
The role of arousal in maintaining the relationship between insomnia and gastrointestinal conditions
Introduction
In recent years, research examining interaction between gastrointestinal (GI) conditions and sleep disturbances has accelerated. This relationship has been established in both fields: rates of sleep disorders are elevated in people with many GI conditions, while GI conditions are more common in people with sleep disorders. Broadly, poor sleep quality is endorsed by around 25% of the general population (1), with rates two to three times higher in GI populations (2-5). Research has focused primarily on restless legs syndrome (also called Willis-Ekbom disease), obstructive sleep apnea, and insomnia. Restless legs syndrome involves very unpleasant sensations in ones’ legs combined with the urge to move, predominantly at night. Across GI conditions, it appears to be elevated most in people with irritable bowel syndrome (IBS) (6), with either limited research or similar rates to the general populations in other GI conditions (7,8). Obstructive sleep apnea, a sleep-related breathing disorder, involves decreased airflow during sleep resulting in snoring and/or gasping during sleep and daytime sleepiness. Research suggests a complex association between obstructive sleep apnea and gastroesophageal reflux disease (GERD) and elevated rates of IBS in people with obstructive sleep apnea (4,9-12). Most commonly investigated is insomnia—difficulty falling asleep, staying asleep, or early morning awakening combined with daytime impairment (13). Rates of insomnia may be at least five times those of the general population in inflammatory bowel disease (IBD), IBS, and functional dyspepsia (FD) (2,5,8,14).
To detail the interactions between sleep disturbances and GI conditions, recent reviews have described the prevalence rates, clinical considerations, and emerging evidence for both physiological mechanisms and interventions (15,16). However, comprehensive consideration of numerous GI and sleep diagnoses naturally necessitated broad content. Herein, we sought to dive more deeply into the specific relationship between insomnia and GI symptoms, given the high prevalence rates and more robust literature. In particular, we highlight arousal as both a unifying feature of insomnia and GI symptoms and a possible mechanism of action for the bidirectional relationship. Heightened arousal is a key maintenance factor in both insomnia and numerous GI conditions (17-20).
The current review considers evidence for arousal as a common factor potentially responsible for the co-occurrence of insomnia and GI symptoms. To facilitate a wide range of focus for the literature review, the authors posed the following initial research questions to guide the search process:
- What are factors that impact both sleep and GI conditions?
- How are these common factors related to arousal in general?
Following the preliminary search, we then aimed to identify all articles addressing specific factors that were relevant to both sleep and GI conditions. A comprehensive search was conducted in PubMed, PsychInfo and Google Scholar. The research team met and agreed on the databases to be used for this review. For the study selection, articles were included if they (I) were in English, (II) involved patients with insomnia and/or patients with GI conditions, and (III) involved mental health, physical comorbidities, and social factors seen in insomnia and GI conditions. Articles that were excluded were non-peer reviewed materials, including dissertations, manuscripts, and conference abstracts. No other exclusion criteria were set. All authors were in agreement with the included articles. The articles chosen for the final analysis ranged from years 2007 to 2023. Since this was a review article, ethical approval was not required.
Based on the results of the literature search, we developed an organizational schematic that depicts some of the proximal and distal influences of arousal on the sleep/GI relationship across four domains: psychosocial factors, physical health factors, daily living factors, and sociocultural factors. Figure 1 provides a graphical overview of this model.
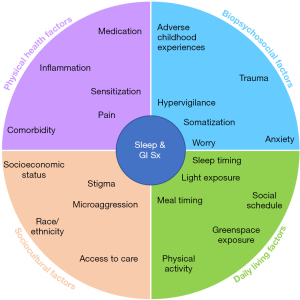
Psychosocial factors
Complex interactions between biological, environmental, and psychological factors may impact arousal and often contribute to the development and maintenance of GI conditions and sleep disorders. Psychosocial factors that may impact this relationship include early adverse life events, perceived stress and stressful life events, psychological comorbidities, and cognitive and affective patterns.
Early adverse events
Adverse childhood experiences (ACEs) including abuse, neglect, poverty, exposure to domestic violence, or family members with mental illness or incarceration may increase the risk for developing GI conditions such as IBS and IBD (21,22). Ju et al. (21) examined the prevalence of early adverse events amongst a large group of IBS patients and found that a higher number of adverse life events was associated with increased odds of having the disease. The study specifically found that experiencing a sexual trauma as well as being a victim of other types of violence increased the chances of IBS in an individual. Other studies have found that relative to other types of ACEs, emotional abuse and having a household member who was diagnosed with mental illness or with a history of incarceration was predictive of IBS (22). Similarly, the presence of ACEs during childhood is associated with sleep pathology in adulthood. A recent systematic review demonstrated that having an adverse childhood event was associated with poor sleep quality and sleep disorders. Specifically, childhood ACEs seemed to be an important predictor of increased sleep onset latency, decreased sleep efficiency and higher movement arousals among individuals with primary insomnia (23). Overall, a higher number of ACEs and the severity of such experiences was associated with increased risk for sleep disorders (24). The occurrence of adverse life events or early childhood events may directly impact both GI conditions and sleep quality through arousal. At a mechanistic level, a history of early adverse life events is known to be associated with increased stress response and hypothalamic pituitary adrenal (HPA) axis dysregulation thought to confer deleterious effects on motility and intestinal permeability in IBS (25,26). Similarly, several studies have shown elevated cortisol activity and hyperarousal of the HPA axis being a key factor in the maintenance of sleep problems (24).
Perceived stress and stressful life events
Although there are mixed results in the literature, the research broadly suggests an association between perceived stress and disease exacerbation in GI symptoms (27). Possible interactions between GI symptoms and insomnia in the context of perceived stress have been explored as well. In a multi-center study, patients with IBS broadly identified psychological stress as the main reason for symptom exacerbation and IBS flares. Cross-sectional analyses demonstrated that insomnia was associated with symptom severity in patients who reported higher levels of perceived stress (28). Perceived stress is also known to precede exacerbations in IBD (27). Similarly, GERD also was related to perceived stress and higher pain ratings (29).
Psychological comorbidities
It has been well established that there is a strong association between GI symptoms and specific mental health conditions, including anxiety and depression. Prevalence of mental health disorders and sleep disturbances is higher in those with GI conditions compared to the general population (30-32).
