
Endoscopic vacuum therapy: pitfalls, tips and tricks, insights, and perspectives
Introduction
Endoscopic vacuum therapy (EVT), also known as endoscopic negative pressure therapy or Endovac (E-Vac), was first reported in 2003 as a new method for sepsis control caused by a colorectal anastomotic leak (1). As standardization of a name is important, we recommend the name EVT following most published studies.
Since the first cases reports, EVT has been used in the management of transmural gastrointestinal (GI) defects (TGIDs) in several organs (2) such as esophagus (3,4), stomach (mainly for post-bariatric surgical leaks) (5-7), small bowel (mostly duodenum) (8), colorectal defects (9), and also in the liver (10) with high efficacy and satisfactory safety profile (2,4,11,12).
EVT allows continuous drainage of GI fluids, reducing edema, increasing local perfusion, and thus promoting healing through a unique mechanism of action, including macro and micro deformation, changes in perfusion (stimulating angioneogenesis), exudate control, and bacterial clearance (4,12).
EVT has the potential to become the first-line endoscopic therapy for most TGIDs, especially for leaks with associated infected collection, presenting higher efficacy and lower adverse events (AEs) than any other endoscopic therapy as proved by several systematic reviews and meta-analyses (13-24). Its effectiveness as rescue treatment is also satisfactory (2,25,26). Mandarino et al. reported dehiscence closure in 75% of post-esophagectomy anastomotic leaks after failed redo surgery or previous endoscopic treatment (25). A recent study including 144 patients (51.39% with prior surgical revision and 9.03% with prior endoscopic attempt) published by our group, demonstrated no association between prior surgery vs. no surgery and prior endoscopic attempt vs. no attempt for TGID closure after EVT as a rescue therapy (2).
Despite its favorable outcomes, EVT is not adopted in many centers worldwide due to several challenges, such as device placement and removal related to the size of the polyurethane sponge, resulting in prolonged procedures, need for multiple exchanges due to tissue ingrowth, patients’ discomfort caused by the device, physicians’ concerns of fatal AEs (due to few reports of massive bleeding), and concerns related to cost increase (2,12,27-35). These factors may deter endoscopists from using this technique, which may lead to undesired outcomes. Furthermore, there is low evidence available in the literature, mainly randomized controlled trials.
To demystify the mentioned limitations of EVT, this narrative review [including all available data in the literature obtained through electronic databases (MEDLINE, Cochrane Library, and SciELO)] describes practical solutions and shares tips and tricks achieved by years of experience to overcome EVT-related challenges, aiming to collaborate with a broader adoption of EVT. Furthermore, future perspectives are discussed, such as prophylactic use of EVT for high-risk anastomosis and treatment of diffuse duodenal bleeding and giant ulcers in the upper GI tract.
Methods
Available data and indications
EVT is associated with high efficacy and low rate of AEs for TGID closure and rarely fails if properly indicated as reported by several systematic review and meta-analyses (Table 1). The ability to achieve negative pressure is critically important. Therefore, understanding the mechanism of action is essential to avoid its use when the system is not functioning (Table 2). Large studies reported factors associated with EVT failures, including neoadjuvant therapy, intraluminal placement, and time from diagnosis of the TGID to initiation of EVT (36,37). It is noteworthy that inadequate drainage can significantly impact the care and clinical outcomes. Thus, all staff must be trained to assess if the EVT system is properly functioning in order to rapidly inform the endoscopist when it is not working. EVT-related problems include sponge migration, connections with leak, battery of the machine, full reservoir, obstruction of the system, and leakage. Warning signs of the machine is usually easily recognized by the machine alarms. Clinical signs include worsening of clinical conditions, as tachycardia, fever, and sepsis. Additionally, complementary exams can also suggest that EVT is not working as expected.
Table 1
| Study author [year] | No. of studies | No. of patients [EVT] | Type of EVT device | Comparative treatment | TGID location | Efficacy EVT vs. other | Safety EVT vs. other |
|---|---|---|---|---|---|---|---|
| Shelygin [2018] | 7 | 158 [158] | OPPS | N/A | Colorectal anastomosis leak | 82% | AEs: 16% |
| Rausa [2018] | 4 | 163 [71] | OPPS | SEMS | Anastomotic leak after esophagectomy | 85.9% vs. 55.8% | Major AEs: 6.3% vs. 16.1% |
| Mortality: 13.27% vs. 29.3% | |||||||
| Scognamiglio [2020] | 5 | 274 [105] | OPPS | SEMS | Anastomotic leak after esophagectomy | 81.8% vs. 61.1% | AEs: 17.8% vs. 22.8% |
| Major AEs: 5.55% vs. 17.5% | |||||||
| Mortality: 11.2% vs. 22.1% | |||||||
| Aziz [2021] | 18 | 423 [423] | OPPS | N/A | Esophageal leaks, fistulas, and perforations | 89.4% | AEs: 13.6% |
| Mortality: 7.1% | |||||||
| do Monte Junior [2021] | 5 | 274 [105] | OPPS | SEMS | Upper GI perforations, leaks, and fistulas | 81.8% vs. 61.1% | AEs: 17% vs. 17.7% |
| Mortality: 10.5% vs. 20.1% | |||||||
| Tavares [2021] | 23 | 559 [559] | OPPS | N/A | Anastomotic leaks after esophagectomy and gastrectomy | 81.6% | Stenosis: 12.5% |
| Tavares [2021] | 5 | 226 [74] | OPPS | SEMS | Anastomotic leaks after esophagectomy and gastrectomy | 75.6% vs. 63.6% | AEs: 12.2% vs. 47.7% |
| Mortality: 12.2% vs. 19.3% | |||||||
| Intriago [2022] | 5 | 55 [55] | OPPS and OPF | N/A | Post-bariatric surgical leaks and fistulas | 87.2% | AEs: 6% |
| Kühn [2022] | 24 | 676 [676] | OPPS | N/A | Colorectal anastomotic leaks, Hartmann stump insufficiency, and iatrogenic colonic perforations | 81.4% | AEs: 12.1% |
| de Lacy [2022] | 29 | 827 [827] | OPPS | N/A | Pelvic intestinal anastomotic leakage | 66.78% | AEs: 6.7% |
| Laopeamthong [2022] | 13 | 298 [47] | OPPS and OPF | Double pigtail stents | Post-bariatric surgical leaks and fistulas | 85.2% vs. 91.6% | AEs: 2.1% vs. 6% |
| Mandarino (Gastrointest Endosc) [2022] | 8 | 357 [152] | OPPS | SEMS | Anastomotic leaks after esophagectomy and gastrectomy | 83.6% vs. 63.3% | AEs: 12.6% vs. 30.2% |
| Dislocation: 10.2% vs. 18.3% | |||||||
| Mortality: 12.6% vs. 21% | |||||||
| Scognamiglio [2022] | 7 | 338 [149] | OPPS | SEMS | Intra-thoracic anastomotic leaks | 85.5% vs. 64.6% | AEs: 11.9% vs. 21.3% |
| Major AEs: 10.4% vs. 18.4% | |||||||
| Mortality: 14.9% vs. 20.6% |
EVT, endoscopic vacuum therapy; TGID, transmural gastrointestinal defect; OPPS, open-pore polyurethane sponge; N/A, not applicable; AEs, adverse events; SEMS, self-expandable metal stent; GI, gastrointestinal; OPF, open-pore film.
Table 2
| Indications |
| • TGID with associated contained undrained collection |
| • TGID with associated contained drainage collection (need to remove or cap the external drain) |
| • Acute iatrogenic perforations |
| • Adjunctive therapy |
| Possible indication |
| • GI-cutaneous fistulas (cutaneous orifice need to be occluded) |
| • TGID with associated uncontained collection (aiming to form a contained collection) |
| Contra-indications/lack of indication |
| • Inability to achieve negative pressure [GI-vaginal, vesical, or respiratory (tracheal/bronchial) fistulas and gastro-enteral/colonic fistulas] |
| • Patient refusal of treatment |
| • Inability to access the TGID |
| • GI-cutaneous fistulas with a thin (<5 mm) and a long-epithelized tract (>2 cm) |
TGID, transmural gastrointestinal defect; GI, gastrointestinal.
