
Heterogeneity and prognosis of single organ metastases in gastric cancer
Highlight box
Key findings
• The prognosis for gastric cancer patients with single organ metastasis varies by the metastatic site, and bone metastasis turns to have the worst prognosis.
What is known and what is new?
• This study made the initial attempt to investigate the prognostic differences of single lung, liver, bone, and brain metastases in gastric cancer.
• Hazard ratios and floating absolute risk hazards were utilized to demonstrate the independent prognostic variations of different metastatic sites in gastric cancer.
What is the implication, and what should change now?
• This discovery can assist physicians in more accurately estimating patients’ prognoses, enable the patients to better understand their disease status, and improve their quality of life.
Introduction
Gastric cancer poses a major global health challenge, ranking as the fifth most common malignancy worldwide, with over one million new cases annually (1). It is also the third leading cause of cancer-related deaths (2). Gastric cancer exhibits distinct regional variations, with higher incidence rates observed in East Asia and Eastern Europe compared to Northern Europe and North America (3). One major contributing factor to the high mortality rate of gastric cancer is the late-stage diagnosis, often characterized by diminished therapeutic possibilities and advanced tumor metastasis, hastening disease progression and even leading to patient death (4). The European Society for Medical Oncology (ESMO) does not recommend gastric resection for metastatic gastric cancer unless it is performed as palliative surgery (5). Meanwhile, gastric cancer usually develops multiple metastases, with the peritoneum and lymph nodes being the most common sites, followed by the liver (6). Multiple metastases often indicate a more aggressive cancer, while single organ metastases may be associated with a better prognosis in certain types of cancer. Examples include bone metastases in breast cancer (7), lymph node metastases in colon cancer (8), liver metastases in gastric adenocarcinoma (AC) (9), brain metastases in non-small cell lung cancer (10), and metastatic uroepithelial carcinoma (11). Besides, it should be particularly noted that these evaluations are based on objective medical research and should be presented as such. Furthermore, studies have demonstrated considerable heterogeneity in cancer metastasis across different organs. For instance, peritoneal metastasis is the most common in gastric cancer (6), while breast cancer tends to develop bone metastasis (12). Even within the context of identical single-site metastases, variations arise in the metastatic distribution from primary tumors of the same type. Breast cancer’s bone metastases tend to concentrate in the thoracic spine, in contrast to lung cancer’s bone metastases, which disseminate more extensively (13). The microenvironment of metastatic cancer differs with the change of metastatic sites. For instance, breast cancer can impact and alter the microenvironment of the bone and lung, thereby promoting cancer metastasis. Similarly, the liver microenvironment may also affect breast cancer metastasis (14). In conclusion, while metastatic cancers from various organs exhibit evident heterogeneity, differences in single organ metastasis of gastric cancer remain unclarified. Indeed, the distant metastasis of gastric cancer has been inadequately studied in terms of prognostic differences between various metastatic sites. Previous research has primarily focused on a generalized analysis of single and combined metastases. Particular attention has been paid to metastatic cancers at specific locations, such as bone metastasis (15) and liver metastasis (16), as well as on single organ metastases of specific tumor types (11,12). These studies have significantly enhanced public understanding of regulatory mechanisms and coincided with rapid advancement in therapeutic strategies and the proliferation of clinical trials. Nevertheless, the examination and comparison of prognostic differences across diverse metastatic sites have been overlooked to certain extent, warranting further investigation into the prognostic differences of single lung, liver, bone, and brain metastases in the era of precision medicine.
Currently, treatment of advanced gastric cancer encompasses not only conventional cytotoxic chemotherapy but also witnesses a rising integration of targeted therapies and immunotherapeutic agents (17). Due to the varied prognoses associated with various treatments, physicians must exercise meticulous judgment in their therapeutic approach selection, which may also impact patients’ treatment preferences, particularly in developing countries. A Swedish-based study revealed that the liver was the most common metastatic site in gastric cancer (accounting for 48% of all metastases), while bone metastasis only accounted for 12% (18). In addition to metastatic sites, the study of metastatic burden, i.e., the number of metastases, remains limited (18). Thus, to date, consensus regarding the prognostic differences of single organ metastases at different sites has not been reached. Both limited patient cases and the challenge of conducting single-center studies have contributed to this lack of consensus. Currently, observational studies have been central in investigating metastatic gastric cancer. However, they are prone to potential biases stemming from the arbitrary selection of control groups. To this end, propensity score matching (PSM) was utilized to match patients, and floating absolute risks were employed to compare relative risks across various exposure levels, effectively mitigating the impact of bias on the study outcomes. The prognosis of cancer metastasis was found to be impacted by various factors, including the stage and size of the primary tumor at the time of diagnosis (2,19) with pathological staging (20), as well as the treatment modality. These factors should be further explored in gastric cancer patients with single metastasis.