Recent evidence has shown that psychological symptoms are present in both disorders of gut-brain interaction (DGBI) as well as in organic GI disorders, including IBD and celiac disease. For instance, patients with IBS reported more anxiety and depressive symptoms compared to controls (33,34); specifically more anxiety symptoms with IBS-diarrhea (IBS-D) and IBS-constipation (IBS-C) and more depressive symptoms in IBS-D (35). Visceral hypersensitivity and GI-specific anxiety, including fear of GI symptoms, were found unique to IBS patients with psychological symptoms (34). Anxiety symptoms have also been identified in other DGBIs including FD and functional constipation.
Post-traumatic stress disorder (PTSD) is another common mental health condition known to be a risk factor in the development of both IBS and IBD. A meta-analytic study by Ng et al. concluded that PTSD was significantly associated with disease emergence among IBD patients (36). Another study found that 9.6% of those with IBD met the criteria for PTSD related to their disease, reporting arousal-eliciting symptoms such as reexperiencing and hypervigilance specifically about GI symptoms. Furthermore, the presence of post-traumatic stress symptoms predicts rates of remission and health care utilization (37). At the same time, the presence of post-traumatic stress impairs sleep quality, including delayed onset, decreased sleep efficiency and frequent awakenings. The number of traumas experienced may be predictive of the experience of trauma related nightmares as well as insomnia (26,38).
In conclusion, there exists a complex relationship between sleep, GI conditions and psychological comorbidities in that not only are anxiety, depression and PTSD related to sleep disturbances, but studies have shown that poor sleep and the presence of depression and PTSD can predict worsening of GI symptoms (15).
Cognitive and affective factors
If arousal plays a role in the maintenance of both insomnia and GI symptoms, the various ways in which psychological processes contribute to arousal are important to consider. Arousal can take the form of hypervigilance of symptoms in certain GI conditions and can contribute to exacerbation of symptoms over time (19,37,39). Other common cognitive patterns include catastrophizing, where the individual tends to focus on worst possible outcomes, and negatively assess one’s ability to cope with pain and illness. Catastrophizing is associated with frequency and severity of abdominal pain and poor quality of life in IBS (40). For example, patients with IBS may hyperfocus on normal abdominal sensations and interpret them as a threat by automatically assuming disastrous medical implications or social rejection, for example, which in turn results in unhelpful behaviors such as social isolation. Similarly, catastrophic thinking patterns are commonly seen in sleep disorders as well (41). Treatment modalities such as cognitive-behavioral therapy (CBT) can help target arousal, reduce negative patterns of thinking, and help with cognitive restructuring to promote a more adaptive response to the illness. Specifically, CBT targeting visceral hypersensitivity and autonomic arousal via interoceptive exposures help reduce GI symptoms in IBS (42). Mindfulness-based therapy (MBT) helps address visceral hypersensitivity using meditation, relaxation and non-judgmental cognitions about one’s experiences (26).
Physical health factors
Physical health factors including disease pathologies, symptoms, and their treatments often play a governing role in arousal state. At a clinical level, these can include both medical comorbidities and treatments, and they can quickly compound when navigating potential treatment side effects. At a systemic level, pathophysiological mechanisms such as inflammation, visceral interoception, and related pain experience are commonly implicated and/or affected by increased arousal state.
Comorbidity
Individuals with certain GI conditions may be at increased likelihood for symptom-related pain or sympathetic arousal based on the pathophysiology and clinical symptoms of that GI condition. Unfortunately, many GI conditions are also associated with comorbid conditions, including co-occurring with other GI illnesses. As such, symptoms causing physiological activation arising from comorbid conditions may predict sleep disturbance independent of the index condition. For instance, IBS, FD, and GERD are common co-occurring conditions (43), each of which is associated with sleep disturbance and can in-turn worsen as a result of sleep deficiency (14,44-46). In another example, approximately 40% of patients with IBD in remission meet symptom criteria for a DGBI, oftentimes IBS. The visceral pain, bowel dysmotility, and psychological stress associated with IBS may drive disturbance in sleep quality and duration even if the IBD is in remission.
Non-GI comorbidities also contribute to insomnia. For example, sleep duration and quality is directly impacted by bladder dysfunction including nocturia and bladder pain (interstitial cystitis) (47,48). And patients with IBS are much more likely to experience bladder dysfunction and report bladder symptoms like nocturia, urgency, and urge incontinence to the extent that some suggest IBS can be considered a risk factor for bladder pain syndrome (interstitial cystitis) (49). This overlap makes sense when considering the common sensory signaling pathways shared between the urological and GI systems as a hypothesized mechanism underlying this connection (50). An individual with this comorbidity may then be more likely to experience urological symptoms driving insomnia.
Medication
Several classes of medication can affect physiological arousal and its influence on the Sleep/GI relationship. Classes of medications commonly used to treat various medical disorders are worth consideration:
Corticosteroids
Many acute inflammatory processes in various disease presentations, including IBDs, are treated with corticosteroid medications (e.g., prednisone). In a very general sense, these medications influence signaling of inflammatory pathways, leading to overall suppression of activated inflammatory response. However, these glucocorticoid-based medications are also known to affect the body’s neuroendocrine processes in the stress response, which relies on the fundamental role of naturally produced glucocorticoids in the body (51). The end result is the now well-documented correlation between corticosteroid treatment and autonomic arousal. Indeed, changes in mood, increase in perceived anxiety symptoms, and insomnia have been associated with any duration of corticosteroid use (52).
Stimulants
Stimulant medications are often prescribed for certain psychiatric disorders, among other conditions. Such medications include methylphenidate, amphetamine, and dextroamphetamine. Some studies indicate that use of these medications is associated with acute cortisol increase and concurrent increase in autonomic response (increased heart rate, arterial pressure). Others show overall no sustained change in cortisol levels over time (6+ months) in individuals regularly taking stimulant medications (53). Therefore, it may be reasonable to conclude that initiation of stimulant use may have near-term effects on arousal-mediated sleep and GI function but these effects may not persist with regular use long term. Stimulant use may also occur through non-prescribed mechanisms. For example, caffeine is known to cause state increase in arousal via common neuroendocrine pathways (e.g., centrally and peripherally acting cortisol, adrenaline, and noradrenaline) (54). Not surprisingly, caffeine’s effect of increased arousal and sleep disturbance has been well described (55,56). As such, medications containing caffeine can affect arousal and the associated sleep/GI relationship.
