
Global burden of adult non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) has been steadily increasing over the past decades and is expected to persist in the future
Highlight box
Key findings
• The burdens of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) in adults escalate as body mass index (BMI), age, and elapsed time increase.
What is known and what is new?
• The prevalence of NAFLD and NASH in adults is on the rise due to the escalating obesity epidemic.
• A predictive model was developed to illustrate the anticipated changes in the burden of NAFLD and NASH with fluctuations in BMI.
What is the implication, and what should change now?
• We need reduce excessive BMI through the adoption of effective strategies to prevent and manage NAFLD/NASH.
Introduction
With persistent efforts to prevent and treat chronic hepatitis B (CHB), there has been a gradual decline in the number of new cases of CHB (1,2). In contrast, the non-alcoholic fatty liver disease (NAFLD) prevalence has been consistently increasing (3,4). NAFLD typically develops as a result of insulin resistance and is often accompanied by metabolic syndrome, which can lead to significant damage both within and outside the liver. The presence of NAFLD has been associated with an increased risk of extrahepatic conditions such as ischemic heart disease and ischemic stroke (5-8). Nonalcoholic steatohepatitis (NASH), a dynamic manifestation of NAFLD, commonly gives rise to intrahepatic complications, namely liver cirrhosis and hepatocellular carcinoma. In recent times, there has been a significant rise in the occurrence of liver cirrhosis associated with NAFLD (4,9). Given that, it is noteworthy that NASH has emerged as the primary cause of cirrhosis, while instances of cirrhosis linked to hepatitis B virus (HBV) and hepatitis C virus (HCV) infections have shown a decline in 2017 (10). Furthermore, it has been demonstrated through accumulating evidence that NAFLD and its comorbidity, metabolic syndrome, exhibit a significant association with both intrahepatic and extrahepatic cancers (11-14).
Despite the evident impact of NAFLD on public health and the substantial economic burden it imposes, there remains a lack of population-based epidemiological data on NAFLD at both global and national levels. To address this knowledge gap, several meta-analyses have been conducted to estimate the pooled incidence of NAFLD in the general population (15-18), as well as in the overweight and obese population (19). Multiple studies have demonstrated a strong association between overweight/obese individuals and a higher incidence of NAFLD compared to those of normal weight (19,20). The likelihood of developing NAFLD is approximately three times higher in individuals who are obese or overweight (15). Furthermore, the prevalence of NASH in severely/morbidly obese patients is significantly elevated compared to the general population (17). These findings underscore the close correlation between body mass index (BMI) and the prevalence of NAFLD/NASH. BMI is the most robust predictor of NAFLD in both genders (21,22). The correlation between BMI and the risk of NAFLD is contingent upon the dosage (22-25). Given the aforementioned evidence, it is posited that the prevalence of NAFLD can be approximated by considering the prevalence of various BMI categories, assuming a well-defined correlation between BMI and NAFLD. Comprehensive data on the burden of NAFLD and NASH will aid in the formulation of tailored preventive measures for these conditions. Despite the potential for meta-analysis to offer relevant insights, caution must be exercised when interpreting its findings due to inherent limitations, including variations in the populations studied, diagnostic methods employed, and diagnostic criteria utilized. We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-118/rc).
Methods
Criteria for NAFLD/NASH
Following the literature (26,27), the present study utilized the subsequent criteria to ascertain the presence of NAFLD or NASH in adults:
- NAFLD was identified by a controlled attenuation parameter (CAP) value of ≥248 dB/m;
- NASH was determined by a CAP value of ≥248 dB/m accompanied by alanine aminotransferase (ALT) levels of ≥29 IU/L (for males) or ≥19 IU/L (for females); alternatively, a CAP value of ≥248 dB/m in conjunction with liver stiffness (E) measuring ≥7.3 kPa indicated the presence of NASH.
NAFLD and NASH were identified in without any indication of other causes of chronic liver disease.
NHANES database
This study enrolled the adults who had completed information on sex, age, BMI, ALT, AST, CAP, and liver stiffness (E), using data obtained from NHANES 2017-March 2020 Pre-Pandemic Examination Data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The data used in this study was sourced from public databases, ethical approval was not required.
Depicting the correlation between BMI and risk of NAFLD/NASH by curve-fitting
Four distinct models were formulated to depict the correlation between BMI and the risk of NAFLD/NASH prevalence among both male and female adults. The construction of these models involved the utilization of a curve-fitting technique, which was executed through the following steps:
- The enrolled adults were evenly distributed into twelve groups based on BMI quantiles, and subsequently, the NAFLD/NASH prevalence within each group was computed;
- The median BMI within each group was denoted as , while the corresponding disease prevalence was denoted as . Utilizing these data points , we employed a curve-fitting approach to identify the optimal function that could be utilized for estimating the prevalence of NAFLD/NASH within the population, specifically for individuals with a given BMI value.
The resulting estimated functions were as follows:
Estimated functions depicting NAFLD/NASH prevalence based on BMI
The prevalence of NAFLD and NASH was determined through the utilization of estimated functions and the prevalence of diverse BMI intervals.
The prevalence of adults with diverse BMI intervals was derived from the database NCD Risk Factor Collaboration (NCD-RisC), accessible at https://ncdrisc.org/data-downloads-adiposity-ado.html, which was published as a pooled analysis in the Lancet (28). By categorizing individuals into five distinct BMI levels, namely obesity, overweight, normal, underweight, and severely underweight, we were able to calculate the prevalence rates associated with each category.
The left and right boundaries for the BMI interval of the level of obesity were denoted as and , respectively. Furthermore, the median BMI value was calculated for subsequent analysis. From the NCD-RisC database, we obtained the prevalence of 5 obesity levels for adults from different countries or regions in the years 1975–2016.
The study designated the as the prevalence of NAFLD/NASH among adolescents based on their level of obesity. To determine the cumulative prevalence of NAFLD/NASH among adults with 5 different levels of obesity, the researchers employed a formula, . This formula incorporated the estimated prevalence of the disease for adults, , from obesity level. Through a weighted summation, the resulting value represented the estimated cumulative prevalence of NAFLD/NASH.
Statistical analysis
Projected prevalence of NAFLD/NASH from 2017 to 2030 based on BMI
Utilizing the data on the prevalence of NAFLD/NASH from 1975 to 2016, we employed the autoregressive integrated moving average (ARIMA) model to predict the prevalence for subsequent years (29). The ARIMA model, a commonly employed technique for time-series forecasting, facilitates the understanding of data patterns and enables the projection of future data points.
Calculation of estimated annual percentage change (EAPC)
The method to generate the EAPC was developed by Hankey et al. (30). The details of the method were also described in our previous study (11).
Results
Study population
The algorithm utilized in this study is presented in Figure 1. A cohort of 3,567 individuals (1,747 males and 1,820 females) aged 19–80 years, who possessed comprehensive data on sex, age, BMI, ALT, AST, CAP, and liver stiffness (E), was selected from the NHANES 2017-March 2020 Pre-Pandemic Examination Data. Additional details can be found in https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, pp 1-97.