In this study, the prognostic differences between different metastatic sites were discussed, and the impact of these single organ metastases was delved into using methods of PSM and adjusted absolute risk. The Surveillance, Epidemiology, and End Results (SEER) database was systematically reviewed to provide evidence on the prognostic differences of single organ metastases in gastric cancer and to explore potential underlying mechanisms for these differences. The primary objective of this study is to investigate the prognostic differences among various single organ metastases in late-stage gastric cancer. We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-11/rc).
Methods
Study population
This study is a retrospective population-based study. Patient information recorded in the SEER database from 2010 to 2016 was collected. According to the study objectives, patients with pathological diagnosis and type of AC [International classification of diseases for oncology, third edition, histological codes: 8140 to 8145, 8210 to 8211, 8220 to 8221, and 8260 to 8263 for AC, 8480 and 8481 for mucinous AC (MAC), 8490 for signet ring cell carcinoma (SRCC)] were selected. Those having died within one month of diagnosis or those with unknown survival status were excluded. Besides, the SEER*Stat software (version 8.3.8) was utilized to extract the information needed, including the age, gender, race, marital status, time of diagnosis, clinical stage of American Joint Commission on Cancer, treatment modality (radiotherapy, surgery), pathological type, pathological grade, primary site, single or multiple lesions, number of malignant tumors, tumor metastases, and survival time [include overall survival [OS] and disease-specific survival (DSS)]. In order to minimize biases, patients with distant lymph nodes or peritoneal metastases were further excluded, and only those with specific single metastases (bone, brain, liver, and lung) were finally included. The specific patient inclusion process is presented in Figure S1. Data collection was conducted by Q.W.Z. and Z.Q. jointly from January to May 2022.
The Ethics Committee of The Affiliated Hospital of Guilin Medical University waived the requirement for formal Institutional Review Board approval and informed consent, given its use of anonymous data and supplementary information obtained from individuals. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
PSM method
Herein, the effect of confounding factors was controlled by performing “post-hoc randomization” through PSM. Matching factors included covariates such as gender, age, and stage, with a 1:1 variable ratio and a caliper value set at 0.02. The “nearest” method within the “MatchIt” package in R was employed for this process (8). A Chi-squared test of P>0.05 and a standardized mean difference <0.1 in the baseline data table were considered balanced between the two groups.
Statistical analysis
Normality and variance Chi-squared were assessed before performing comparisons between groups, and used t-tests or Wilcoxon tests were then conducted. For patients with missing data, we handled the missing values by creating dummy variables. The R (https://www.r-project.org, R version 4.1.1) statistical software was hereby used. Besides, Kaplan-Meier survival curves were employed to exhibit the variation in survival rates between different groups (median survival time represents the survival time at 50%), and log-rank tests were carried out to examine the significance of survival rates between the groups, with the survival R package used for analysis (9). Furthermore, univariate and multivariate Cox regression models were adopted to explore factors affecting patient prognosis. Following that, subgroup Cox regression analysis was performed, and likelihood ratio tests were conducted to analyze the interaction between metastatic sites and other covariates. The “forestplot” package was utilized to draw forest plots for the Cox regressions (10). In comparing the prognostic differences between single lung, liver, bone, and brain metastases of gastric cancer, the liver metastases were used as control, and floating absolute risk method was employed. The floating absolute risk was used to compare the relative risk between different levels of multilevel exposure factors, thus reducing the bias caused by the arbitrary selection of controls. All statistical tests were two-sided, with P<0.05 considered statistically significant.
Results
Baseline characteristics of the study patients
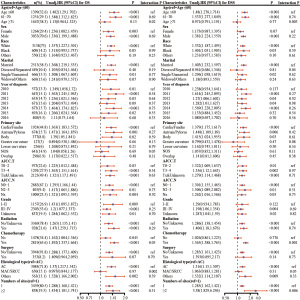
Finally, a total of 4,297 patients with gastric AC were hereby identified as the final study cohort. The predominant sites of single metastases included liver metastases affecting 3,054 patients, followed by bone metastases in 669 patients, lung metastases in 501 patients, and brain metastases in 73 patients. The longest follow-up period was 81 months, with a median follow-up time of 5 months. Among the different metastasis groups, the median survival of brain metastasis was the shortest, being only four months, and the comparison between groups was statistically significant (P<0.001). In addition, the age groups of metastases also differed among sites, except for brain metastases, where patients aged ≤60 years were the least represented. Notably, the highest proportion of patients with liver metastases fell into the age group ≥71 years old, comprising 1,243 patients, which represents 40.7% of all instances of liver metastases and a substantial 28.9% of all patients with a single metastasis. Additional details of each group are shown in Table 1.