Other medications to keep in mind
Oral contraceptives in females have been hypothesized to affect resting autonomic functioning and autonomic reflex based on modulating effect of gonadal hormones. Some studies have found increase in indicators of autonomic arousal with oral contraceptive treatment (57) while others have not (58). A recent systematic review reflects mixed findings based on heterogeneity of study methodology (59). Likewise, the effects of contraceptives on sleep are mixed, and may depend on hormone basis of the oral contraceptive and/or the route of administration [e.g., local intrauterine contraceptive device (IUD) vs. oral] (60). There is some evidence to suggest that individuals treated with thyroid replacement medications experience an increase in anxiety compared to controls (61). Finally certain antihistamines, although commonly associated with sedation or drowsiness, have been reported to cause paradoxical sympathetic excitation in a subset of individuals with certain genetic profiles (62).
Inflammation, sensitization, and pain
A comprehensive review of the interacting neuroimmune and neuroendocrine systems in the human body is outside the scope of this paper. However, it is by now widely understood that inflammatory processes are intimately intertwined with the stress response, such that sympathetic activation can promote an inflammatory response and prolonged stress response can lead to sympathetic sensitization, dysregulation in inflammatory processes, and in turn further potentiated stress response. And both visceral pain perception and insomnia promote the body’s stress response resulting in downstream endocrine and inflammatory changes. Illustrating these common physiological processes, research indicates that both chronic sleep disturbance, and chronic GI conditions are associated with increased pro-inflammatory signaling. Reduced sleep duration is associated with increased plasma tumor necrosis factor alpha (TNF-α) (63), which is as also elevated in patients with IBS compared to healthy control (HCs) (64-66). Likewise, circulating interleukin-6 (IL-6), another inflammatory cytokine, is relatively higher in those with poor sleep, and as well individuals diagnosed with IBDs. Thus far, research suggests that behavioral insomnia treatment can improve insomnia and IBS symptoms (67,68), though one study found no significant change in IL-6 and C-reactive protein (CRP) with in individuals with insomnia and comorbid IBS (68).
Visceral pain and visceral sensitization are common symptoms of many digestive disorders across the GI spectrum and can be a result of acute inflammatory response to several factors including tissue injury or infection. Chronic GI pain is an endogenous source of physiological and psychological distress in digestive disease and a pain flare recruits the sympathetic stress response in the same way an external stressor might. Chronic GI pain, and more broadly visceral distress, can eventuate increased visceral interoceptive sensitivity wherein sensations of pain, pressure, or even heat are more readily detected, and therefore more easily stimulate a stress response (69). Many of the same physiological processes perpetuating chronic pain are as well involved in the sleep regulation and, unsurprisingly, the mutually influential relationship between chronic pain, interoception, and sleep disturbance has been well established (70,71). Implicated overlapping neurobiological mechanisms are numerous, including the serotonergic and noradrenergic systems at both central and peripheral locations (72). Noradrenaline, for example, is produced centrally in the locus coeruleus of the brain, projecting to other brain areas but as well via adrenal glands where it is released in the bloodstream playing a key role in the sympathetic stress response. This adrenergic receptor-mediated physiological state change has the effect of, among others, changes in motility. Furthermore, sleep quality appears to be a reliable predictor of somatic pain perception and sleep deficiency has been associated with increased perceived bowel urgency and dysmotility in individuals with functional constipation (71,73). Considering common inflammatory and endocrine pathways along with documented clinical interrelationships, we can start to draw a reasonably clear line connecting arousal, sleep disturbance, and GI functioning where both GI distress and sleep disturbance are simultaneously affected by stress, and are stressors themselves.
Daily living factors
Direct and indirect daily living factors may interact with one another to regulate when arousal or activity levels are high or low, or whether they match the demand characteristics of a situation. Some daily living factors may be modifiable (e.g., exercise, caffeine intake) whereas others may require intervention or major changes in lifestyle to address (e.g., circadian rhythms).
Circadian rhythms
While the body’s central circadian clock in the suprachiasmatic nucleus is regulated by the light/dark cycle, other systems in the body have their own clocks, coordinated by, but semi-independent from, the central pacemaker and entrainable to other processes. In the GI tract, the hepato-intestinal clock is regulated by food/eating timing (74,75). In healthy individuals, the central and hepatointestinal clocks are aligned; when desynchrony between these clocks occurs, individuals can experience both metabolic and GI changes (74). Thus, in the relationship between sleep and GI conditions, both light and food exposure are critical timekeepers (or zeitgebers) for regulating internal processes.
Further supporting the relationship between GI symptoms and circadian rhythms, research suggests that shift work is associated with an increased risk for a variety of GI conditions and diseases, including GERD (76), erosive esophagitis (77), and IBS (78). Researchers investigating shiftwork and IBS also demonstrated that increased risk generally persists above and beyond the impact of poor sleep quality and may be particularly pronounced in people with rotating shift schedules. The importance of understanding circadian rhythms also applies to IBD, where research has identified a relationship between IBD and dysregulation of clock genes (79). Research in IBD has also demonstrated an association between evening chronotype (i.e., night owls) and greater fatigue (80), more self-reported Crohn’s symptoms (8), and reduced IBD-related quality of life (81). Finally, emerging experimental research suggests that nausea while eating is more likely to occur in one’s biological night (82).
Examples of environmental or behavioral processes to consider in the sleep/GI relationship are as follows:
Sleep timing
Broadly, individuals with evening chronotypes may be more likely to report symptoms of insomnia (83). This phenomenon may be explained by behavioral/social factors: people may attempt to go to sleep earlier than their natural bedtime to prepare for morning social obligations, resulting in excess time spent in bed awake. Over time, this persistent time spent awake in bed and resulting arousal may contribute to the development of insomnia (84).
Social (e.g., school, work) schedules
When social schedules require people to wake up or go to sleep at times that are inconsistent with their natural circadian rhythms, they may experience circadian misalignment. Evening chronotype in particular may result in social jet lag—a regular discrepancy between one’s endogenous and actual sleep phase that often manifests as insufficient sleep during the week and sleeping in on the weekends (85). In Crohn’s disease, social jet leg has been associated with more aggressive disease course, including greater likelihood of both fistulizing/stricturing phenotypes and Crohn’s related surgery (81).
Meal timing
As food intake is the primary zeitgeber for the hepato-intestinal clock, meal patterns have the potential to significantly impact circadian rhythms and GI function. Eating at different times from one day to the next or regularly skipping meals have the potential to result in circadian misalignment. In IBD, eating breakfast or dinner at inconsistent times was associated with reduced IBD-related quality of life (81). Research also suggests that people with IBS are more likely to follow an irregular meal pattern than healthy controls (86). The timing of particular foods may be important as well. For example, as described above, caffeine has been shown to reduce total sleep time, decrease the percentage of time spent in deep sleep, and increase arousal (55,56).