Choosing the best approach based on patient’s characteristics
The management of TGID must consider patient’s clinical condition and the characteristics of the TGID. There is as lack of evidence to state the best approach for this challenging condition. However, based on our experience and data available, we summarized our recommendations in Table 3. For unstable patients, immediately intervention is critical. Thus, imaging exams can be obviated. On the other hand, for stable patients, imaging should be performed as it provides additional information, allowing for a suitable treatment plan (8,9).
Table 3
| Clinical scenarios | Recommended approaches | Alternative approaches |
|---|---|---|
| Undrained uncontained collection (extremely rare in late and chronic leaks) | Unstable patients | Unstable patients |
| • Surgical lavage + drainage ± surgical repair ± endoscopic therapy: intraluminal EVT, cap-mounted clips (TGID ≤2 cm), endoscopic suturing (TGID >2 cm), and/or stents (downstream stenosis) | • In centers of reference, less invasive therapies such as IGPD + endoscopic therapy can be performed | |
| • Endoscopic lavage of the abdominal cavity followed by intracavitary EVT placement aiming to create a compartment (turn an uncontained into a contained collection) may be considered in referral centers with close monitoring in ICU | ||
| Stable patients | Stable patients | |
| • IGPD + endoscopic therapy: intraluminal EVT, cap-mounted clips (TGID ≤2 cm), endoscopic suturing (TGID >2 cm), and/or stents (downstream stenosis) or intracavitary EVT | • In scarce-resource centers, surgical management is indicated to avoid worsening of patient´s clinical condition | |
| Undrained contained collection (unstable or stable patients)—most patients are stable due to the contained collection | • Endoscopic internal drainage | • Choosing the correct endoscopic therapy is imperative to achieve clinical success and must consider several factors, such as time: |
| ∘ Collection ≥3 cm: intracavitary EVT or EID with DPS | ∘ Acute/early: cap-mounted clips (TGID ≤2 cm), endoscopic suturing (TGID >2 cm), stents (downstream stenosis), and/or intraluminal EVT | |
| ∘ Collection <3 cm: intraluminal EVT | ∘ Late/chronic: CSDO or cap-mounted clips (TGID ≤2 cm) | |
| • Although we recommend EID, IGPD associated with an endoscopic therapy is a reasonable approach, mainly for unstable patients | • If a septum is identified, septotomy must be performed | |
| • In scarce-resource centers, surgical management is indicated to avoid worsening of patient’s clinical condition | ∘ Adjunctive therapies such as tissue sealants/glues injection and intraluminal EVT is usually helpful |
EVT, endoscopic vacuum therapy; TGID, transmural gastrointestinal defect; IGPD, image-guided percutaneous drainage; ICU, intensive care unit; CSDO, cardiac septal defect occluder; EID, endoscopic internal drainage; DPS, double pigtail stents.
Hemodynamic unstable with undrained uncontained collection (without wall—free fluid is present in the extraluminal compartment)
Surgical lavage and drainage are recommended. Surgical repair of the TGID can also be performed but due to the unhealthy tissue surrounding the TGID, this approach is usually not effective. If unsuccessful surgical closure, endoscopic therapy is indicated. The traditional technique is self-expandable metal stent (SEMS) placement for large TGID (>2 cm or unsuccessful closure with clips) and clipping for small TGID (≤2 cm) as recommended by the American Gastroenterological Association (AGA) (38) and European Society of Gastrointestinal Endoscopy (ESGE) position updates (39). However, based on our experience and available data, EVT should always be considered, at least as an adjunctive therapy (2). As detailed in Table 1, EVT is not only a promising therapy as mentioned by both societies’ statements (38,39), but also a valuable tool in the armamentarium to treat this critical condition. Six recent meta-analyzes (13,15,16,20,21,23) comparing EVT vs. SEMS for upper TGID, reported higher rates of clinical success and lower rates of AEs, and mortality, favoring EVT. Despite the evident superior outcomes of EVT, the selection of the best therapy should be individualized considering TGID characteristics, personal and local experience, devices availability, costs, and patient’s preference (2). Furthermore, combining techniques, applied simultaneously or sequentially, that employ different mechanisms of action are being evaluated (7,40).
Hemodynamic stable with undrained uncontained collection
Both surgical and less-invasive therapies (radiologic or endoscopic intervention) can be performed. If image-guided drainage is performed, endoscopic therapy must be indicated for TGID closure. If an endoscopic internal drainage (EID) technique is selected, the external drain should be capped or removed to obtain negative pressure. In our experience (no evidence available), intracavitary EVT is effective for selected cases (2,5). Prior to intracavitary EVT placement, the extraluminal compartment is accessed through the TGID followed by lavage with saline solution and aspiration of the fluids until the cavity is cleaned. After lavage, “intracavitary” EVT is placed aiming to control the infection with the formation of a contained collection. In addition, an intraluminal modified triple-lumen tube (TLT) EVT is also placed, mainly for anastomotic leaks (2,8,35). The goal of this approach is to model the anastomosis, reduce aggressive factors such as gastric and biliopancreatic secretions, and allow enteral nutrition reducing the need for parenteral nutrition. A recent multicenter study sharing our experience, reported a very high clinical success rate when EVT was used in cases without contra-indications. The rate of successful closure was 98.11% for esophagus, 95.34% for stomach, 92.30% for small bowel, mainly duodenum, and 90% for colorectal TGIDs (2).
Hemodynamic stable or unstable with undrained or drained contained collection
Endoscopic therapies are now considered the best approach for contained collection associated to a TGID. If closure or cover techniques are performed, external drainage must be accomplished. Therefore, EID should be preferred, avoiding the inconvenience of a percutaneous drain. In our daily practice, EVT is the first approach in most cases. We always lavage the contained collection with a solution containing saline, hydrogen peroxide (we suggest only one 20-cc syringe), and n-acetylcysteine, until the cavity is cleaned. It is important to state that there is no evidence regarding the safety and efficacy of the use of hydrogen peroxide and n-acetylcysteine for associated cavity lavage. After granulation tissue is identified, we replace the EVT with double pigtail stents (DPS) (2,8,35). This approach allows fast infection control, which improves the tissue healing process and patient clinical condition, resulting in early hospital discharge, without the need to wait for complete TGID closure.
Hemodynamic stable (or unstable although it is rare) with no associated collection
Endoscopic therapies should always be preferred. In our clinical practice, if an iatrogenic perforation is identified, closure is performed as fast as possible. For small TGID (up to 1.5 cm), through the scope clips (TTSCs) are preferred. Cap-mounted clip is reserved for TGID measuring between 1.5 and 2 cm and endoscopic suturing is performed for larger TGID (>2 cm). After closure, usually intraluminal EVT is placed as an adjunctive therapy to avoid fluid extravasation to the extraluminal compartment. We do not use intraluminal EVT alone to reduce the risk of late severe AEs (SAEs) as an iatrogenic perforation sometimes is not well accepted by patients. If complete closure is not confirmed, intraluminal EVT must be placed (2,8,35). When fluid extravasation occurs, we place the EVT in the extraluminal compartment to avoid contamination. Although, we rarely use SEMS due to the considerable rates of AEs, including the high risk of migration, especially if there is no downstream stenosis, SEMS is also an option to manage TGID. A recent matched case-control study (41) comparing EVT vs. SEMS for treatment of anastomotic leaks <3 cm post-oncologic Ivor-Lewis showed no statistically significant results in terms of leaks resolution (90.9% EVT × 72.7% SEMS) with similar number of procedures. SEMS migration occurred in 15.3%. In our opinion, the lack of statistical difference in terms of leaks resolution is strongly associated with the small sample size (22 patients per group). Furthermore, SEMS migration rates are very high, regardless of the type of SEMS. Migration and other reported SEMS-related SAEs can be catastrophic, especially in patients with deteriorated clinical condition (3,41-45).
Management of external drains
For TGID with associated collections EID should always be preferred for two reasons. First, EID is highly effective and second, EID eliminates the need for a percutaneous drain, which is always undesired by patients due to the risk of having pain, local infection, and chronic GI-cutaneous fistula. However, in most cases endoscopic evaluation is requested after image-guided percutaneous drainage. Thus, knowledge regarding its management is essential.