Table 1
| Variables | Total (n=4,297) | Met.bone (n=669) | Met.brain (n=73) | Met.liver (n=3,054) | Met.lung (n=501) | P |
|---|---|---|---|---|---|---|
| Sex | <0.001 | |||||
| Female | 1,264 (29.4) | 239 (35.7) | 17 (23.3) | 841 (27.5) | 167 (33.3) | |
| Male | 3,033 (70.6) | 430 (64.3) | 56 (76.7) | 2,213 (72.5) | 334 (66.7) | |
| Race | <0.001 | |||||
| Black | 609 (14.2) | 61 (9.1) | 5 (6.8) | 487 (15.9) | 56 (11.2) | |
| Others | 550 (12.8) | 105 (15.7) | 7 (9.6) | 376 (12.3) | 62 (12.4) | |
| White | 3,138 (73.0) | 503 (75.2) | 61 (83.6) | 2,191 (71.7) | 383 (76.4) | |
| Marital | 0.53 | |||||
| Divorced/separated | 449 (10.4) | 66 (9.9) | 9 (12.3) | 315 (10.3) | 59 (11.8) | |
| Married | 2,513 (58.5) | 382 (57.1) | 41 (56.2) | 1,803 (59) | 287 (57.3) | |
| Single/unmarried | 666 (15.5) | 125 (18.7) | 11 (15.1) | 453 (14.8) | 77 (15.4) | |
| Widowed/unknown | 669 (15.6) | 96 (14.3) | 12 (16.4) | 483 (15.8) | 78 (15.6) | |
| Year of diagnosis | 0.32 | |||||
| 2010 | 572 (13.3) | 78 (11.7) | 3 (4.1) | 419 (13.7) | 72 (14.4) | |
| 2011 | 647 (15.1) | 89 (13.3) | 13 (17.8) | 479 (15.7) | 66 (13.2) | |
| 2012 | 633 (14.7) | 113 (16.9) | 13 (17.8) | 424 (13.9) | 83 (16.6) | |
| 2013 | 671 (15.6) | 104 (15.5) | 13 (17.8) | 474 (15.5) | 80 (16.0) | |
| 2014 | 673 (15.7) | 100 (14.9) | 13 (17.8) | 478 (15.7) | 82 (16.4) | |
| 2015 | 693 (16.1) | 119 (17.8) | 13 (17.8) | 483 (15.8) | 78 (15.6) | |
| 2016 | 408 (9.5) | 66 (9.9) | 5 (6.8) | 297 (9.7) | 40 (8.0) | |
| AJCC T stage | 0.002 | |||||
| T0–2 | 973 (22.6) | 133 (19.9) | 14 (19.2) | 693 (22.7) | 133 (26.5) | |
| T3–4 | 1,201 (27.9) | 170 (25.4) | 15 (20.5) | 862 (28.2) | 154 (30.7) | |
| Tx & unknown | 2,123 (49.4) | 366 (54.7) | 44 (60.3) | 1,499 (49.1) | 214 (42.7) | |
| AJCC N stage | 0.69 | |||||
| N0–1 | 2,883 (67.1) | 440 (65.8) | 46 (63) | 2,054 (67.3) | 343 (68.5) | |
| N2–3 | 405 (9.4) | 57 (8.5) | 7 (9.6) | 297 (9.7) | 44 (8.8) | |
| Nx | 1,009 (23.5) | 172 (25.7) | 20 (27.4) | 703 (23) | 114 (22.8) | |
| Chemotherapy | 0.53 | |||||
| No | 1,478 (34.4) | 228 (34.1) | 29 (39.7) | 1,038 (34.0) | 183 (36.5) | |
| Yes | 2,819 (65.6) | 441 (65.9) | 44 (60.3) | 2,016 (66.0) | 318 (63.5) | |
| Surgery | 0.003 | |||||
| No/unknown | 3,944 (91.8) | 637 (95.2) | 65 (89.0) | 2,780 (91.0) | 462 (92.2) | |
| Yes | 353 (8.2) | 32 (4.8) | 8 (11.0) | 274 (9.0) | 39 (7.8) | |
| Radiation | <0.001 | |||||
| No/unknown | 3,369 (78.4) | 422 (63.1) | 22 (30.1) | 2,549 (83.5) | 376 (75.0) | |
| Yes | 928 (21.6) | 247 (36.9) | 51 (69.9) | 505 (16.5) | 125 (25.0) | |
| Histological type | <0.001 | |||||
| AC | 3,084 (71.8) | 347 (51.9) | 47 (64.4) | 2,376 (77.8) | 314 (62.7) | |
| MAC/SRCC | 650 (15.1) | 255 (38.1) | 17 (23.3) | 248 (8.1) | 130 (25.9) | |
| Others† | 563 (13.1) | 67 (10.0) | 9 (12.3) | 430 (14.1) | 57 (11.4) | |
| Grade | <0.001 | |||||
| I–II | 1,157 (26.9) | 63 (9.4) | 22 (30.1) | 958 (31.4) | 114 (22.8) | |
| III–IV | 2,303 (53.6) | 459 (68.6) | 37 (50.7) | 1,526 (50) | 281 (56.1) | |
| Unknown | 837 (19.5) | 147 (22.0) | 14 (19.2) | 570 (18.7) | 106 (21.2) | |
| Primary site | <0.001 | |||||
| Antrum/pylorus | 674 (15.7) | 92 (13.8) | 3 (4.1) | 512 (16.8) | 67 (13.4) | |
| Body | 377 (8.8) | 68 (10.2) | 3 (4.1) | 261 (8.5) | 45 (9.0) | |