Light exposure
Light increases both subjective and objective alertness/arousal, which if experienced at night can suppress melatonin, reduce sleepiness, and increase the time it takes to fall asleep (87). Blue light/short wavelength in particular may increase alertness at night more than other colors (88,89). Thus, poorly timed light exposure could result in sleep disturbance, therefore worsening or perpetuating symptoms of various GI conditions.
Stress
While the central clock appears to be resilient in the face of acute and chronic stressors (90), stress may cause cognitive and behavioral changes to sleep or meal timing (e.g., those described above), resulting in circadian misalignment.
Physical activity
Broadly, meta-analytic evidence suggests that engagement in physical activity may reduce anxiety, depression, and stress reactivity (91-93). While intense physical activity within an hour of bedtime may be disruptive to sleep (94), overall exercise has a small positive impact on time to fall asleep, sleep efficiency (time spent asleep compared to time in bed), total sleep time, and sleep quality both broadly (i.e., on average over time) and immediately (i.e., sleep is improved that night) (95). Additionally, researchers have demonstrated that regular engagement in physical activity may improve GI symptoms in people with IBS and quality of life and disease specific anxiety in people with IBD (96,97). In one very large longitudinal study, researchers demonstrated that sedentary behavior and both insufficient and excessive sleep were associated with increased risk for developing IBS (98), highlighting the importance of regular activity in disease management. The relationship between sleep and physical activity is also likely bidirectional; in one study in people with IBD, time spent awake during the night was associated with reduced physical activity the next day (99).
Importantly, despite the health promotion benefits of regular activity, it may be difficult for patients to prioritize exercise during times of stress. Research broadly suggests that both acute stress (e.g., major life events) and chronic stress (e.g., caregivers) are associated with decreased overall activity (100). Thus, as is described above, it is possible that decreases in exercise contribute to the impact of stress on GI conditions.
Natural environment exposure
Green space and blue space (e.g., lakes, rivers, oceans) exposure is hypothesized to improve health in a number of ways, including through promotion of physical activity, reduction of stress, and increased microbiome diversity. Indeed, a recent meta-analysis of randomized controlled trials demonstrated that exposure to the natural environment may lead to reductions in both self-reported stress and objectively measured physiological arousal (101). Research also suggests that exposure to natural environments may be protective against the development of both IBD and insomnia (102-104). Additionally, as research suggests that virtual exposure to natural environments may also reduce stress (105), there may be a connection to the emerging literature on virtual reality treatments for DGBIs (106). Specifically, preliminary research has demonstrated that brief, daily exposure to immersive, nature-oriented, virtual reality scenes improved symptoms of FD above and beyond watching nature videos (107). Future research on the role of both real life and immersive reality greenspace exposure is clearly warranted.
Broadly, a number of these daily living factors are consistent with sleep hygiene guidelines (108-110). However, while sleep hygiene is important for maintaining a healthy sleep schedule and relationship with one’s bed/bedroom, it is not a sufficient treatment once a sleep problem has progressed to the level of chronic insomnia (i.e., occurring at least 3x/week for at least 3 months) (111,112).
Sociocultural factors
Factors such as race, socioeconomic status, perceived microaggressions, and stigma can play a role in the sleep/GI relationship through their impact on access to healthcare, healthcare utilization, and disease outcomes. For example, one large multicenter retrospective study using healthcare utilization as a lens to dissect racial disparities found that racial minority patients were less likely to be referred to specialty GI care than White controls and instead received higher number of primary care visits for their IBS related issues. The study also found that minority patients were more likely to undergo GI procedures compared to White controls. The authors propose problems with communication as an explanatory mechanism for increased procedures (113). Another review that examined the role of race and socioeconomic status in IBD, concluded that disparities based on socioeconomic status and race existed in healthcare delivery and effectiveness among minority patients (114).
Similarly, perception of discrimination could contribute to disease maintenance both across GI diseases as well as in sleep disorders (115,116). Discrimination as a contributory factor for the dysregulation of the brain-gut microbiome system was examined in a recent study. Not surprisingly, discrimination was associated with anxiety, depressive symptoms and visceral sensitivity. Structural and functional changes in the gut microbiome were also associated with feelings of perceived discrimination (115). Discrimination was also seen to be consistently associated with poor sleep in a large review study (117). Again, arousal and hypervigilance for threat may play a role in the experience of discrimination impacting sleep quality (118).
Finally, the experience of stigma can heavily impact care utilization, treatment seeking and adherence, and can contribute to decreased self-esteem and self-efficacy. Although stigma is considered a global health burden, very few studies exist examining its effect on GI diseases. Shame related to their GI symptoms, including having to use the bathroom several times in public, fear about accidents in public, and bowel sounds can lead to social isolation and concealment of disease condition. Similarly, miscommunication and perceived invalidation coming from the medical care provider about their GI experiences may play a role in reduced treatment seeking and adherence in patients with IBD (119). Moreover, the emotional experience associated with shame, embarrassment, and invalidation could potentially perpetuate the psychophysiological comorbidities associated with GI and insomnia.
Conclusions
Understanding arousal as a confluence of direct and indirect factors converging to play a role in sleep and GI disturbance provides guidance for clinical assessment and treatment. In a more direct sense, treating GI symptoms with medication and/or behavioral therapies can improve sleep symptoms, and vice versa (68). More broadly, assessing for the factors outlined in this review and how they may play a role in presenting clinical symptoms based on the underlying arousal pathway can inform treatment decision-making in the biomedical and psychosocial domains. For example, changing from a stimulant to a non-stimulant medication, addressing symptoms of generalized anxiety, targeting motivational enhancement to increase physical and recreational activity, or considering comprehensive treatment for chronic pain may improve insomnia and GI symptoms. In another instance, connecting community support resources for an individual who acknowledges chronic stress related to living circumstances or finances may help reduce environmentally driven chronic stress, improve baseline arousal magnitude and thus in turn reduce chronic insomnia and GI symptom severity. This model fits well with a multidisciplinary approach to care where multiple specialists can collaboratively address these various areas. For example, alongside the care of the treating gastroenterologist, a GI psychologist can address psychologic comorbidities and help improve sleep behaviors, a clinical pharmacist can review for possible medication interactions, and social work support may help with resource accrual to address environmental barriers. Each of these can help reduce sources of chronic arousal perpetuating GI and insomnia symptoms. Fortunately, this model of care is becoming more available in medical centers (120), although it is far from ubiquitous.