Remove or cap the external drain
When there is a communication between the percutaneous and the transmural GI drains (both draining the same collection), the external drain must be removed or at least capped to allow internal drainage, mainly for EVT to achieve negative pressure (5). Prior to remove the percutaneous drain, it can be used for contrast injection allowing for a better understand of the anatomy. Furthermore, in our clinical practice, we always introduce a 0.035-in guidewire through the drain and then capture and remove it using a forceps biopsy through the gastroscope. This allows traction providing an easy placement of the EVT system. We only maintain the external drain for very large infected collection with purulent content. We cap the external drain to allow the internal drainage and uses it for lavage, injecting saline solution twice daily, to help the EVT system to clean dense fluids. In selected patients with pleural effusion, a thoracic drain with negative pressure might be applied (10).
Keep the external drain
It should be maintained only if there is no communication with the collection receiving EID.
Cutaneous orifice occlusion after drain removal
To achieve negative pressure, there must be no air leakage. Therefore, as closure is very challenging in this condition, occlusion of the cutaneous orifice is usually needed. However, in some cases, the subcutaneous tissue collapses and closes the tunnel of the drain.
Devices/EVT system selection
As EVT continues to gain more and more prominence to address TGID, diverse EVT types have been developed, each with distinct advantages and disadvantages (Figure 1) (27-35) as summarized in Table 4.
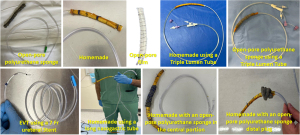
Table 4
| EVT types | Device characteristics | Advantages | Disadvantages | Our experience |
|---|---|---|---|---|
| OPPS | • Sponge connected at the tip of a NGT covering all the fenestrations (homemade) | • Widely available materials | • Challenging placement and removal due to its large size (impedes passage through the nostril) | • Requires additional maneuvers for placement and removal, leading to prolonged procedures (homemade OPPS system) |
| • Can be manufactured based on each defect characteristics | • Both require multiple exchanges due to tissue ingrowth, increasing AEs risk (both devices) | • Very challenging placement when two EVT systems are used | ||
| • Larger tubes diameter | • High cost | • Faster granulation tissue formation compared to other EVT types | ||
| • Fast and effective in promoting granulation tissue (both devices) | • Not available in several countries | • OPPS is our preferred initial device for 7–10 days | ||
| • Commercially available OPPS | • Not off-label | • 9 Fr tube | • Commercially available OPPS system: not available in our country | |
| • Less challenging placement due to the devices available in the commercially device (overtube) | ||||
| OPF | • Permeable film connected at the tip of a NGT covering all the fenestrations | • Easy and fast placement and removal (through the nostrils) due to its small diameter | • High cost | • Very similar characteristics, outcomes, advantages, and disadvantages compared to the H-EVT device, except costs |
| • Allows longer intervals between exchanges | • Higher risk for device migration compared to the OPPS | • Not widely available | ||
| • Not available in all countries (e.g., United States of America) | ||||
| H-EVT | • Homemade device with similar characteristics to the OPF | • Widely available low-cost materials for device manufacturing | • Off-label | • Very easy placement and removal through the nostrils, with no need for endotracheal intubation in most cases |
| • A half-gauze is placed at the tip of a NGT covering all the fenestrations. Then, a surgical drape is wrapped around the gauze. The homemade “sponge” is then fixed, and several perforations (pores) are made | • Small diameter allowing easy placement and removal | • High risk of device dislocation compared to the OPPS and OPF systems | • Allows the creation of devices designed based on the specific characteristics of each TGID | |
| • Slippery surface allows prolonged interval between exchanges | • Preferable for scarce resources centers | |||
| • Low risk of obstruction and AEs | ||||
| TT | • Manufactured with two Levin tubes. A 12 Fr NGT is inserted in the lumen of a 20 Fr NGT. Then, with the distal fenestrations of the tubes not aligned, fixation with suturing is performed. The vacuum pump is connected to the inner tube. The external tube prevents clogging | • Easy to manufacture | • Off-label | • Less effective in stimulating granulation tissue and aspirating thick secretions |
| • Allows lavage of the collection | • Poor granulation tissue formation | • Useful for large cavities with high volume of fluid and thin fistulous tract | ||
| • Slippery surface and small diameter allowing easy placement and removal | • High risk of device obstruction | • Easy placement and removal through the nostrils, with no need for endotracheal intubation in most cases | ||
| • Low risk of AEs | ||||
| TLT | • Any type of EVT system (OPPS, OPF, H-EVT) is connected at the gastric (aspiration) portion of the tube, covering all the fenestration | • Allows simultaneous drainage (EVT) and nutrition with only one tube through the nostril | • Allows only intraluminal placement | • Reduces patient’s discomfort and need for parenteral nutrition |
| • Reduces patient’s discomfort | • High risk of enteral tube obstruction (small diameter) | • It is our preferred device for intraluminal EVT | ||
| • Enteral nutrition reduces the risk of intestinal bacterial translocation | • Not widely available | • Challenging placement compared to other enteral feeding tubes | ||
| • Low risk of AEs | • Off-label | • Simultaneous placement with intracavitary EVT, allows enteral nutrition, provides anastomosis remodeling, and reduces aggressive factors such as biliopancreatic secretions leakage to the extraluminal compartment | ||
| SOS | • OPPS combined with FCSEMS | • Allows oral intake | • High cost, mainly if multiple exchanges are needed | • No personal experience: not available in South America |
| • FCSEMS isolates the OPPS from saliva and other GI secretions | • Allows both intracavitary and intraluminal EVT | • High risk of migration, similar to others FCSEMS | • May be useful for TGID with large associated collections with downstream stenosis | |
| • Commercially available | • Not widely available | • Does not appear to change paradigms, as SEMS-related AEs are expected | ||
| VACStent | • FCSEMS coated with OPPS and connected to a suction tube | • FCSEMS seals the GI lumen, and the OPPS promotes healing and secures the device in position (reduced risk of migration) | • High cost, mainly if multiple exchanges are needed | • No personal experience: not available in South America |
| • Allows for oral intake | • Allows only intraluminal placement due to the cylindrical shape of the OPPS | • May be useful for TGID associated with downstream stenosis | ||
| • Commercially available | • Not widely available | • May become an option for esophageal iatrogenic perforation without fluid extravasation to the extraluminal compartment |
EVT, endoscopic vacuum therapy; OPPS, open-pore polyurethane sponge; NGT, nasogastric tube; OPF, open-pore film; AEs, adverse events; Fr, French; H-EVT, homemade-endoscopic vacuum therapy; TT, tube-in-tube; TLT, triple-lumen tube; SOS, stent-over-sponge; FCSEMS, fully covered self-expandable metal stent; GI, Gastrointestinal; TGID, transmural gastrointestinal defect; SEMS, self-expandable metal stent.
The “Traditional Sponge” system customized with an open-pore polyurethane sponge (OPPS) connected to the tip of a nasogastric tube (NGT) has proven its merit. Notably, it enables faster healing by promoting granulation tissue. It is commercially available favoring its use compared to off-label devices. However, its larger diameter poses challenges during placement and removal, increasing procedure time. Moreover, due to tissue ingrowth, the OPPS necessitates frequent exchanges due to tissue ingrowth, increasing the risk of AEs and costs. These limitations may be associated with the low EVT adoption worldwide (29).
To overcome the limitations of the OPPS, several techniques have been described including open-pore film (OPF), homemade EVT (H-EVT), tube-in-tube, modified TLT (Figure 2), and novel systems combing EVT with SEMS as stent-over-sponge (SOS) and VACStent.
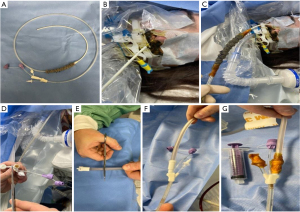
The selection of the EVT system should consider several factors such as personal and local experience, device availability, costs, and mainly TGID characteristics such as location, presence of an associated collection, size, and fluids/secretions volume. Furthermore, patient clinical condition, anesthesiologist assistance, and fluoroscopic image guidance during the endoscopic procedure should be considered.