| Cardia/fundus | 1,924 (44.8) | 242 (36.2) | 52 (71.2) | 1,408 (46.1) | 222 (44.3) | |
| Greater curvature | 127 (3.0) | 21 (3.1) | 1 (1.4) | 94 (3.1) | 11 (2.2) | |
| Lesser curvature | 256 (6.0) | 37 (5.5) | 3 (4.1) | 188 (6.2) | 28 (5.6) | |
| NOS | 641 (14.9) | 153 (22.9) | 8 (11.0) | 390 (12.8) | 90 (18) | |
| Overlap | 298 (6.9) | 56 (8.4) | 3 (4.1) | 201 (6.6) | 38 (7.6) | |
| Numbers of sites | 0.92 | |||||
| 1 | 3,459 (80.5) | 533 (79.7) | 58 (79.5) | 2,462 (80.6) | 406 (81.0) | |
| ≥2 | 838 (19.5) | 136 (20.3) | 15 (20.5) | 592 (19.4) | 95 (19.0) | |
| Age (years) | <0.001 | |||||
| 61–70 | 1,251 (29.1) | 184 (27.5) | 30 (41.1) | 901 (29.5) | 136 (27.1) | |
| ≤60 | 1,399 (32.6) | 294 (43.9) | 27 (37) | 910 (29.8) | 168 (33.5) | |
| ≥71 | 1,647 (38.3) | 191 (28.6) | 16 (21.9) | 1,243 (40.7) | 197 (39.3) | |
| Survival months | 5 [2, 12] | 5 [2, 9] | 4 [2, 11] | 6 [2, 12] | 6 [2, 12] | <0.001 |
| OS | 0.03 | |||||
| Alive | 548 (12.8) | 65 (9.7) | 13 (17.8) | 409 (13.4) | 61 (12.2) | |
| Dead | 3,749 (87.2) | 604 (90.3) | 60 (82.2) | 2,645 (86.6) | 440 (87.8) | |
| DSS | 0.40 | |||||
| Alive | 1,378 (32.1) | 198 (29.6) | 27 (37.0) | 990 (32.4) | 163 (32.5) | |
| Dead | 2,919 (67.9) | 471 (70.4) | 46 (63.0) | 2,064 (67.6) | 338 (67.5) |
Data are presented as n (%) or median [Q1, Q3]. †, pathological types include mucin-producing adenocarcinoma, adenocarcinoma in tubulovillous adenoma, papillary adenocarcinoma, adenocarcinoma in multiple adenomatous polyps, tubular adenocarcinoma, adenocarcinoma in adenomatous polyp, diffuse type carcinoma, adenocarcinoma, intestinal type, superficial spreading adenocarcinoma, linitis plastica. AJCC, American Joint Commission on Cancer; AC, adenocarcinoma; MAC, mucinous adenocarcinoma; SRCC, signet ring cell carcinoma; NOS, not otherwise specified; OS, overall survival; DSS, disease-specific survival; Met., metastasis; Q1, 25th quartile; Q3, 75th quartile.
Survival analysis of different single metastatic lesions in gastric cancer
Kaplan-Meier curves were hereby plotted to clarify the survival curves for different metastatic sites (Figure 1), and median survival times and 95% confidence intervals (CI) were calculated (Table S1). We observed an all-cause mortality rate of 67.9%. The survival times of patients with metastases differed between all-cause and disease-specific deaths (P<0.001), with bone metastasis patients experiencing the poorest prognosis (Figure 1).

Additionally, Cox proportional risk models were established to examine factors associated with survival. The results revealed that bone metastases (vs. liver metastases) were significantly associated with reduced OS in patients in a univariate regression analysis [hazard ratio (HR), 1.319; 95% CI: 1.207–1.442; P<0.001] (Figure S2), while the brain (P=0.88) and lung metastases (P=0.75) were not significantly associated with patients’ OS. Similar results were also observed in the DSS (Figure 2). When adjusting all variables for multivariate regression analysis, no significant association between bone metastases and patients OS relative to liver metastases was observed (HR, 1.1; 95% CI: 0.998–1.214; P=0.05). However, there existed a significant association with patients DSS (HR, 1.181; 95% CI: 1.055–1.323; P=0.004). Additional results are shown in Figure 2.