Research into the mechanistic and contributory elements of central nervous system arousal as they pertain to digestive and sleep disorders continues to emerge, allowing further precision in characterizing these issues. For instance, the role of gut microbial composition is a by now well-established player in digestive disease, but it may play a key role in the presence of insomnia, and other arousal-based syndromes (121,122). As we look to the future of comprehensive clinical care for GI disorders and Insomnia, assessing and addressing these various factors with a multidisciplinary plan will likely be essential.
Acknowledgments
Funding: This work was supported by
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sara H Marchese and Tiffany H Taft) for the series “Social and Emotional Impacts of Chronic Digestive Diseases” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-126/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-126/coif). The series “Social and Emotional Impacts of Chronic Digestive Diseases” was commissioned by the editorial office without any funding or sponsorship. J.K.S.D. reports that this work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (No. K23DK134814) and reports grant from Crohn’s & Colitis Foundation Litwin IBD Pioneers Award, honoraria from Crohn’s & Colitis Foundation, ROME Foundation and Johns Hopkins University and research support through supplies from Buhlmann Diagnostics. Honoraria do not pertain directly to the manuscript’s content. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buman MP, Phillips BA, Youngstedt SD, et al. Does nighttime exercise really disturb sleep? Results from the 2013 National Sleep Foundation Sleep in America Poll. Sleep Med 2014;15:755-61. [Crossref] [PubMed]
- Ballou S, Alhassan E, Hon E, et al. Sleep Disturbances Are Commonly Reported Among Patients Presenting to a Gastroenterology Clinic. Dig Dis Sci 2018;63:2983-91. [Crossref] [PubMed]
- Ananthakrishnan AN, Long MD, Martin CF, et al. Sleep disturbance and risk of active disease in patients with Crohn's disease and ulcerative colitis. Clin Gastroenterol Hepatol 2013;11:965-71. [Crossref] [PubMed]
- Vela MF, Kramer JR, Richardson PA, et al. Poor sleep quality and obstructive sleep apnea in patients with GERD and Barrett's esophagus. Neurogastroenterol Motil 2014;26:346-52. [Crossref] [PubMed]
- Lacy BE, Everhart K, Crowell MD. Functional dyspepsia is associated with sleep disorders. Clin Gastroenterol Hepatol 2011;9:410-4. [Crossref] [PubMed]
- Guo J, Pei L, Chen L, et al. Bidirectional association between irritable bowel syndrome and restless legs syndrome: a systematic review and meta-analysis. Sleep Med 2021;77:104-11. [Crossref] [PubMed]
- Becker J, Berger F, Schindlbeck KA, et al. Restless legs syndrome is a relevant comorbidity in patients with inflammatory bowel disease. Int J Colorectal Dis 2018;33:955-62. [Crossref] [PubMed]
- Salwen-Deremer JK, Smith MT, Haskell HG, et al. Poor Sleep in Inflammatory Bowel Disease Is Reflective of Distinct Sleep Disorders. Dig Dis Sci 2022;67:3096-107. [Crossref] [PubMed]
- El Hage Chehade N, Fu Y, Ghoneim S, et al. Association between obstructive sleep apnea and gastroesophageal reflux disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 2023; [Crossref] [PubMed]
- Modolell I, Esteller E, Segarra F, et al. Proton-pump inhibitors in sleep-related breathing disorders: clinical response and predictive factors. Eur J Gastroenterol Hepatol 2011;23:852-8. [Crossref] [PubMed]
- Al Momani L, Alomari M, Patel A, et al. 2875 The Prevalence of Irritable Bowel Syndrome in Patients With Obstructive Sleep Apnea: A Meta-Analysis and Systematic Review. Am J Gastroenterol 2019;114:S1576-7. [Crossref]
- Ghiasi F, Amra B, Sebghatollahi V, et al. Association of irritable bowel syndrome and sleep apnea in patients referred to sleep laboratory. J Res Med Sci 2017;22:72. [Crossref] [PubMed]
- Mai E, Buysse DJ. Insomnia: Prevalence, Impact, Pathogenesis, Differential Diagnosis, and Evaluation. Sleep Med Clin 2008;3:167-74. [Crossref] [PubMed]
- Wuestenberghs F, Melchior C, Desprez C, et al. Sleep Quality and Insomnia Are Associated With Quality of Life in Functional Dyspepsia. Front Neurosci 2022;16:829916. [Crossref] [PubMed]
- Salwen-Deremer JK, Ballou S, Painful GI. Conditions and Their Bidirectional Relationships with Sleep Disturbances. CCurr Sleep Med Rep 2022;8:105-13. [Crossref]
- Salwen-Deremer JK, Sun M. Management of Sleep and Fatigue in Gastrointestinal Patients. Gastroenterol Clin North Am 2022;51:829-47. [Crossref] [PubMed]
- Robertson JA, Broomfield NM, Espie CA. Prospective comparison of subjective arousal during the pre-sleep period in primary sleep-onset insomnia and normal sleepers. J Sleep Res 2007;16:230-8. [Crossref] [PubMed]
- Yang YY, Jun S. Prevalence and associated factors of insomnia in college students with irritable bowel syndrome. Korean J Adult Nurs 2018;30:235-44. [Crossref]
- Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev 2010;14:19-31. [Crossref] [PubMed]
- Lackner JM, Jaccard JIBS Outcome Study Research Group. Factors Associated With Efficacy of Cognitive Behavior Therapy vs Education for Patients With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol 2019;17:1500-1508.e3. [Crossref] [PubMed]
- Ju T, Naliboff BD, Shih W, et al. Risk and Protective Factors Related to Early Adverse Life Events in Irritable Bowel Syndrome. J Clin Gastroenterol 2020;54:63-9. [Crossref] [PubMed]
- Park SH, Videlock EJ, Shih W, et al. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil 2016;28:1252-60. [Crossref] [PubMed]
- Bader K, Schäfer V, Schenkel M, et al. Increased nocturnal activity associated with adverse childhood experiences in patients with primary insomnia. J Nerv Ment Dis 2007;195:588-95. [Crossref] [PubMed]