In our clinical practice, to reduce the costs related to the vacuum machine, wall suction is used in scarce resources hospitals. After connecting the NGT to the suction tube, a 20 gauge (G) intravenous (IV) catheter is inserted into the tube allowing for a stable negative pressure (between 75 and 150 mmHg). This idea was initial used only with the H-EVT. However, this approach can be used with other EVT devices, such as OPPS, OPF and TLT.
Placement
EVT placement is historically recognized to be challenging and time-consuming. However, this procedure can be pleasant when performed under appropriate conditions in compliance with some technical aspects. The perfect scenario encompasses anesthesiologist assistance, fluoroscopic assistance, trained nurses, high-quality endoscopy equipment’s including high-definition scopes (conventional gastroscope, ultra-slim gastroscope, double-channel scope, pediatric colonoscope, and echoendoscope), electrosurgical machine, and water pump machine. We recommend to not start a procedure without having everything you might need available (28). Additionally, an EVT-toolbox is helpful to ensure device availability (Table 5).
Table 5
| EVT-toolbox |
| EVT system manufacture |
| NGT (24 Fr, 20 Fr, 18 Fr) |
| Triple-lumen tube |
| Rectal tube (24 Fr, 28 Fr, 30 Fr) |
| Open-pore polyurethane sponge |
| Gauze |
| Surgical plastic drape |
| Scissor |
| 18 G needle |
| 0 cotton suture |
| 2-0 nylon suture |
| Needle holder |
| Kelly forceps |
| EVT placement |
| Two 0.035-inch hydrophilic guidewire |
| Rat tooth forceps |
| Overtube |
| Forceps biopsy |
| Lubricating jelly |
EVT, endoscopic vacuum therapy; NGT, nasogastric tube; Fr, French; G, gauge.
Before placement, endoscopic evaluation under water infusion (water-immersion technique) or with minimal carbon dioxide (CO2) insufflation is imperative to prevent severe complications such as inadvertent disruption of a contained associated collection.
Several EVT placement techniques have been described and the selection of each approach is based on each patient clinical scenario, TGID characteristic, and materials availability. Based on our experience, below we describe our approaches in different scenarios.
TGID with no external drains and no GI-cutaneous communication
In most cases, we place the EVT system over a 0.035-inch guidewire. However, depending on EVT system, placement through an overtube or with forceps, grasper, or polypectomy snares assistance is preferred. In very challenging cases, surgical and interventional radiology assistance may be useful (11,12).
TGID with external drains or GI-cutaneous fistula
We consider it as the best scenario for endoscopists. First, a 0.035-inch guidewire is introduced through the external drain and placed inside the associated collection. Then, with a gastroscope, the associated cavity is accessed, and the guidewire placed via the external drain inside the collection is captured with a biopsy forceps and its proximal tip is removed with the gastroscope. Then, with both tips of the guidewire in hands, the external drain is removed and the EVT system placement turns into an easy procedure. Furthermore, in this scenario, the intracavitary EVT system can be fixed in the skin using a suture to facilitate the next EVT exchange session. We recommend this technique for large contained collections, previous challenge placement, and critically ill patients, aiming to reduce the need for prolonged procedure. After fixation, cover or closure of the cutaneous orifice to achieve negative pressure is critically important (11,12,21).
Ultra-slim gastroscope
Due to its diameter, it can be introduced through the nostril, allowing the positioning of the guidewire and subsequent insertion of the EVT tube directly through the nostril, without the need for mouth-to-nose transfer. This technique is recommended for small diameter EVT devices as larger devices can damage the nostril. Additionally, it is very helpful in cases with external drains or GI-cutaneous fistula as the guidewire can be placed through the external drain and then captured and removed directly through the nostril (5,10). In few cases, OPPS-EVT systems may also be used (8,9,28). Ideally, the NGT is inserted through the nostril and externalized through the cutaneous orifice to manufacture the system. After the device is assembled, the NGT is retracted until it is properly positioned in the TGID. For OPPS-TLT-EVT placement through the nostril can be performed with careful, and the OPPS must have a small diameter. Additionally, gentle introduction using lubricating jelly is required. Ultra-slim gastroscope is our preferred endoscope for EVT placement, whether in intracavitary or intraluminal positions. The ultra-slim gastroscope is used only for guidewire placement to avoid mouth-to-nose transfer. Then, the ultra-slim gastroscopy is exchanged for a standard gastroscope to facilitate placement.
Larger diameter EVT devices or unavailability of ultra-slim gastroscope
For these cases, EVT systems should be introduced through the mouth. This approach can be performed by two different techniques. (I) The NGT is introduced through the nostril and removed through the mouth for device confection. Then, the EVT system is re-introduced through the mouth for adequate positioning; (II) EVT system placement through the mouth. After adequate positioning, a small diameter tube/catheter (i.e., ureteral catheter) is introduced through the nostril and removed through the mouth. Then, the small tube is connected to the NGT and pulled through the nostril. This maneuver should be performed with the EVT system functioning to avoid migration.
Small orifices
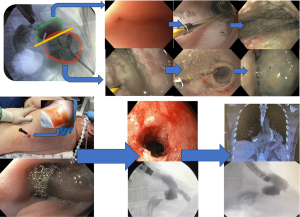
Fluoroscopy assistance is recommended as water-soluble contrast can be injected, allowing the study of the external compartment and evaluation of the associated cavity and/or the fistula tract (fistulogram). If a contained cavity is identified, dilation of the orifice should be performed until the gastroscope can enter inside the cavity, allowing a better lavage. For associated cavities larger than 3 cm, intracavitary placement is recommended. Thus, dilation of small orifices is mandatory to allow intracavitary access (Figure 3).
Pediatric population
We recommend small diameter EVT systems, mostly the H-EVT, for patients under 3 years old. As this population has small nostrils, some patients will need to be intubated during EVT to allow the EVT system through the mouth. Rendezvous is the preferred technique for patients with percutaneous gastrostomy. This situation is common in pediatric patients.
Position
Adequate position is critical for treatment success. The device can be placed in intracavitary or intraluminal position (Figure 4).
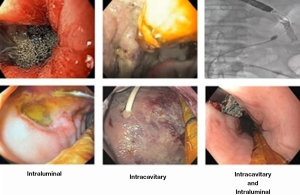
For TGID with associated contained collections larger than 3 cm, EVT system must be placed in intracavitary position. Conversely, intraluminal EVT placement is recommended for associated collection smaller than 3 cm or for TGID with no associated collection. This strategy is mainly used for acute perforation. In our clinical practice we always used intraluminal EVT for iatrogenic perforations as a primary or adjunctive therapy. Hospital stay for these cases, varies from 48 hours to 7 days, depending on the on clinical status, defect size and location, and laboratorial and imaging exams.
In our experience simultaneous intracavitary and intraluminal placement is associated with higher successful closure rates than individual intraluminal or intracavitary placement (2). The best indication for this combined approach is leaks associated with a large infected collection. This technique promotes infection control, cavity reduction, granulation tissue, reduction in GI fluids extravasation to the cavity and remodeling of anastomotic dehiscence. Furthermore, if a TLT is used, it allows for enteral nutrition without needing a third tube inserted through the nostril.
EVT system exchange
There are no ideal intervals for EVT exchange, time for exchange should consider several factors, including TGID characteristics, device functioning, EVT system type and position, and patient’s clinical condition.
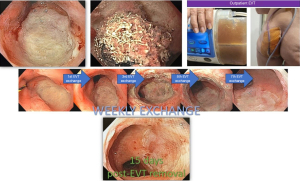
The OPPS is traditionally exchanged every 3 to 7 days due to tissue ingrowth as reported by most studies. In our clinical experience, intervals of up to 10 days are safe for very large collections, although minor bleeding might be expected. However, some steps must be followed for technical success during EVT system removal (Table 6). The modified EVT systems can be in place for a longer period as tissue ingrowth is very rare. However, this benefit is rarely used during intracavitary EVT as cavity lavage and endoscopic evaluation are required at least every 10 days. However, for intraluminal EVT, the continuous use for longer periods (7 to 15 days) is a huge advantage as it reduces the need for repeat procedures and thus, reduce cost and risk of AEs. For TGID without associated collection, only one EVT session is usually required for successful closure (35,46).