Table 2 presents the HR and corresponding 95% CI, with bone metastases being significantly associated with reduced OS in patients (HR, 1.10; 95% CI: 1.01–1.20). However, brain and lung metastases were not significantly associated with OS in patients. The application of DSS as an outcome indicator yielded similar results (HR, 1.18; 95% CI: 1.07–1.30).
Table 2
| Met.type | HR (95% CI) | |
|---|---|---|
| OS | DSS | |
| Met.liver | 1.00 (0.95–1.05) | 1.00 (0.95–1.06) |
| Met.bone | 1.10 (1.01–1.20) | 1.18 (1.07–1.30) |
| Met.brain | 1.03 (0.79–1.33) | 0.99 (0.74–1.33) |
| Met.lung | 0.92 (0.84–1.01) | 0.93 (0.83–1.03) |
Met., metastasis; HR, hazard ratio; CI, confidence interval; OS, overall survival; DSS, disease-specific survival.
Subgroup analysis and interaction of bone metastases from gastric AC
To explore possible interactions, subgroup analysis and interaction testing of metastatic sites were conducted (bone metastases vs. other metastases, Figure 3). In the subgroup analysis, bone metastases were significantly associated with reduced OS in patients in the ≤60 and 61–70 years age subgroups (≤60 years: HR, 1.482; 95% CI: 1.29–1.702; 61–70 years: HR, 1.548; 95% CI: 1.312–1.825). In the ≥71 years age subgroup, bone metastases were not significantly associated with patients OS (P=0.77). Furthermore, there was an interaction between bone metastases and OS in the ≥71 years group (vs. ≤60 years group) (P=0.02), but not in the 61–70 years group (vs. ≤60 years group) (P=0.69). Additionally, tumor pathology type emerged as an interacting element with bone metastasis. The findings revealed that the association between bone metastasis and prognosis was not statistically significant in the MAC or SRCC (MAC/SRCC) subgroup compared to the AC subgroup (HR, 0.997; 95% CI: 0.844–1.177; P for interaction =0.002). In the subgroup with DSS as the outcome, interactions were detected between age (P=0.003), divorced/separated (P=0.04), chemotherapy (P=0.001), and the number of tumorigenesis (P=0.006) and bone metastases.
Analysis of prognostic differences among different metastases after PSM
PSM was hereby used to correct confounding factors between groups, including age, sex, race, primary site, T-stage, pathological grade, pathological type, radiotherapy, and surgical treatment. Finally, a cohort of 605 sample size pairs were generated. Table S2 shows the baseline information before and after PSM. Following PSM matching, the data distribution between groups became homogeneous. Figure S3 shows the results of the post-matching equilibrium test. The histogram exhibited good symmetry between the liver metastasis and lung/bone/brain metastasis groups after matching, presenting a well-balanced distribution of covariates between the two groups.
After PSM, the relationship between each factor and the occurrence of survival outcomes was investigated using univariate and multivariate Cox regression (Table S3). Interestingly, after controlling for confounding factors, bone metastases were still strongly correlated with prognosis compared to other metastases (Figure 4). Meanwhile, the multivariate regression results clearly showed a strong correlation with OS, including single/unmarried marital status, 2016 diagnosis, overlap site, stage T3–T4, pathological grade III–IV, chemotherapy, and surgical treatment. In terms of the DSS, distinct and robust correlations with overlap site, stage T3–T4, chemotherapy, and surgical treatment were observed.

Subgroup analysis was performed in post-PSM patients, and interactions between age (P=0.01), radiotherapy (P=0.02), chemotherapy (P=0.03), and bone metastasis for OS were detected (Figure S4). In addition, regarding DSS, there were interactions for age (P=0.006), T-stage (P=0.03), chemotherapy (P=0.006), and bone metastasis (Figure S5).
