) | 97 (16%) | |
| Q2 | 2,687 (27%) | 2,524 (27%) | 163 (24%) | |
| Q3 | 2,649 (25%) | 2,442 (25%) | 207 (27%) | |
| Q4 | 2,450 (21%) | 2,221 (20%) | 229 (33%) | |
The data are n (unweighted) (%). Chi-squared test with Rao & Scott’s second-order correction. BMI, body mass index; DII, Dietary Inflammatory Index; Q1, the first quartile; Q2, the second quartile; Q3, the third quartile; Q4, the highest quartile.
Using the number of bowel movements as the diagnostic criterion, we included 10,418 participants, among whom age, gender, race, income, physical activity level, alcohol consumption, and the DII showed differences between the constipated and normal groups (Table S1).
Relationship between the DII and constipation in NHANES
Using stool characteristics as the diagnostic criterion, we constructed three models to assess the relationship between the DII and constipation. In Model 1, we found that participants in the third quartile (Q3) of the DII had a higher risk of constipation compared to those in the first quartile (Q1) [odds ratio (OR): 1.85; 95% confidence interval (CI): 1.02–3.35; P=0.042], and participants in the highest quartile (Q4) had a higher risk of constipation compared to those in Q1 (OR: 2.85; 95% CI: 1.78–4.56; P<0.001). Additionally, using the DII as a continuous variable, we found that as the DII value increased, the risk of constipation also increased (OR: 1.25; 95% CI: 1.09–1.45; P=0.003). A trend test revealed a significant linear trend between the DII and constipation (P<0.001). In Model 2, only participants in Q4 had a higher risk of constipation compared to those in Q1 (OR: 1.91; 95% CI: 1.12–3.27; P=0.02), and there was no statistical difference in the continuous variable of the DII and constipation. The trend test still showed a linear trend between the DII and constipation (P=0.040). Finally, Model 3, which adjusted for all covariates, showed results consistent with Model 2 (Table 2).
Table 2
| Characteristic | Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |||
| DII | |||||||||||
| Q1 | – | – | – | – | – | – | |||||
| Q2 | 1.52 | 0.84, 2.78 | 0.2 | 1.40 | 0.78, 2.50 | 0.2 | 1.42 | 0.77, 2.60 | 0.2 | ||
| Q3 | 1.85 | 1.02, 3.35 | 0.042 | 1.42 | 0.75, 2.70 | 0.3 | 1.45 | 0.76, 2.77 | 0.3 | ||
| Q4 | 2.85 | 1.78, 4.56 | <0.001 | 1.91 | 1.12, 3.27 | 0.02 | 1.96 | 1.15, 3.36 | 0.02 | ||
| P for trend | <0.001 | 0.040 | 0.03 | ||||||||
| P for continuous | 1.25 | 1.09, 1.45 | 0.003 | 1.14 | 0.97, 1.33 | 0.11 | 1.14 | 0.97, 1.34 | 0.10 | ||
Model 1, without adjustment; Model 2, adjusted for age, sex, race, education, income, BMI, physical activity, and alcohol consumption; and Model 3, adjusted for all covariates. DII, Dietary Inflammatory Index; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; CI, confidence interval; Q1, the first quartile; Q2, the second quartile; Q3, the third quartile; Q4, the highest quartile; BMI, body mass index.
Using the frequency of bowel movements as the diagnostic criterion, we constructed three models to assess the relationship between the DII and constipation. The results of these three models were consistent, indicating that participants in the second quartile (Q2), third quartile (Q3), and highest quartile (Q4) of the DII had a higher risk of constipation compared to those in Q1 (P<0.05). In all three models, the DII as a continuous variable was also a risk factor for constipation (P<0.05), and trend tests revealed a linear trend between the DII and constipation in all three models (P<0.05, Table S2).
IPTW
Due to the uneven distribution of covariates across different DII groups in this study (Table S3), we employed generalized boosted modeling to weight the data for constipation diagnosis based on stool characteristics. After IPTW adjustment, all standardized mean differences (SMDs) of covariates were less than 0.1, indicating that differences between groups had been effectively eliminated (Figure 2A). On this basis, we reconstructed three models to assess the relationship between the DII and constipation. We found that in all models, participants in Q3 and Q4 had a higher risk of constipation compared to those in Q1 (P<0.05), and the DII as a continuous variable was also a risk factor for constipation in all three models (P<0.05). Trend tests confirmed a linear trend between the DII and constipation in all three models (P<0.05, Table 3).