Table 6
| Primary approaches |
| (I) Turn-off the vacuum machine |
| (II) Disconnect the EVT tube from the vacuum machine |
| (III) Flush approximately 80 mL of water or saline |
| (IV) Gently pull the EVT system |
| Unsuccessful removal → alternative approaches |
| (V) Flush approximately 20 mL of hydrogen peroxide (not evidence based) followed by 20 mL of water or saline |
| (VI) Gently pull the EVT system |
| (VII) Endoscopic interventions |
| • Water immersion |
| • Distal attachment cap on the endoscope, manoeuvring the endoscope between the mucosa and sponge to carefully loosen it |
| • Foreign body forceps/grasper assistance and polypectomy snare, if possible, to grab the sponge |
EVT, endoscopic vacuum therapy.
For non-function devices, EVT system should be changed as quickly as possible to mitigate the risks of an undrained TGID, including sepsis and clinical deterioration.
Perioperative management—the anesthesiologist’s point of view
Procedure complexity and patient’s clinical condition is pivotal to choosing the best approach, mainly considering the risk for aspiration. General anesthesia is recommended for patients with high risk of gastric stasis (peritonitis, sepsis, GI obstruction, and recent use of glucagon-like peptide-1 receptor agonists) (47). Although there is no evidence to recommend general anesthesia for EVT-related procedures. Table 7 summarized indications for general anesthesia based in our clinical practice.
Table 7
| Scenario | Comments |
|---|---|
| First endoscopic evaluation + EVT placement | • Usually a prolonged procedure, most requiring water-immersion evaluation and contrast injection. Furthermore, these patients frequently present with sepsis, increasing the risk of aspiration |
| Need for water-soluble contrast injection and/or water-immersion for upper TGID evaluation | • Reflux can occur and may lead to aspiration and severe pneumonia |
| Prolonged procedure | • For challenging cases prolonged procedure is expected. For these cases general anesthesia is safer than sedation, avoiding CO2 retention and reducing the risk of aspiration |
| High risk of bleeding | • Anticoagulation and/or antiplatelet use |
| • Previous bleeding related to the TGID | |
| • Critically ill patients under ECMO | |
| • Previous coagulation disorders | |
| Esophagus/gastric—pleural fistula | • Not essential for contained collections |
| • Recommended when lavage is required to reduce the risk of aspiration | |
| • For uncontained collections, orotracheal intubation must be performed as gas insufflation or sterile solution infusion can lead to pneumothorax or hydrothorax, respectively. Both conditions can cause hemodynamic instability and respiratory failure if not adequately managed. Mechanical ventilation facilitates the management of these frightful conditions | |
| Painful procedures | • Manipulation of external drains and percutaneous access through the abdominal or thoracic orifice may not be tolerated by patients due to considerable pain stimuli |
| • Placement of two tubes cause more pain and is less traumatic under general anesthesia |
EVT, endoscopic vacuum therapy; TGID, transmural gastrointestinal defect; ECMO, extra corporeal membrane oxygenation.
It is critical to understand that most patients presenting with acute TGID require intensive care unit monitoring after the first interventions. Hypothermia prophylaxis is crucial when the estimated procedure time exceeds 60 minutes or when a high volume of fluid is used (lavage/underwater evaluation). Warm solutions (37 ℃) and forced-air warming blankets are recommended.
Sedation is usually preferred for stable patient during EVT exchanges, mainly for colorectal procedures, and upper GI therapies without requiring lavage or water-soluble contrast injection.
Regarding pain control, analgesic requirements significantly vary among patients, and management should be individualized.
Outpatient procedures can be performed for stable patients with low risk of complications.
Management during EVT and follow-up
Close follow-up is imperative during EVT to achieve clinical success. In most cases 7 to 30 days of hospitalization are needed. Thus, continuous functioning of the device is required, even when there is no infection and a low risk of clinical deterioration.
The ideal vacuum settings are controversial. Most studies reported a negative pressure between 125 and 175 mmHg. In our clinical practice, we select the settings based on the TGID characteristics. Continuous suction with maximum intensity is preferred when a H-EVT system is placed, usually—200 mmHg. When using OPPS, we recommend continuous suction with negative pressure between −125 and 175 mmHg and maximum intensity. Wall suction is used in hospitals with scarce resources to reduce costs. For this approach, after connecting and sealing the NGT with the suction tube, a 20 Fr IV catheter is attached to the tube to maintain a negative pressure between −75 and −150 mmHg (28). This low-cost alternative has several disadvantages compared to the commercially available machine including negative pressure control, no alarms in case of leakage or obstruction, need to disconnected suction every time patient wants to move.
During intracavitary EVT, when an extensive granulation tissue is identified and there are no more signs of infection, we change the EID technique. EVT is concluded and DPS is placed, reducing hospital stay (2). Prior to patient discharge, imaging with water-soluble contrast is carried out. In most cases, an esophagogastroduodenoscopy (EGD) with fluoroscopic assistance is performed.
After 15 to 60 days of hospital discharge, a follow-up EGD is planned. However, if the patient presents GI symptoms, an early EGD is performed.
Avoiding AEs
EVT is safe with a low rate of adverse evets. Substantial discomfort related to the NGT is reported. Additionally, patients under treatment for upper TGID may experience nausea and emesis, particularly when combined with an additional nasoenteral tube. Patients receiving EVT for lower TGID may present tenesmus and secretions extravasation (18,22). Furthermore, prolonged hospital stay and recurrent EVT systems exchanges may cause distress for patients.
Nowadays, in our clinical practice we rarely experience these reported symptoms. Probably, because prior to the procedure, we inform patients and relatives about all advantages and disadvantages of the procedure. Additionally, close follow-up and daily doctor visit is performed. However, in our prior experience, severe pain was reported by EVT for lower TGID. After several reports, we identified that the pain was related to EVT system fixation with suture. Thus, suture is now precluded and the EVT system is fixed with only surgical drapes (28,31,35).
Most AEs are not severe. Device dislocation, system obstruction, and minor bleeding during removal due to the granulation tissue, mainly related to OPPS EVT system are the most common AEs (27,29).
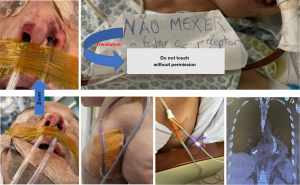
It is mandatory to have extra care with NGT fixation as nasal wing necrosis and nose deformation can occur if the NGT cause continuous compression of the nasal wall. If this happens, EVT placement through the nostrils should terminate. However, alternative approaches such as fixation between the nose and the mouth may be performed (Figure 5).
The main concern regarding EVT for upper TGID is the risk of major bleeding related to the risk of development of a fistula with the aorta or its branches. Formation and rupture of pseudoaneurysm due to the ongoing healing process is also considered a risk for bleeding (48,49). Unfortunately, some major bleeding related to EVT were reported. A prospective study with 52 patients reported two deaths due to major bleeding (50). One death occurred due to acute hemorrhage after 56 days of EVT and the other during the third EVT exchange session after 12 days of therapy. In this case, authors believe that a rupture of the descending aorta occurred. For massive bleeding immediate surgical or radiological management are required.
Post-EVT stricture is also reported as demonstrated in a metanalysis (16), with a mean rate of 12.5%. Recent, a large retrospective study reported a high rate (18.5%) of stenosis post-EVT treatment. The cause of post-EVT strictures is not well known, but this condition also occurs after anastomotic leakage treated with other modalities (36). Occasionally, we face cases of strictures after EVT, all in the esophagus and stomach. Fortunately, most cases are successfully treated with endoscopic dilation. Based on our experience, predictors for post-EVT strictures include esophageal intraluminal OPPS EVT for more than 10 days, esophagogastric anastomotic disconnection, and long ischemic segment after Roux-en-Y gastric bypass (27,29). All cases were successfully treated with endoscopic dilations, with bougies and/or hydrostatic balloon. However, some cases required multiple sessions for achieve clinical success.