- Kajeepeta S, Gelaye B, Jackson CL, et al. Adverse childhood experiences are associated with adult sleep disorders: a systematic review. Sleep Med 2015;16:320-30. [Crossref] [PubMed]
- Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014;63:1293-9. [Crossref] [PubMed]
- Person H, Keefer L. Psychological comorbidity in gastrointestinal diseases: Update on the brain-gut-microbiome axis. Prog Neuropsychopharmacol Biol Psychiatry 2021;107:110209. [Crossref] [PubMed]
- Black J, Sweeney L, Yuan Y, et al. Systematic review: the role of psychological stress in inflammatory bowel disease. Aliment Pharmacol Ther 2022;56:1235-49. [Crossref] [PubMed]
- Araki M, Shinzaki S, Yamada T, et al. Psychologic stress and disease activity in patients with inflammatory bowel disease: A multicenter cross-sectional study. PLoS One 2020;15:e0233365. [Crossref] [PubMed]
- Edman JS, Greeson JM, Roberts RS, et al. Perceived Stress in Patients with Common Gastrointestinal Disorders: Associations with Quality of Life, Symptoms and Disease Management. Explore (NY) 2017;13:124-8. [Crossref] [PubMed]
- Alkhayyat M, Abou Saleh M, Coronado W, et al. Increasing Prevalence of Anxiety and Depression Disorders After Diagnosis of Chronic Pancreatitis: A 5-Year Population-Based Study. Pancreas 2021;50:153-9. [Crossref] [PubMed]
- Trindade IA, Hreinsson JP, Melchior C, et al. Global Prevalence of Psychological Distress and Comorbidity With Disorders of Gut-Brain Interactions. Am J Gastroenterol 2024;119:165-75. [Crossref] [PubMed]
- Alvaro PK, Roberts RM, Harris JK. A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep 2013;36:1059-68. [Crossref] [PubMed]
- Simpson CA, Mu A, Haslam N, et al. Feeling down? A systematic review of the gut microbiota in anxiety/depression and irritable bowel syndrome. J Affect Disord 2020;266:429-46. [Crossref] [PubMed]
- Midenfjord I, Polster A, Sjövall H, et al. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol Motil 2019;31:e13619. [Crossref] [PubMed]
- Fond G, Loundou A, Hamdani N, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014;264:651-60. [Crossref] [PubMed]
- Ng QX, Soh AYS, Loke W, et al. Systematic review with meta-analysis: The association between post-traumatic stress disorder and irritable bowel syndrome. J Gastroenterol Hepatol 2019;34:68-73. [Crossref] [PubMed]
- Taft TH, Quinton S, Jedel S, et al. Posttraumatic Stress in Patients With Inflammatory Bowel Disease: Prevalence and Relationships to Patient-Reported Outcomes. Inflamm Bowel Dis 2022;28:710-9. [Crossref] [PubMed]
- Milanak ME, Zuromski KL, Cero I, et al. Traumatic Event Exposure, Posttraumatic Stress Disorder, and Sleep Disturbances in a National Sample of U.S. Adults. J Trauma Stress 2019;32:14-22. [Crossref] [PubMed]
- Ljótsson B, Hesser H, Andersson E, et al. Mechanisms of change in an exposure-based treatment for irritable bowel syndrome. J Consult Clin Psychol 2013;81:1113-26. [Crossref] [PubMed]
- Sherwin LB, Leary E, Henderson WA. The association of catastrophizing with quality-of-life outcomes in patients with irritable bowel syndrome. Qual Life Res 2017;26:2161-70. [Crossref] [PubMed]
- Hiller RM, Lovato N, Gradisar M, et al. Trying to fall asleep while catastrophising: what sleep-disordered adolescents think and feel. Sleep Med 2014;15:96-103. [Crossref] [PubMed]
- Craske MG, Wolitzky-Taylor KB, Labus J, et al. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behav Res Ther 2011;49:413-21. [Crossref] [PubMed]
- de Bortoli N, Tolone S, Frazzoni M, et al. Gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome: common overlapping gastrointestinal disorders. Ann Gastroenterol 2018;31:639-48. [Crossref] [PubMed]
- Kurin M, Shibli F, Kitayama Y, et al. Sorting out the Relationship between Gastroesophageal Reflux Disease and Sleep. Curr Gastroenterol Rep 2021;23:15. [Crossref] [PubMed]
- Grover S, Chauhan SG, Karia S, et al. A cross-sectional study of sleep disturbances in patients diagnosed with Functional Dyspepsia. Indian J Ment Health 2020;7:136-42. [Crossref]
- Tu Q, Heitkemper MM, Jarrett ME, et al. Sleep disturbances in irritable bowel syndrome: a systematic review. Neurogastroenterol Motil 2017; [Crossref] [PubMed]
- Ancoli-Israel S, Bliwise DL, Nørgaard JP. The effect of nocturia on sleep. Sleep Med Rev 2011;15:91-7. [Crossref] [PubMed]
- Troxel WM, Booth M, Buysse DJ, et al. Sleep disturbances and nocturnal symptoms: relationships with quality of life in a population-based sample of women with interstitial cystitis/bladder pain syndrome. J Clin Sleep Med 2014;10:1331-7. [Crossref] [PubMed]
- Chang KM, Lee MH, Lin HH, et al. Does irritable bowel syndrome increase the risk of interstitial cystitis/bladder pain syndrome? A cohort study of long term follow-up. Int Urogynecol J 2021;32:1307-12. [Crossref] [PubMed]
- Grundy L, Erickson A, Brierley SM. Visceral Pain. Annu Rev Physiol 2019;81:261-84. [Crossref] [PubMed]
- Nicolaides NC, Kyratzi E, Lamprokostopoulou A, et al. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 2015;22:6-19. [Crossref] [PubMed]
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci 2011;65:549-60. [Crossref] [PubMed]
- Subramaniam A, LoPilato A, Walker EF. Psychotropic medication effects on cortisol: Implications for research and mechanisms of drug action. Schizophr Res 2019;213:6-14. [Crossref] [PubMed]
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev 1992;17:139-70. [Crossref] [PubMed]
- Sanchis C, Blasco E, Luna FG, et al. Effects of caffeine intake and exercise intensity on executive and arousal vigilance. Sci Rep 2020;10:8393. [Crossref] [PubMed]
- Gardiner C, Weakley J, Burke LM, et al. The effect of caffeine on subsequent sleep: A systematic review and meta-analysis. Sleep Med Rev 2023;69:101764. [Crossref] [PubMed]