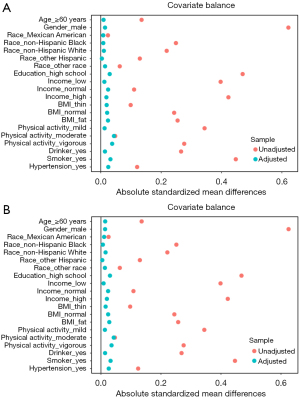
Table 3
| Characteristic | Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |||
| DII | |||||||||||
| Q1 | – | – | – | – | – | – | |||||
| Q2 | 1.46 | 0.96, 2.22 | 0.08 | 1.44 | 0.94, 2.21 | 0.09 | 1.44 | 0.94, 2.21 | 0.09 | ||
| Q3 | 1.65 | 1.11, 2.44 | 0.01 | 1.67 | 1.11, 2.53 | 0.02 | 1.67 | 1.10, 2.54 | 0.02 | ||
| Q4 | 1.85 | 1.34, 2.56 | <0.001 | 1.87 | 1.33, 2.64 | <0.001 | 1.88 | 1.33, 2.66 | <0.001 | ||
| P for trend | <0.001 | 0.001 | 0.001 | ||||||||
| P for continuous | 1.14 | 1.05, 1.23 | 0.003 | 1.13 | 1.03, 1.23 | 0.007 | 1.13 | 1.04, 1.23 | 0.007 | ||
Model 1, without adjustment; Model 2, adjusted for age, sex, race, education, income, BMI, physical activity, and alcohol consumption; and Model 3, adjusted for all covariates. DII, Dietary Inflammatory Index; IPTW, inverse probability of treatment weighting; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; CI, confidence interval; Q1, the first quartile; Q2, the second quartile; Q3, the third quartile; Q4, the highest quartile; BMI, body mass index.
In the analysis using the frequency of bowel movements as the diagnostic criterion, we also used the IPTW method for propensity score weighting and model construction (Figure 2B, Table S4), finding that the results of these three models were consistent with those obtained without propensity score weighting (P<0.05, Table S5).
Nonlinear relationship between the DII and constipation in NHANES
We used RCS to analyze the nonlinear relationship between the DII as a continuous variable and constipation. With stool characteristics serving as the diagnostic criterion, we generated RCS curves under various conditions: unadjusted, adjusted for covariates in Model 2, and adjusted for covariates in Model 3 (Figure 3A-3C). Figure 3 demonstrated a nonlinear relationship between the DII and constipation across different adjustment conditions. The risk of constipation remained stable or slightly decreased until the DII value reached a threshold of 0.974. Surpassing this threshold, the risk of constipation markedly increased as the DII value continued to rise.
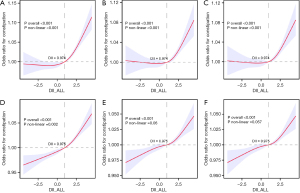
Using the frequency of bowel movements as the diagnostic criterion, we did not find a statistically significant nonlinear relationship between the DII and constipation. Nevertheless, we observed that the risk of constipation did not escalate rapidly as the DII increased, until it reached the threshold of 0.975. Beyond this threshold, the risk of constipation rose sharply as the DII continued to increase (Figure 3D-3F).
NHANES subgroup analysis
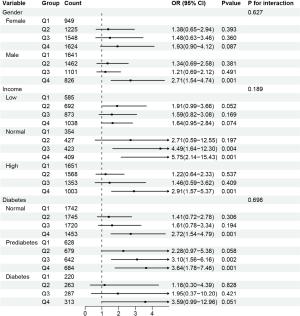
To further explore whether the relationship between the DII and constipation varies across different population subgroups, we conducted subgroup analyses for all categorical covariates (Table S6). The analysis did not reveal significant interactions between subgroups, indicating that the impact of the DII on constipation does not change with covariate variation. However, subgroup analysis still suggested that constipation in women is not affected by the DII, whereas in men, participants in Q4 had a higher risk of constipation compared to those in Q1 (OR: 2.71; 95% CI: 1.54–4.74; P=0.001; Figure 4). Constipation in the low-income group is not affected by the DII, whereas in the normal income group, participants in Q3 and Q4 had a higher risk of constipation compared to those in Q1 (P<0.05), and participants in the high-income group in Q4 had a higher risk of constipation compared to those in Q1 (P<0.05; Figure 4). Additionally, constipation in the diabetes group is not affected by the DII, whereas in the non-diabetes group, participants in Q3 had a higher risk of constipation compared to those in Q1 (P<0.05), and participants in the prediabetes group in Q3 and Q4 had a higher risk of constipation compared to those in Q1 (P<0.05; Figure 4).