Discussion
To the best of our knowledge, while numerous studies have reported a poorer prognosis for bone metastases (18), this study is pioneering in exploring the prognostic differences of single lung, liver, bone, and brain metastases in gastric cancer. The present study was conducted primarily to evaluate the prognostic survival differences among single organ metastases at different sites in gastric cancer. The findings uncovered that among single organ metastases in gastric cancer, the liver was the most prevalent site, with brain metastases being the least common. Furthermore, bone metastases demonstrated the worst prognosis. Of particular note, in examining bone metastases, 68.6% were identified as poorly differentiated, consistent with prior research implicating a heightened tendency for poorly differentiated gastric cancers to disseminate to the bones (21). This study leveraged HRs and floating absolute risk, and revealed that distinct metastatic sites in gastric cancer carried their own prognostic implications. Notably, gastric cancer bone metastases exhibited the shortest DSS (HR, 1.181; 95% CI: 1.055−1.323; P=0.004). In addition, the sub-dataset of PSM with “post-hoc randomization” further confirmed a poorer OS (HR, 1.167; 95% CI: 1.033−1.318; P=0.01) and DSS (HR, 1.277; 95% CI: 1.11−1.468; P<0.001) for bone metastases, reinforcing the credibility of the present study. Subgroup analyses were specifically conducted for bone metastases to evaluate potential interacting factors, such as age, pathological type, chemotherapy, and number of metastatic sites. Significant interactions were observed between bone metastases and these factors. Indeed, surgical treatment is not routinely recommended for metastatic gastric cancer. However, opinions within the academic community diverge on this issue. The REGATTA phase III trial demonstrated that the combination of primary tumor resection and chemotherapy failed to improve patient survival (22). Conversely, the AIO-FLOT3 trial indicated favorable outcomes for patients with limited metastatic gastric cancer who underwent gastrectomy along with the resection of metastatic lesions following chemotherapy (23). In this study, surgical treatment (accounting for 8.2% of the total patients) was associated with improved OS and DSS. Notably, improved OS for patients after 2015 and improved DSS for patients in 2016 were observed, which was speculated to be attributed to the increasing utilization of targeted therapy and immunotherapy as adjunctive measures to conventional cytotoxic chemotherapy (24). Given the low incidence, the ESMO and the National Comprehensive Cancer Network guidelines do not endorse routine bone scintigraphy for gastric cancer patients during diagnosis or treatment, which may lead to the neglect of some cases (25). However, abnormally elevated bone alkaline phosphatase levels require particular attention as well as further investigation. Alkaline phosphatase is primarily produced by osteoblasts in bone. Elevated levels of alkaline phosphatase may indicate bone tissue damage or abnormal proliferation. In the case of cancerous bone metastases, cancer cells invading bone tissue may increase osteoblast activity in the bone marrow, leading to higher alkaline phosphatase levels in the blood. Blood alkaline phosphatase levels can thus be used as an indicator for assessing bone metabolic activity. This is clinically significant as it aids in the detection of bone metastasis and its influence on patient prognosis.
Regarding reasons for worse prognosis in gastric cancer with bone metastasis, gastric cancer bone metastasis, in addition to the primary changes caused by gastric cancer, can also lead to skeletal-related events, such as pathological fractures, paralysis, hematologic disorders, poor chemotherapy response, and pain, significantly impacting patients’ quality of life and reducing their survival rates (26). Spine is the most common site of bone metastasis, with the pelvis, ribs, sternum, and the long bones of the limbs following in prevalence. Therefore, compared to other metastases, bone metastases are more likely to lead to skeletal-related events (SREs) and necessitate longer periods of bed rest (27). A study has also reported that patients with SREs have a shorter median survival time compared to those without SREs, which may thus contribute to a poorer prognosis (15). Additionally, tumor invasion of the bone can cause hematological abnormalities and even trigger disseminated intravascular coagulation, leading to rapid disease progression and death (28). Current research suggests that bone metastasis affects the function of osteoblasts and osteoclasts, leading to either osteolytic destruction or osteoblastic proliferation (29). Besides, tumor cell proliferation produces factors such as IL-6 and parathyroid hormone-related peptide (PTHrP), which activate the receptor activator of nuclear factor kappa-b ligand pathway and stimulate osteoclast proliferation (30). In addition to osteolytic lesions, bone metastasis often induces sclerotic proliferation. Bone proliferative diseases are also associated with abnormalities in the number and function of osteoblasts and osteoclasts. On one hand, tumor cells release endothelin-1 to inhibit osteoclast movement (31). Additionally, the BB isoform, a subtype of platelet-derived growth factor secreted by tumor cells, has been found to stimulate osteoblast activation and promote bone proliferation (32). On the other hand, osteoblasts express osteoprotegerin and hepatocyte growth factors, thereby promoting tumor cell survival (33). In addition to bone cells, endothelial cells in the bone marrow not only provide an adhesive surface for tumor cells entering the bone (34), but can also be induced to produce growth factors and PTHrP, which are involved in the process of angiogenesis (35).