Some measures need to be followed to avoid AEs related to EVT. Figure 5 shows some approaches we use to avoid undesired outcomes, such as EVT systems not fixed in the nose due to partial necrosis in both nasal wings that occurred in other institution prior to transference to our hospital. Interestingly, this severe lesion was caused after only 5 days of EVT due to inadequate fixation on the nose. This shows how important it is to understand the mechanism of action and how to manage this patient. Other images demonstrate careful device fixation and measures to avoid EVT system disfunction. The key figure shows a message attached to the EVT system, informing that nobody can manage the system without our authorization. This approach reduces the risk of dislodgement, disconnection of the tubes or inadequate sealing, which could affect the system’s functioning. This message is dedicated to hospital staff without experience with the device. Our trained nursing staff are always allowed to take care of the patient with authorization to manipulate the connections and change the reservoir when needed.
How to deal with patients and companions
The physician’s ability to understand the illness that the patient is going through, communicate and act collaboratively regarding the proposed treatment provides substantial benefits to both patients and physicians (51). The absence of empathy is a real problem when EVT is indicated and can cause severe impacts on outcomes.
EVT is not only an endoscopic therapy that can be rapidly explained to patient’s and relatives. Empathy involves understanding patient’s past experiences, concerns, fears, and expectations. Empathy and trust are key factors for EVT. Without these factors, we advise that the treatment might fail, because in this scenario, patients do not usually support the difficulties related to EVT such as discomfort due to the tube, restrictive oral diet, multiple interventions, and possible prolonged hospital stay.
It is critical to inform the challenges of the disease they are going through and explain in detail all endoscopic approaches available including advantages and disadvantages. Particularly, we show slides from conferences and endoscopic images to improve their understanding and discuss the available data in the literature and our experience including efficacy and safety rates. But we make sure to let them know that besides efficacy is very high, we cannot guarantee treatment success. As EVT is not covered by insurance in our country, costs are informed, and patients and companions are guided to the financial department to make sure they know possible costs in detail. Furthermore, decision-making incorporates patients and companions. If EVT is preferred, we teach patients and companions how the vacuum machine works properly and ask them to immediately inform our team when the machine is not properly functioning. This approach offers the opportunity for them to participate in patient care. Approaching patients and companions with the described empathic behavior and clear communication, avoiding overlap, and raising expectations as described are vital to reaching a friendly relationship, which is critical to achieving a successful treatment. Additionally, the gratitude of critically ill patients after EVT treatment makes this technique astonishing (11,12).
Future perspectives
Pre-emptive EVT
Until now, there is no consensus if pre-emptive EVT (prophylactic EVT) reduces the incidence of anastomotic leaks. Additionally, the best candidates for this approach are unknown. Initial studies demonstrated a low incidence of post-esophagectomy anastomotic leaks when EVT was placed intraoperatively or after early EGD detection of anastomotic ischemia (52,53). In a small series evaluating the outcomes of pre-emptive EVT, 75% of patients had complete healing. Despite 25% of patients presented leaks, all of them were successfully closed maintaining EVT (52). A systematic review with low quality of evidence due to the lack of data, concluded that pre-emptive EVT is safe and could lead to potential benefit for preventing anastomotic leaks, mainly in high-risk anastomosis (54).
A recent pilot study evaluating a novel device combining EVT and stent, named VACStent, reported satisfactory results of its prophylactic use to reduce anastomotic leaks after Ivor-Lewis hybrid esophagectomy. Of nine patients, only one developed a leak, which was successfully treated with the same device (55).
The largest study reporting the results of the pre-emptive EVT to reduce morbidity after minimally invasive Ivor-Lewis esophagectomy showed that 73% of the 67 patients had no complications and 19% required to keep EVT for a longer period to achieve complete healing. Contained leak occurred in 6% of patients and only one patient (1.5%) had an uncontained leak due to gastric conduit necrosis, resulting in an overall anastomotic leak rate of 7.5% (56). Comparing these results with the data provided by a recent meta-analysis (57) evaluating the outcomes (morbidity: 39%, major morbidity: 20%, anastomotic leak: 8%, and mortality: 2%) of the same surgical technique but without the use of pre-emptive EVT. It appears that pre-emptive EVT will play an important role to reduce postoperative morbidity after minimally invasive Ivor-Lewis esophagectomy, remarkably in high-risk anastomosis and in patients with deteriorated clinical and nutritional status.
The prophylactic use of EVT for anastomotic dehiscence after rectal anterior resection is also successful reported encouraging its use, especially in redo surgery and in cases of inflammation, extensive radiation, and after severe stapler dysfunction (58-60).
In our practice, we perform pre-emptive EVT in high-risk anastomosis after GI surgery, mainly for colorectal anastomosis. The procedure is easy and does not disturb the surgical and anesthesia teams. Furthermore, it is performed in less than five minutes, thus, does not lengthen the anesthesia. The pre-emptive EVT use is planned for three days. However, if the device migrates and the patient has no complaints, we do not perform rectosigmoidoscopy and/or EVT replacement. In our opinion, pre-emptive should be indicated for high-risk anastomosis and if the surgeon is not confident. Additionally, if a nasoenteral feeding tube will be placed after a high-risk upper GI surgery, why not place a TLT with an EVT system in its gastric (aspiration) portion as the patient will have a tube through the nostril anyway. This approach will provide enteral nutrition with an additional benefit of reducing morbidity.
Treatment of AEs after endoscopic resection
Data evaluating the use of EVT after endoscopic resection is scarce. Recent, its successful use was reported for a leak after endoscopic submucosal dissection (61). In our experience intraluminal EVT use is associated with favorable outcomes after large endoscopic resection, especially in the duodenum and right colon. EVT is only used in cases that a perforation occurred or as a pre-emptive measure if we considered that there is a high risk of late complications and mucosal closure was not performed. Additionally, EVT is profitable after endoscopic full-thickness resections.
EVT for giant GI ulcers and upper GI bleeding
First, it is important to state that there are few case reports of EVT for upper GI ulcers and bleeding, all performed by our group. Thus, more data are warranted to confirm our findings (8,62,63).
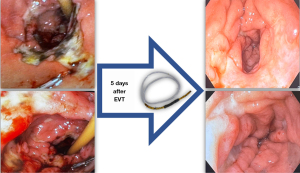
The mechanisms of action of EVT have a powerful effect on healing and might contribute to hemostasis, especially in cases of diffuse bleeding related to severe inflammatory response, tissue ischemia, and radiation induced ulcer (Figure 6). EVT benefits include tissue microdeformation and macrodeformation, gastric and biliopancreatic secretions clearance, exudate control, reduction in intraluminal pressure, and changes in perfusion.
Considering giant duodenal and antral ulcers refractory to conservative management (lifestyle modification and medications), EVT may be considered prior to surgery, aspiring to reduce the high rate of complications related to surgical intervention. For upper GI bleeding, EVT might be used for bleeding related to TGID, diffuse bleeding due to severe local inflammation), large ulcers, and refractory bleeding after conventional therapies hemostasis (Figure 7) (62-64).
As most patients with life-threatening illnesses need to use an enteral feeding tube and a considerable portion of patients with GI hemorrhage and/or giant ulcers do not receive oral diet, the advantages of the TLT with the H-EVT (TLT-H-EVT) must be highlighted. As the majority of these patients will have one tube through the nostril anyway, the TLT-H-EVT should be preferred, allowing for both healing and enteral nutrition as the enteral portion of the TLT is positioned distally from the ulcer and/or bleeding site, usually at the proximal jejunum. This strategy appears to be safe and effective and can be considered an option when conventional therapies fail.
Conclusions
EVT may be considerer the first-line therapy for the management of TGID due to its safety and efficacy as a primary or rescue therapy, as an individual or adjunctive therapy, with or without associated collection, regardless of defect location or duration.
Despite its favorable outcomes in multiple studies, EVT is not adopt in many centers worldwide. The reasons are probably related to the low quality of evidence available in the literature, mainly randomized controlled trials and to technical aspects, such as challenging device placement and removal, need for multiple exchanges, and patients’ discomfort caused by the device.
In the last decade, EVT use has been increasing, and more possible indications are now under investigation. The prophylactic use of EVT seems to reduce post-surgical morbidity and may turn into a common approach for high-risk anastomosis. Furthermore, based on our initial experience, EVT may play a role in the treatment of GI hemorrhage, mainly for refractory bleeding after conventional therapies hemostasis.