- Harvey RE, Hart EC, Charkoudian N, et al. Oral Contraceptive Use, Muscle Sympathetic Nerve Activity, and Systemic Hemodynamics in Young Women. Hypertension 2015;66:590-7. [Crossref] [PubMed]
- Middlekauff HR, Park J, Gornbein JA. Lack of effect of ovarian cycle and oral contraceptives on baroreceptor and nonbaroreceptor control of sympathetic nerve activity in healthy women. Am J Physiol Heart Circ Physiol 2012;302:H2560-6. [Crossref] [PubMed]
- Pereira TJ, Bouakkar J, Johnston H, et al. The effects of oral contraceptives on resting autonomic function and the autonomic response to physiological stressors: a systematic review. Clin Auton Res 2023;33:859-92. [Crossref] [PubMed]
- Partonen T, Toffol E, Latvala A, et al. Hormonal contraception use and insomnia: A nested case-control study. Sleep Med 2023;109:192-6. [Crossref] [PubMed]
- Romero-Gómez B, Guerrero-Alonso P, Carmona-Torres JM, et al. Mood Disorders in Levothyroxine-Treated Hypothyroid Women. Int J Environ Res Public Health 2019;16:4776. [Crossref] [PubMed]
- de Leon J, Nikoloff DM. Paradoxical excitation on diphenhydramine may be associated with being a CYP2D6 ultrarapid metabolizer: three case reports. CNS Spectr 2008;13:133-5. [Crossref] [PubMed]
- Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep 2009;32:200-4. [Crossref] [PubMed]
- Norlin AK, Walter S, Icenhour A, et al. Fatigue in irritable bowel syndrome is associated with plasma levels of TNF-α and mesocorticolimbic connectivity. Brain Behav Immun 2021;92:211-22. [Crossref] [PubMed]
- Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007;132:913-20. [Crossref] [PubMed]
- Martin-Viñas JJ, Quigley EM. Immune response in irritable bowel syndrome: A systematic review of systemic and mucosal inflammatory mediators. J Dig Dis 2016;17:572-81. [Crossref] [PubMed]
- Ballou S, Katon J, Rangan V, et al. Brief Behavioral Therapy for Insomnia in Patients with Irritable Bowel Syndrome: A Pilot Study. Dig Dis Sci 2020;65:3260-70. [Crossref] [PubMed]
- Yang YY, Jun S. The Effects of Cognitive Behavioral Therapy for Insomnia among College Students with Irritable Bowel Syndrome: A Randomized Controlled Trial. Int J Environ Res Public Health 2022;19:14174. [Crossref] [PubMed]
- Pace-Schott EF, Amole MC, Aue T, et al. Physiological feelings. Neurosci Biobehav Rev 2019;103:267-304. [Crossref] [PubMed]
- Sun Y, Laksono I, Selvanathan J, et al. Prevalence of sleep disturbances in patients with chronic non-cancer pain: A systematic review and meta-analysis. Sleep Med Rev 2021;57:101467. [Crossref] [PubMed]
- Wei Y, Van Someren EJW. Interoception relates to sleep and sleep disorders. Curr Opin Behav Sci 2020;33:1-7. [Crossref]
- Haack M, Simpson N, Sethna N, et al. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology 2020;45:205-16. [Crossref] [PubMed]
- Liu J, Wang W, Tian J, et al. Sleep Deficiency Is Associated With Exacerbation of Symptoms and Impairment of Anorectal and Autonomic Functions in Patients With Functional Constipation. Front Neurosci 2022;16:912442. [Crossref] [PubMed]
- Bishehsari F, Levi F, Turek FW, et al. Circadian Rhythms in Gastrointestinal Health and Diseases. Gastroenterology 2016;151:e1-5. [Crossref] [PubMed]
- Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol 2011;62:139-50. [PubMed]
- Li YM, Du J, Zhang H, et al. Epidemiological investigation in outpatients with symptomatic gastroesophageal reflux from the Department of Medicine in Zhejiang Province, east China. J Gastroenterol Hepatol 2008;23:283-9. [Crossref] [PubMed]
- Chung TH, Lee J, Kim MC. Impact of night-shift work on the prevalence of erosive esophagitis in shipyard male workers. Int Arch Occup Environ Health 2016;89:961-6. [Crossref] [PubMed]
- Nojkov B, Rubenstein JH, Chey WD, et al. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol 2010;105:842-7. [Crossref] [PubMed]
- Weintraub Y, Cohen S, Chapnik N, et al. Clock Gene Disruption Is an Initial Manifestation of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2020;18:115-122.e1. [Crossref] [PubMed]
- Chrobak AA, Nowakowski J, Zwolińska-Wcisło M, et al. Associations between chronotype, sleep disturbances and seasonality with fatigue and inflammatory bowel disease symptoms. Chronobiol Int 2018;35:1142-52. [Crossref] [PubMed]
- Chakradeo PS, Keshavarzian A, Singh S, et al. Chronotype, social jet lag, sleep debt and food timing in inflammatory bowel disease. Sleep Med 2018;52:188-95. [Crossref] [PubMed]
- Zitting KM, Isherwood CM, Yuan RK, et al. Eating during the biological night is associated with nausea. Sleep Health 2024;10:S144-8. [Crossref] [PubMed]
- Kivelä L, Papadopoulos MR, Antypa N. Chronotype and Psychiatric Disorders. Curr Sleep Med Rep 2018;4:94-103. [Crossref] [PubMed]
- Ebben MR, Spielman AJ. Non-pharmacological treatments for insomnia. J Behav Med 2009;32:244-54. [Crossref] [PubMed]
- Wittmann M, Dinich J, Merrow M, et al. Social jetlag: misalignment of biological and social time. Chronobiol Int 2006;23:497-509. [Crossref] [PubMed]
- Hajishafiee M, Keshteli AH, Saneei P, et al. Healthy lifestyle score and irritable bowel syndrome: A cross-sectional study in adults. Neurogastroenterol Motil 2020;32:e13793. [Crossref] [PubMed]
- Fisk AS, Tam SKE, Brown LA, et al. Light and Cognition: Roles for Circadian Rhythms, Sleep, and Arousal. Front Neurol 2018;9:56. [Crossref] [PubMed]
- Mu YM, Huang XD, Zhu S, et al. Alerting effects of light in healthy individuals: a systematic review and meta-analysis. Neural Regen Res 2022;17:1929-36. [Crossref] [PubMed]
- Xu Q, Lang CP. Revisiting the alerting effect of light: A systematic review. Sleep Med Rev 2018;41:39-49. [Crossref] [PubMed]
- Ota SM, Kong X, Hut R, et al. The impact of stress and stress hormones on endogenous clocks and circadian rhythms. Front Neuroendocrinol 2021;63:100931. [Crossref] [PubMed]