Herein, over half of the patients in the study sample received chemotherapy, and the management of SRE symptoms was considered equally important. Besides, a significant interaction between bone metastases and gastric cancer pathological types was observed. Previous studies have reported that the preferred site of metastasis varies for different subtypes of breast cancer (36-39). Additionally, the ccA/ccB subtype of clear cell renal cell carcinoma has been documented to be significantly associated with the location and grading of metastatic cancer (20). These findings are comparable to the present observations. The pain experienced by patients with bone metastasis severely affects their quality of life. Analgesic opioids or radiation therapy (more commonly used for neuropathic pain) can be considered (40). In this study sample, radiation therapy was administered to 36.9% of patients, representing the largest proportion after those with brain metastasis. This group of patients receiving radiation therapy likely encompassed those treated for both tumor control and pain management. Additionally, due to the active function of osteoclasts in patients with bone metastasis, bisphosphonate drugs can not only inhibit their function but also induce tumor cell apoptosis and inhibit bone metastasis (41). The underlying mechanisms are still under exploration. In general, therefore, even if patients are diagnosed with stage M1 gastric cancer, it remains essential to conduct a comprehensive assessment of factors such as histological type, age, site of metastasis, and number of metastases to formulate personalized treatment strategies.
Despite the relatively longer progression time of metastases in other sites such as the liver, brain, and lungs compared to bone metastasis, their median survival time still does not exceed six months. However, it is reassuring that the prognostic differences among various metastatic sites are being increasing clarified. Further research will be conducted to actively and specifically monitor these patients with targeted follow-up to offer novel insights into future treatments. Overall, this study reinforces the notion that the prognosis varies among different metastases of gastric cancer. This finding is expected to provide physicians with the tools to more accurately assess patient prognoses. Meanwhile, it can also empower patients with a clearer understanding of their disease status, enhance their quality of life, and reduce their suffering.
However, this study still has the following main limitations: first, the study lacked access to more detailed patient information, such as patients’ transcriptomic features, relevant imaging data, and hematological tumor markers. While a relatively poorer prognosis for bone metastasis was predicted, reasons for its worse prognosis were not clarified, significantly hampering further research. Besides, it should be noted that this study was conducted on a specific population-based cohort. While the underlying pathology and physiology of the disease may be consistent across different groups, variations in lifestyle, environment, and genetics should still be taken into account, necessitating further validation of these conclusions. Prospective research should be also pursued to investigate the detailed reasons for poorer prognosis. Lastly, acknowledging the paramount importance of quality of life for patients in the advanced stages of cancer, future research should also consider incorporating quality of life as an important efficacy reference. There are still several questions to be answered. A natural progression of this work will be to analyze the prognosis of different sites of gastric cancer metastasis. From a broader perspective, means and methods for early detection of bone metastasis should be further identified. Significant efforts are also required to elucidate the mechanisms of gastric cancer metastasis, and the issue of metastasis poses an intriguing question to be explored in further studies. Moreover, studying the pathological and signaling pathway alterations following metastasis may contribute to establishing higher accuracy in this field.
Conclusions
In conclusion, this study elucidates the prognostic differences in single organ metastases of gastric cancer, offers insights into subsequent clinical treatment, and lays the groundwork for exploring the mechanisms of single organ metastasis in gastric cancer.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-11/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-11/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-24-11/coif). H.N.G. reports funding supports from the Guangxi Natural Science Foundation, and the Research Capability Improvement Project for Young and Middle-aged Teachers in Guangxi Universities. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of The Affiliated Hospital of Guilin Medical University waived the requirement for formal Institutional Review Board approval and informed consent, given its use of anonymous data and supplementary information obtained from individuals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol 2020;18:534-42. [Crossref] [PubMed]
- Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol 2023;20:338-49. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Sexton RE, Al Hallak MN, Diab M, et al. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev 2020;39:1179-203. [Crossref] [PubMed]
- Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:1005-20. [Crossref] [PubMed]