Relationship between DII components and constipation in NHANES
To further explore the relationship between the components constituting the DII and constipation, we conducted a component-wise analysis using the 27 constituents of DII from the NHANES dataset. With the exception of vitamin B12, caffeine, carbohydrates, folic acid, vitamin A, and vitamin C, significant distribution differences were observed between all other components among individuals with and without constipation (Table S7). In the three constructed assessment models, beta-carotene, fiber, omega-3 fatty acids, omega-6 fatty acids, and polyunsaturated fatty acids were consistently identified as factors influencing constipation (P<0.05; Table S8). Further analysis using RCS revealed a non-linear relationship between these five components and constipation (non-linear P<0.05). The risk of constipation decreased with increased intake of beta-carotene and omega-3 fatty acids until a threshold was exceeded, beyond which the risk increased. Conversely, the risk of constipation decreased with increasing intake of fiber, omega-6 fatty acids, and polyunsaturated fatty acids, with little change in risk beyond a certain threshold (Figure 5).
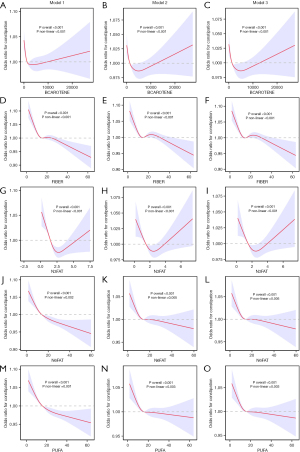
Causal relationship between DII components and constipation
After screening, 11 DII components were included as exposures (Table S9). Mendelian randomization analysis revealed no significant causal relationship between these dietary components, with the exception of energy intake, and the DII (Table S10). To further validate the directionality of the causal relationship, we employed the Steiger test, which confirmed a correct causal direction with a P value significantly below 0.05.
Discussion
This study primarily investigates the association between the DII and functional constipation. Previous research has linked high DII scores to various gastrointestinal diseases, as well as inflammatory and metabolic disorders (12-14). Constipation, a symptom-based disorder resulting from functional or organic impairments in the digestive system, is influenced by emotions, metabolism, and other factors, significantly affecting patients’ quality of life and work (1). Dietary intervention is recognized as the most cost-effective treatment, however, the impact of dietary patterns on constipation has not been fully elucidated. Hence, our study utilized large-scale population data and Mendelian randomization to explore the relationship between DII and constipation.
Our findings suggest that an increase in DII is associated with a higher risk of constipation. This relationship remained consistent across multivariate adjustment models and after IPTW adjustment. High-DII diets may elevate the risk of constipation by increasing systemic inflammation levels. In 2022, Costa et al. found a positive correlation between constipation patients’ DII and gut microbiota composition (26). As DII scores increased, the populations of Bacteroides, Butyrivibrio and Desulfovibrio also increased. These microbiota are closely related to inflammatory bowel disease and gastrointestinal tumors (27-30). This suggests that DII may influence constipation through disruptions in gut microbiota.
Although studies have shown that certain foods such as cheese, peas, milk, meat, poultry, and beverages can exacerbate constipation symptoms, while tea, coffee, and kiwifruit may alleviate them, these findings do not clarify the specific effects of different nutrients (31-35). Research by You et al. discussed the relevance of calories, protein, fat, and carbohydrates to constipation but did not address the combined effect of nutrients (8). Our RCS analysis revealed a complex non-linear relationship between DII and constipation risk, exhibiting a J-shaped trend in the general US population, with 0.974 as a significant threshold. Beyond this threshold, an increase in DII significantly elevates the risk of constipation. In clinical settings, adjusting patients’ diets to control DII scores can alleviate constipation symptoms, and individuals can also monitor and adjust their daily diets to prevent or mitigate constipation.
Further analysis through adjustment models identified carotenoids, omega-3 fatty acids, omega-6 fatty acids, and polyunsaturated fatty acids as independent factors affecting constipation, demonstrating U-shaped or L-shaped non-linear relationships. This highlights the nutrients are related to constipation and suggests that nutrient intake should be maintained within a reasonable range rather than being maximized.