Finally, the homemade/modified EVT types will expand EVT use by providing less-invasive treatment to more patients worldwide, especially in resource-scarce settings.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-86/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-86/coif). D.T.H.d.M. serves as an unpaid editorial board member of Translational Gastroenterology and Hepatology from June 2023 to May 2025. D.T.H.d.M. reports consulting fees from BariaTek Advanced Bariatric Solutions as advisory board member and honoraria for lectures from Boston Scientific. H.G.G. reports honoraria for lectures from TOPMED Medical Supplies. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weidenhagen R, Spelsberg F, Lang R, et al. New method for sepsis control caused by anastomotic leakage in rectal surgery: the Endo-VAC. Color Dis 2003;5:1-4. [Crossref]
- de Moura DTH, Hirsch BS, McCarty TR, et al. Homemade endoscopic vacuum therapy device for the management of transmural gastrointestinal defects. Dig Endosc 2023;35:745-56. [Crossref] [PubMed]
- de Moura DTH, Brunaldi VO, Minata M, et al. Endoscopic vacuum therapy for a large esophageal perforation after bariatric stent placement. VideoGIE 2018;3:346-8. [Crossref] [PubMed]
- de Moura DTH, Hirsch BS, de Medeiros Neto HC, et al. Endoscopic Treatment of Non-malignant Esophageal Perforation: Time to Go Vacuum? Curr Treat Options Gastroenterol 2023;21:95-124. [Crossref]
- Bestetti AM, Santo MA, Trasolini RP, et al. Sequential Endoscopic Therapies for Treatment of Complex Gastrointestinal Transmural Leak Following Bariatric Surgery. Obes Surg 2022;32:4113-4. [Crossref] [PubMed]
- Loske G, Müller CT. Tips and tricks for endoscopic negative pressure therapy. Chirurg 2019;90:7-14. [Crossref] [PubMed]
- Dos Santos NT, De Moura DTH, Galvão Neto M, et al. Novel laparo-endoscopic hybrid procedure to treat a disconnected Roux limb after bariatric surgery. Endoscopy 2019;51:E341-2. [Crossref] [PubMed]
- de Moura DTH, do Monte ES Junior, Hathorn KE, et al. Modified endoscopic vacuum therapy in the management of a duodenal transmural defect. Endoscopy 2021;53:E17-8. [Crossref] [PubMed]
- de Moura DTH, do Monte ES Junior, Hathorn KE, et al. The use of novel modified endoscopic vacuum therapies in the management of a transmural rectal wall defect. Endoscopy 2021;53:E27-8. [Crossref] [PubMed]
- de Moura DTH, Sasso JGRJ, Ribas PHBV, et al. Novel less-invasive therapy for liver abscess: combining lavage and draining through a single device. iGIE 2023;2:18-21.
- de Moura DTH, Sachdev AH, Thompson CC. Endoscopic Full-Thickness Defects and Closure Techniques. Curr Treat Options Gastroenterol 2018;16:386-405. [Crossref] [PubMed]
- de Moura DTH, de Moura BFBH, Manfredi MA, et al. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc 2019;11:329-44. [Crossref] [PubMed]
- Scognamiglio P, Reeh M, Karstens K, et al. Endoscopic vacuum therapy versus stenting for postoperative esophago-enteric anastomotic leakage: systematic review and meta-analysis. Endoscopy 2020;52:632-42. [Crossref] [PubMed]
- Aziz M, Haghbin H, Sharma S, et al. Safety and effectiveness of endoluminal vacuum-assisted closure for esophageal defects: Systematic review and meta-analysis. Endosc Int Open 2021;9:E1371-80. [Crossref] [PubMed]
- do Monte ES Junior, de Moura DTH, Ribeiro IB, et al. Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: Systematic review and meta-analysis. Dig Endosc 2021;33:892-902. [PubMed]
- Tavares G, Tustumi F, Tristão LS, et al. Endoscopic vacuum therapy for anastomotic leak in esophagectomy and total gastrectomy: a systematic review and meta-analysis. Dis Esophagus 2021;34:doaa132. [Crossref] [PubMed]
- Intriago JMV, de Moura DTH, do Monte ES Junior, et al. Endoscopic Vacuum Therapy (EVT) for the Treatment of Post-Bariatric Surgery Leaks and Fistulas: a Systematic Review and Meta-analysis. Obes Surg 2022;32:3435-51. [Crossref] [PubMed]
- Kühn F, Schardey J, Wirth U, et al. Endoscopic vacuum therapy for the treatment of colorectal leaks - a systematic review and meta-analysis. Int J Colorectal Dis 2022;37:283-92. [Crossref] [PubMed]
- Laopeamthong I, Akethanin T, Kasetsermwiriya W, et al. Vacuum Therapy and Internal Drainage as the First-Line Endoscopic Treatment for Post-Bariatric Leaks: A Systematic Review and Meta-Analysis. Visc Med 2022;38:63-71. [Crossref] [PubMed]
- Mandarino FV, Barchi A, Fanti L, et al. Endoscopic vacuum therapy in the treatment of postesophagectomy leaks: Is intracavitary the way? Gastrointest Endosc 2022;96:873. [Crossref] [PubMed]
- Scognamiglio P, Reeh M, Melling N, et al. Management of intra-thoracic anastomotic leakages after esophagectomy: updated systematic review and meta-analysis of endoscopic vacuum therapy versus stenting. BMC Surg 2022;22:309. [Crossref] [PubMed]
- Shelygin YA, Nagudov MA, Ponomarenko AA, et al. Meta-analysis of management of colorectal anastomotic leakage. Khirurgiia (Mosk) 2018;(8. Vyp. 2):30-41.
- Rausa E, Asti E, Aiolfi A, et al. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus 2018;31: [Crossref] [PubMed]
- de Lacy FB, Talboom K, Roodbeen SX, et al. Endoscopic vacuum therapy and early surgical closure after pelvic anastomotic leak: meta-analysis of bowel continuity rates. Br J Surg 2022;109:822-31. [Crossref] [PubMed]
- Mandarino FV, Barchi A, Fanti L, et al. Endoscopic vacuum therapy for post-esophagectomy anastomotic dehiscence as rescue treatment: a single center case series. Esophagus 2022;19:417-25. [Crossref] [PubMed]
- De Pasqual CA, Mengardo V, Tomba F, et al. Effectiveness of endoscopic vacuum therapy as rescue treatment in refractory leaks after gastro-esophageal surgery. Updates Surg 2021;73:607-14. [Crossref] [PubMed]
- Loske G, Rucktaeschel F, Schorsch T, et al. Endoscopic negative pressure therapy (ENPT) for duodenal leakage - novel repair technique using open-pore film (OFD) and polyurethane-foam drainages (OPD). Endosc Int Open 2019;7:E1424-31. [Crossref] [PubMed]
- de Moura DTH, Hirsch BS, Do Monte ES Junior, et al. Cost-effective modified endoscopic vacuum therapy for the treatment of gastrointestinal transmural defects: step-by-step process of manufacturing and its advantages. VideoGIE 2021;6:523-8. [Crossref] [PubMed]
- Loske G, Schorsch T, Rucktaeschel F, et al. Open-pore film drainage (OFD): a new multipurpose tool for endoscopic negative pressure therapy (ENPT). Endosc Int Open 2018;6:E865-71. [Crossref] [PubMed]
- Sánchez-Luna SA, Thompson CC, De Moura EGH, et al. Modified endoscopic vacuum therapy: Are we ready for prime time? Gastrointest Endosc 2022;95:1281-2. [Crossref] [PubMed]
- de Lima MS, Figueiredo LZ, Furuya CK Jr, et al. Tube-in-tube endoscopic vacuum therapy for treatment of colorectal anastomotic leaks: A low-cost, patient-friendly, feasible and efficient technical modification of sponge-based endoscopic vacuum therapy. Colorectal Dis 2023;25:1519-22. [Crossref] [PubMed]
- Valli PV, Mertens JC, Kröger A, et al. Stent-over-sponge (SOS): a novel technique complementing endosponge therapy for foregut leaks and perforations. Endoscopy 2018;50:148-53. [Crossref] [PubMed]