- Rebar AL, Stanton R, Geard D, et al. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev 2015;9:366-78. [Crossref] [PubMed]
- Stubbs B, Vancampfort D, Rosenbaum S, et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: A meta-analysis. Psychiatry Res 2017;249:102-8. [Crossref] [PubMed]
- Mariano IM, Amaral AL, Ribeiro PAB, et al. Exercise training improves blood pressure reactivity to stress: a systematic review and meta-analysis. Sci Rep 2023;13:10962. [Crossref] [PubMed]
- Stutz J, Eiholzer R, Spengler CM. Effects of Evening Exercise on Sleep in Healthy Participants: A Systematic Review and Meta-Analysis. Sports Med 2019;49:269-87. [Crossref] [PubMed]
- Kredlow MA, Capozzoli MC, Hearon BA, et al. The effects of physical activity on sleep: a meta-analytic review. J Behav Med 2015;38:427-49. [Crossref] [PubMed]
- Zhou C, Zhao E, Li Y, et al. Exercise therapy of patients with irritable bowel syndrome: A systematic review of randomized controlled trials. Neurogastroenterol Motil 2019;31:e13461. [Crossref] [PubMed]
- Eckert KG, Abbasi-Neureither I, Köppel M, et al. Structured physical activity interventions as a complementary therapy for patients with inflammatory bowel disease - a scoping review and practical implications. BMC Gastroenterol 2019;19:115. [Crossref] [PubMed]
- Gao X, Tian S, Huang N, et al. Associations of daily sedentary behavior, physical activity, and sleep with irritable bowel syndrome: A prospective analysis of 362,193 participants. J Sport Health Sci 2024;13:72-80. [Crossref] [PubMed]
- Guadagnoli L, Horrigan J, Walentynowicz M, et al. Sleep Quality Drives Next Day Pain and Fatigue in Adults With Inflammatory Bowel Disease: A Short Report. J Crohns Colitis 2024;18:171-4. [Crossref] [PubMed]
- Stults-Kolehmainen MA, Sinha R. The effects of stress on physical activity and exercise. Sports Med 2014;44:81-121. [Crossref] [PubMed]
- Shuda Q, Bougoulias ME, Kass R. Effect of nature exposure on perceived and physiologic stress: A systematic review. Complement Ther Med 2020;53:102514. [Crossref] [PubMed]
- Zhang Z, Chen L, Qian ZM, et al. Residential green and blue space associated with lower risk of adult-onset inflammatory bowel disease: Findings from a large prospective cohort study. Environ Int 2022;160:107084. [Crossref] [PubMed]
- Elten M, Benchimol EI, Fell DB, et al. Residential Greenspace in Childhood Reduces Risk of Pediatric Inflammatory Bowel Disease: A Population-Based Cohort Study. Am J Gastroenterol 2021;116:347-53. [Crossref] [PubMed]
- Shin JC, Parab KV, An R, et al. Greenspace exposure and sleep: A systematic review. Environ Res 2020;182:109081. [Crossref] [PubMed]
- Syed Abdullah SS, Awang Rambli DR, Sulaiman S, et al. The impact of virtual nature therapy on stress responses: A systematic qualitative review. Forests 2021;12:1776. [Crossref]
- Lacy BE, Cangemi DJ, Spiegel BR. Virtual Reality: A New Treatment Paradigm for Disorders of Gut-Brain Interaction? Gastroenterol Hepatol (N Y) 2023;19:86-94. [PubMed]
- Cangemi DJ, Montenegro M, Spiegel BMR, et al. Virtual Reality Improves Symptoms of Functional Dyspepsia: Results of a Randomized, Double-Blind, Sham-Controlled, Pilot Study. Am J Gastroenterol 2024;119:210-3. [Crossref] [PubMed]
- Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Med Rev 2015;22:23-36. [Crossref] [PubMed]
- Mastin DF, Bryson J, Corwyn R. Assessment of sleep hygiene using the Sleep Hygiene Index. J Behav Med 2006;29:223-7. [Crossref] [PubMed]
- Chung KF, Lee CT, Yeung WF, et al. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract 2018;35:365-75. [Crossref] [PubMed]
- Taylor DJ, Pruiksma KE. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int Rev Psychiatry 2014;26:205-13. [Crossref] [PubMed]
- Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med 2021;17:263-98. [Crossref] [PubMed]
- Silvernale C, Kuo B, Staller K. Racial disparity in healthcare utilization among patients with Irritable Bowel Syndrome: results from a multicenter cohort. Neurogastroenterol Motil 2021;33:e14039. [Crossref] [PubMed]
- Sewell JL, Velayos FS. Systematic review: The role of race and socioeconomic factors on IBD healthcare delivery and effectiveness. Inflamm Bowel Dis 2013;19:627-43. [Crossref] [PubMed]
- Dong TS, Gee GC, Beltran-Sanchez H, et al. How Discrimination Gets Under the Skin: Biological Determinants of Discrimination Associated With Dysregulation of the Brain-Gut Microbiome System and Psychological Symptoms. Biol Psychiatry 2023;94:203-14. [Crossref] [PubMed]
- Kingsbury JH, Buxton OM, Emmons KM. Sleep and its Relationship to Racial and Ethnic Disparities in Cardiovascular Disease. Curr Cardiovasc Risk Rep 2013; [Crossref] [PubMed]
- Slopen N, Lewis TT, Williams DR. Discrimination and sleep: a systematic review. Sleep Med 2016;18:88-95. [Crossref] [PubMed]
- Harrell JP, Hall S, Taliaferro J. Physiological responses to racism and discrimination: an assessment of the evidence. Am J Public Health 2003;93:243-8. [Crossref] [PubMed]
- Taft TH, Keefer L. A systematic review of disease-related stigmatization in patients living with inflammatory bowel disease. Clin Exp Gastroenterol 2016;9:49-58. [PubMed]
- Salwen-Deremer JK, Bardach SH, Tormey LK, et al. Redesigning a Gastroenterology Behavioral Health Program to Improve Patient Access. Clin Gastroenterol Hepatol 2024;22:12-15.e1. [Crossref] [PubMed]
- Zhang Q, Yun Y, An H, et al. Gut Microbiome Composition Associated With Major Depressive Disorder and Sleep Quality. Front Psychiatry 2021;12:645045. [Crossref] [PubMed]
- Laudani S, Torrisi SA, Alboni S, et al. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain Behav Immun 2023;107:385-96. [Crossref] [PubMed]
Cite this article as: Rameshkumar S, Arizmendi BJ, Salwen-Deremer JK. The role of arousal in maintaining the relationship between insomnia and gastrointestinal conditions. Transl Gastroenterol Hepatol 2024;9:41.