Subgroup analysis did not reveal significant interaction effects, however, the impact of DII on constipation varied across different genders, income levels, and diabetes statuses. The study showed no significant effect of DII on female constipation incidence, possibly due to differences in physiological structure, hormone levels, and fertility conditions (34). Similar findings were observed in low-income groups, potentially due to higher economic stress, poorer diet quality, and inadequate healthcare coverage (36-38). DII did not significantly affect constipation incidence in diabetic populations, possibly because diabetes is a metabolic disease with a high level of inflammation (39,40). This finding suggests which populations are more suitable for adjusting their diet through the DII to alleviate symptoms of constipation, facilitating more precise guidance by clinicians.
Mendelian randomization analysis found that there is a causal relationship between energy intake and constipation, which may be attributed to the common pro-inflammatory food components including meat, sugary drinks, white flour and refined carbohydrates. Carbohydrates and lipids are the two major sources of energy for the human body. Therefore, the higher the energy intake, the higher the DII score, and consequently, the higher the risk of constipation. This is consistent with the results of Mendelian randomization. However, no causal relationship was found between other dietary components and constipation. This might be due to the minor effects of individual food components, which are insufficient to be detected in the sample size of this study, as well as the complex interplay of genetic and environmental factors.
However, the study has limitations. First, as a cross-sectional study, participants’ dietary habits could have changed over time, potentially affecting the outcomes. There may be unknown confounding factors and recall bias with the 24-hour dietary recall method used to calculate DII. Second, since the study was based on the US population, factors like race, economy, and geography could influence the results. Further research involving a more diverse geographic population and more precise dietary data is needed to validate these findings. Lastly, Mendelian randomization studies may not fully capture the interactions between genes and the environment, and the distribution of genetic variations may vary among different ethnicities and populations.
Conclusions
In summary, this study, based on NHANES data, analyzed the association between the DII and constipation in adults. After adjusting for multiple potential confounding factors and performing IPTW, higher DII scores were significantly associated with increased constipation risk. Subgroup analysis indicated that the association between DII and constipation risk varies across different genders, income levels, and diabetes statuses. This study highlights the potential importance of improving dietary patterns in preventing and treating constipation, providing reference thresholds for DII, identifying suitable populations for dietary intervention, and offering evidence to support dietary intervention strategies for constipation patients.
Acknowledgments
We extend our gratitude to the participants and the research team for their invaluable contributions to this study.
Footnote
Reporting Checklist: The authors have completed the STROBE-MR reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-99/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-99/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-99/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bharucha AE, Wald A. Chronic Constipation. Mayo Clin Proc 2019;94:2340-57. [Crossref] [PubMed]
- Wald A. Constipation: Advances in Diagnosis and Treatment. JAMA 2016;315:185-91. [Crossref] [PubMed]
- Shah BJ, Rughwani N, Rose S. In the clinic. Constipation. Ann Intern Med 2015;162:ITC1. [Crossref] [PubMed]
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007;25:599-608. [Crossref] [PubMed]
- Pijpers MA, Bongers ME, Benninga MA, et al. Functional constipation in children: a systematic review on prognosis and predictive factors. J Pediatr Gastroenterol Nutr 2010;50:256-68. [Crossref] [PubMed]
- Podzemny V, Pescatori LC, Pescatori M. Management of obstructed defecation. World J Gastroenterol 2015;21:1053-60. [Crossref] [PubMed]
- Singh S, Rao SS. Pharmacologic management of chronic constipation. Gastroenterol Clin North Am 2010;39:509-27. [Crossref] [PubMed]
- You JS, Park JY, Chang KJ. A case-control study on the dietary taurine intake, nutrient status and life stress of functional constipation patients in Korean male college students. J Biomed Sci 2010;17:S41. [Crossref] [PubMed]
- Xiao Y, Kashyap PC. Microbially derived polyunsaturated fatty acid as a modulator of gastrointestinal motility. J Clin Invest 2022;132:e161572. [Crossref] [PubMed]
- Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther 2011;33:895-901. [Crossref] [PubMed]
- Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17:1689-96. [Crossref] [PubMed]
- Bian D, Liu X, Wang C, et al. Association between Dietary Inflammatory Index and Sarcopenia in Crohn's Disease Patients. Nutrients 2022;14:901. [Crossref] [PubMed]
- Sreeja SR, Le TD, Eom BW, et al. Association between the Dietary Inflammatory Index and Gastric Disease Risk: Findings from a Korean Population-Based Cohort Study. Nutrients 2022;14:2662. [Crossref] [PubMed]
- Marx W, Veronese N, Kelly JT, et al. The Dietary Inflammatory Index and Human Health: An Umbrella Review of Meta-Analyses of Observational Studies. Adv Nutr 2021;12:1681-90. [Crossref] [PubMed]
- Hariharan R, Odjidja EN, Scott D, et al. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes Rev 2022;23:e13349. [Crossref] [PubMed]
- Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016;315:2284-91. [Crossref] [PubMed]
- Scott SM, Simrén M, Farmer AD, et al. Chronic constipation in adults: Contemporary perspectives and clinical challenges. 1: Epidemiology, diagnosis, clinical associations, pathophysiology and investigation. Neurogastroenterol Motil 2021;33:e14050. [Crossref] [PubMed]
- Black CJ, Ford AC. Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med J Aust 2018;209:86-91. [Crossref] [PubMed]
- Bharucha AE, Lacy BE. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology 2020;158:1232-1249.e3. [Crossref] [PubMed]
- Markland AD, Palsson O, Goode PS, et al. Association of low dietary intake of fiber and liquids with constipation: evidence from the National Health and Nutrition Examination Survey. Am J Gastroenterol 2013;108:796-803. [Crossref] [PubMed]
- Huang X, Zhao L, Li Z, et al. Association of niacin intake with constipation in adult: result from the National Health and Nutrition Examination. Eur J Med Res 2023;28:377. [Crossref] [PubMed]
- Tan Y, Fu Y, Yao H, et al. Relationship between phthalates exposures and hyperuricemia in U.S. general population, a multi-cycle study of NHANES 2007-2016. Sci Total Environ 2023;859:160208. [Crossref] [PubMed]
- Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. [Crossref] [PubMed]
- Flatby HM, Ravi A, Damås JK, et al. Circulating levels of micronutrients and risk of infections: a Mendelian randomization study. BMC Med 2023;21:84. [Crossref] [PubMed]
- Kurki MI, Karjalainen J, Palta P, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023;613:508-18. [Crossref] [PubMed]
- Costa LM, Mendes MM, Oliveira AC, et al. Dietary inflammatory index and its relationship with gut microbiota in individuals with intestinal constipation: a cross-sectional study. Eur J Nutr 2022;61:341-55. [Crossref] [PubMed]
- Rowan F, Docherty NG, Murphy M, et al. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis Colon Rectum 2010;53:1530-6. [Crossref] [PubMed]
- Dimidi E, Christodoulides S, Scott SM, et al. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv Nutr 2017;8:484-94. [Crossref] [PubMed]
- Wei B, Dalwadi H, Gordon LK, et al. Molecular cloning of a Bacteroides caccae TonB-linked outer membrane protein identified by an inflammatory bowel disease marker antibody. Infect Immun 2001;69:6044-54. [Crossref] [PubMed]
- Vacca M, Celano G, Calabrese FM, et al. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020;8:573. [Crossref] [PubMed]
- Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol 2003;98:1790-6. [Crossref] [PubMed]
- Voderholzer WA, Schatke W, Mühldorfer BE, et al. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol 1997;92:95-8. [PubMed]
- Gearry R, Fukudo S, Barbara G, et al. Consumption of 2 Green Kiwifruits Daily Improves Constipation and Abdominal Comfort-Results of an International Multicenter Randomized Controlled Trial. Am J Gastroenterol 2023;118:1058-68. [Crossref] [PubMed]
- Sandler RS, Jordan MC, Shelton BJ. Demographic and dietary determinants of constipation in the US population. Am J Public Health 1990;80:185-9. [Crossref] [PubMed]
- Ormarsson OT, Asgrimsdottir GM, Loftsson T, et al. Free fatty acid suppositories are as effective as docusate sodium and sorbitol enemas in treating constipation in children. Acta Paediatr 2016;105:689-94. [Crossref] [PubMed]
- Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr 2004;79:6-16. [Crossref] [PubMed]
- Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology 1990;98:380-6. [Crossref] [PubMed]
- Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology 2011;140:761-5. [Crossref] [PubMed]
- Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822-32. [Crossref] [PubMed]
- Jin C, Henao-Mejia J, Flavell RA. Innate immune receptors: key regulators of metabolic disease progression. Cell Metab 2013;17:873-82. [Crossref] [PubMed]
Cite this article as: Wei S, Qian J, Zou M, Qian Y, Zhou W, Gu Y, Tang L, Liu H, Zhang C. Non-linear relationship between Dietary Inflammatory Index and constipation: threshold identification and insights from NHANES [2005–2010] and Mendelian randomization analysis. Transl Gastroenterol Hepatol 2025;10:25.