- Lange J, Dormann A, Bulian DR, et al. VACStent: Combining the benefits of endoscopic vacuum therapy and covered stents for upper gastrointestinal tract leakage. Endosc Int Open 2021;9:E971-6. [Crossref] [PubMed]
- Chon SH, Töx U, Lorenz F, et al. A Novel Hybrid Stent with Endoscopic Vacuum Therapy for Treating Leaks of the Upper Gastrointestinal Tract. Visc Med 2021;37:403-9. [Crossref] [PubMed]
- de Moura DTH, Hirsch BS, Boghossian MB, et al. Low-cost modified endoscopic vacuum therapy using a triple-lumen tube allows nutrition and drainage for treatment of an early post-bariatric surgery leak. Endoscopy 2022;54:E376-7. [Crossref] [PubMed]
- Jung DH, Huh CW, Min YW, et al. Endoscopic vacuum therapy for the management of upper GI leaks and perforations: a multicenter retrospective study of factors associated with treatment failure (with video). Gastrointest Endosc 2022;95:281-90. [Crossref] [PubMed]
- Momblan D, Gimeno Garcia AZ, Busquets D, et al. Endoscopic Vacuum Therapy for Upper Gastrointestinal Leaks and Perforations: Analysis From a Multicenter Spanish Registry. Am J Gastroenterol 2023;118:1797-806. [Crossref] [PubMed]
- Lee JH, Kedia P, Stavropoulos SN, et al. AGA Clinical Practice Update on Endoscopic Management of Perforations in Gastrointestinal Tract: Expert Review. Clin Gastroenterol Hepatol 2021;19:2252-2261.e2. [Crossref] [PubMed]
- Paspatis GA, Arvanitakis M, Dumonceau JM, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement - Update 2020. Endoscopy 2020;52:792-810. [Crossref] [PubMed]
- Schäfer C. Don't be afraid of black holes: Vacuum sponge and vacuum stent treatment of leaks in the upper GI tract-a case series and mini-review. Front Surg 2023;10:1168541. [Crossref] [PubMed]
- Mandarino FV, Barchi A, Leone L, et al. Endoscopic vacuum therapy versus self-expandable metal stent for treatment of anastomotic leaks < 30 mm following oncologic Ivor-Lewis esophagectomy: a matched case-control study. Surg Endosc 2023;37:7039-50. [Crossref] [PubMed]
- de Moura DTH, de Moura EGH, Neto MG, et al. Outcomes of a novel bariatric stent in the management of sleeve gastrectomy leaks: a multicenter study. Surg Obes Relat Dis 2019;15:1241-51. [Crossref] [PubMed]
- Okazaki O, Bernardo WM, Brunaldi VO, et al. Efficacy and Safety of Stents in the Treatment of Fistula After Bariatric Surgery: a Systematic Review and Meta-analysis. Obes Surg 2018;28:1788-96. [Crossref] [PubMed]
- Sánchez-Luna SA, De Moura EGH, De Moura DTH. Bigger is not always better for the endoscopic treatment of sleeve gastrectomy (SG) leaks using fully covered stents. Obes Surg 2022;32:1764-7. [Crossref] [PubMed]
- Hamid HKS, Emile SH, Saber AA, et al. Customized bariatric stents for sleeve gastrectomy leak: are they superior to conventional esophageal stents? A systematic review and proportion meta-analysis. Surg Endosc 2021;35:1025-38. [Crossref] [PubMed]
- Ribeiro Jordão Sasso JG, Yvamoto EY, Rocha RSP, et al. Modified endoscopic vacuum therapy for hypopharyngeal acute leakage after foreign body perforation. Endoscopy 2022;54:E1022-3. [Crossref] [PubMed]
- Silveira SQ, da Silva LM, de Campos Vieira Abib A, et al. Relationship between perioperative semaglutide use and residual gastric content: A retrospective analysis of patients undergoing elective upper endoscopy. J Clin Anesth 2023;87:111091. [Crossref] [PubMed]
- Virgilio E, Ceci D, Cavallini M. Surgical Endoscopic Vacuum-assisted Closure Therapy (EVAC) in Treating Anastomotic Leakages After Major Resective Surgery of Esophageal and Gastric Cancer. Anticancer Res 2018;38:5581-7. [Crossref] [PubMed]
- Pournaras DJ, Hardwick RH, Safranek PM, et al. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J Surg 2018;42:2507-11. [Crossref] [PubMed]
- Laukoetter MG, Mennigen R, Neumann PA, et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 2017;31:2687-96. [Crossref] [PubMed]
- Gertsman S, Ene IC, Palmert S, et al. Clinical empathy as perceived by patients with chronic illness in Canada: a qualitative focus group study. CMAJ Open 2023;11:E859-68. [Crossref] [PubMed]
- Neumann PA, Mennigen R, Palmes D, et al. Pre-emptive endoscopic vacuum therapy for treatment of anastomotic ischemia after esophageal resections. Endoscopy 2017;49:498-503. [Crossref] [PubMed]
- Gubler C, Vetter D, Schmidt HM, et al. Preemptive endoluminal vacuum therapy to reduce anastomotic leakage after esophagectomy: a game-changing approach? Dis Esophagus 2019;32:doy126. [Crossref] [PubMed]
- Adamenko O, Ferrari C, Seewald S, et al. Prophylactic endoluminal vacuum therapy after major gastrointestinal surgery: a systematic review. Updates Surg 2022;74:1177-86. [Crossref] [PubMed]
- Lange J, Eisenberger CF, Knievel J, et al. Preemptive endoluminal vacuum therapy with the VACStent-A pilot study to reduce anastomotic leakage after Ivor Lewis hybrid esophagectomy. Front Surg 2023;10:1133083. [Crossref] [PubMed]
- Müller PC, Morell B, Vetter D, et al. Preemptive Endoluminal Vacuum Therapy to Reduce Morbidity After Minimally Invasive Ivor Lewis Esophagectomy: Including a Novel Grading System for Postoperative Endoscopic Assessment of GI-Anastomoses. Ann Surg 2021;274:751-7. [Crossref] [PubMed]
- Casas MA, Angeramo CA, Bras Harriott C, et al. Surgical outcomes after totally minimally invasive Ivor Lewis esophagectomy. A systematic review and meta-analysis. Eur J Surg Oncol 2022;48:473-81. [Crossref] [PubMed]
- Lehwald-Tywuschik NC, Alexander A, Alkhanji N, et al. The "impossible" rectal anastomosis: a novel use for endoluminal vacuum-assisted therapy. Tech Coloproctol 2021;25:125-30. [Crossref] [PubMed]
- Mandarino FV, Barchi A, Biamonte P, et al. The prophylactic use of endoscopic vacuum therapy for anastomotic dehiscence after rectal anterior resection: is it feasible for redo surgery? Tech Coloproctol 2022;26:319-20. [Crossref] [PubMed]
- Lehwald-Tywuschik NC, Alexander A, Alkhanji N, et al. Author's reply to Mandarino et al.: The prophylactic use of endoscopic vacuum therapy for anastomotic dehiscence after rectal anterior resection-isit feasible for redo surgery? Tech Coloproctol 2022;26:321. [Crossref] [PubMed]
- Chaves J, Ventura S, Vasconcelos AC, et al. Efficacy of endoluminal vacuum therapy for the treatment of a rectal leak after endoscopic submucosal dissection. Gastrointest Endosc 2024;99:657-9. [Crossref] [PubMed]
- de Moura DTH, de Moura EGH, Hirsch BS, et al. Modified endoscopic vacuum therapy for duodenal hemorrhage in patients with severe acute respiratory syndrome coronavirus 2. Endoscopy 2022;54:E837-9. [Crossref] [PubMed]
- de Moura DTH, de Moura EGH, Hirsch BS, et al. Endoscopic Vacuum Therapy for Duodenal Hemorrhage in Critically Ill Patients With COVID-19. Am J Gastroenterol 2022;117:688. [Crossref] [PubMed]
- de Moura DTH, Hirsch BS, da Conceicao Vasconcelos KGM, et al. A novel less-invasive therapy for a bleeding eroded artery in a giant duodenal ulcer: principles and technical description. iGIE 2022;1:15-18.
Cite this article as: de Moura DTH, Hirsch BS, Ribas PHBV, Silveira SQ, Guedes HG, Bestetti AM. Endoscopic vacuum therapy: pitfalls, tips and tricks, insights, and perspectives. Transl Gastroenterol Hepatol 2024;9:50.