- Kwee RM, Kwee TC. Modern imaging techniques for preoperative detection of distant metastases in gastric cancer. World J Gastroenterol 2015;21:10502-9. [Crossref] [PubMed]
- Koizumi M, Yoshimoto M, Kasumi F, et al. Comparison between solitary and multiple skeletal metastatic lesions of breast cancer patients. Ann Oncol 2003;14:1234-40. [Crossref] [PubMed]
- Li Q, Wang Y, Cai G, et al. Solitary lymph node metastasis is a distinct subset of colon cancer associated with good survival: a retrospective study of surveillance, epidemiology, and end-results population-based data. BMC Cancer 2014;14:368. [Crossref] [PubMed]
- Okano K, Maeba T, Ishimura K, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg 2002;235:86-91. [Crossref] [PubMed]
- Fuchs J, Früh M, Papachristofilou A, et al. Resection of isolated brain metastases in non-small cell lung cancer (NSCLC) patients - evaluation of outcome and prognostic factors: A retrospective multicenter study. PLoS One 2021;16:e0253601. [Crossref] [PubMed]
- Acciuffi S, Meyer F, Bauschke A, et al. Solitary colorectal liver metastasis: overview of treatment strategies and role of prognostic factors. J Cancer Res Clin Oncol 2022;148:657-65. [Crossref] [PubMed]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93. [Crossref] [PubMed]
- Maccauro G, Spinelli MS, Mauro S, et al. Physiopathology of spine metastasis. Int J Surg Oncol 2011;2011:107969. [Crossref] [PubMed]
- Liang Y, Zhang H, Song X, et al. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin Cancer Biol 2020;60:14-27. [Crossref] [PubMed]
- Petrillo A, Giunta EF, Pappalardo A, et al. Bone Metastases from Gastric Cancer: What We Know and How to Deal with Them. J Clin Med 2021;10:1777. [Crossref] [PubMed]
- Ciner AT, Jones K, Muschel RJ, et al. The unique immune microenvironment of liver metastases: Challenges and opportunities. Semin Cancer Biol 2021;71:143-56. [Crossref] [PubMed]
- Fuchs CS. Chemotherapy for advanced gastric cancer: where do we stand? J Clin Oncol 1997;15:3299-300. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist K, et al. Metastatic spread in patients with gastric cancer. Oncotarget 2016;7:52307-16. [Crossref] [PubMed]
- Neville AM, Bettelheim R, Gelber RD, et al. Factors predicting treatment responsiveness and prognosis in node-negative breast cancer. The International (Ludwig) Breast Cancer Study Group. J Clin Oncol 1992;10:696-705. [Crossref] [PubMed]
- Serie DJ, Joseph RW, Cheville JC, et al. Clear Cell Type A and B Molecular Subtypes in Metastatic Clear Cell Renal Cell Carcinoma: Tumor Heterogeneity and Aggressiveness. Eur Urol 2017;71:979-85. [Crossref] [PubMed]
- Qiu MZ, Shi SM, Chen ZH, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med 2018;7:3662-72. [Crossref] [PubMed]
- Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 2016;17:309-18. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol 2017;3:1237-44. [Crossref] [PubMed]
- Zeng Y, Jin RU. Molecular pathogenesis, targeted therapies, and future perspectives for gastric cancer. Semin Cancer Biol 2022;86:566-82. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Kim YJ, Kim SH, Kim JW, et al. Gastric cancer with initial bone metastasis: a distinct group of diseases with poor prognosis. Eur J Cancer 2014;50:2810-21. [Crossref] [PubMed]
- Silvestris N, Pantano F, Ibrahim T, et al. Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey. PLoS One 2013;8:e74402. [Crossref] [PubMed]
- Gomi D, Fukushima T, Kobayashi T, et al. Gastric cancer initially presenting as bone metastasis: Two case reports and a literature review. Oncol Lett 2018;16:5863-7. [Crossref] [PubMed]
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004;350:1655-64. [Crossref] [PubMed]
- de la Mata J, Uy HL, Guise TA, et al. Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J Clin Invest 1995;95:2846-52. [Crossref] [PubMed]
- Mohammad KS, Guise TA. Mechanisms of osteoblastic metastases: role of endothelin-1. Clin Orthop Relat Res 2003;S67-74. [Crossref] [PubMed]
- Achbarou A, Kaiser S, Tremblay G, et al. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res 1994;54:2372-7. [PubMed]
- Vallet S, Bashari MH, Fan FJ, et al. Pre-Osteoblasts Stimulate Migration of Breast Cancer Cells via the HGF/MET Pathway. PLoS One 2016;11:e0150507. [Crossref] [PubMed]
- Esposito M, Kang Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol Ther 2014;141:222-33. [Crossref] [PubMed]
- Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology 2001;142:5050-5. [Crossref] [PubMed]
- Molnár IA, Molnár BÁ, Vízkeleti L, et al. Breast carcinoma subtypes show different patterns of metastatic behavior. Virchows Arch 2017;470:275-83. [Crossref] [PubMed]
- Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res 2008;68:3108-14. [Crossref] [PubMed]
- Ignatov A, Eggemann H, Burger E, et al. Patterns of breast cancer relapse in accordance to biological subtype. J Cancer Res Clin Oncol 2018;144:1347-55. [Crossref] [PubMed]
- Grote I, Poppe A, Lehmann U, et al. Frequency of genetic alterations differs in advanced breast cancer between metastatic sites. Genes Chromosomes Cancer 2024;63:e23199. [Crossref] [PubMed]
- Rich SE, Chow R, Raman S, et al. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol 2018;126:547-57. [Crossref] [PubMed]
- Roelofs AJ, Thompson K, Ebetino FH, et al. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Des 2010;16:2950-60. [Crossref] [PubMed]
Cite this article as: Zhao QW, Quan Z, Liu SS, Wang YD, Guo HN. Heterogeneity and prognosis of single organ metastases in gastric cancer. Transl Gastroenterol Hepatol 2024;9:61.