The correlation between BMI and risk of NAFLD/NASH
The relevant parameters of the models generated were presented in Table 1, while the fitting curves were displayed in Figure 2. The curves demonstrated that the risk of NAFLD/NASH increased with higher BMI, although the relationship was not linear. There was a strong agreement between the predicted and actual risk of NAFLD/NASH. The NASH model for females had the lowest R2 value, yet it remained high at 0.971. Conversely, the NAFLD model for males had the highest R2 value, reaching as high as 0.994.
Table 1
| Model | a | b | c | R2 |
|---|---|---|---|---|
| NAFLD, males | 0.94 | 25.46 | 3.37 | 0.99 |
| NAFLD, females | 0.86 | 25.89 | 3.88 | 0.98 |
| NASH, males | 0.69 | 30.45 | 3.96 | 0.99 |
| NASH, females | 0.61 | 31.57 | 5.90 | 0.97 |
NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.

Estimated global NAFLD/NASH prevalence
From 1975 to 2016, there was a consistent increase in the global prevalence of NAFLD and NASH. The prevalence of NAFLD/NASH was found to be higher in males compared to females. Specifically, the estimated prevalence of NAFLD in males ranged from 30.64% in 1975 to 41.12% in 2016, while in females it ranged from 29.07% in 1975 to 37.32% in 2016 (Figure 3A,3B; https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, pp 98–99). For NASH, the estimated prevalence in males ranged from 10.35% in 1975 to 15.79% in 2016, while in females it ranged from 12.68% in 1975 to 16.48% in 2016 (Figure 3C,3D; https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, pp 100-101). From 1975 to 2016, the EAPC of NAFLD prevalence for males was 0.78% [95% confidence interval (CI): 0.77–0.79%], whereas for females it was 0.63% (95% CI: 0.63–0.64%) (https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 102). Similarly, the EAPC of NASH prevalence for males was 1.13% (95% CI: 1.11–1.15%), while for females it was 0.68% (95% CI: 0.67–0.69%) (https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 103). It should be noted that the EAPC of NASH was higher than that of NAFLD. By 2030, the projected global prevalence of NAFLD was expected to increase to 46.10% in males and 41.02% in females (https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 104). It is projected that the global prevalence of NASH will rise to 18.90% in males and 18.41% in females by the year 2030 (https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 105).

Assessment of regional NAFLD/NASH prevalence
Between 1975 and 2016, there was a notable increase in the prevalence of NAFLD and NASH among adults across various regions, as depicted in Figure 4. Over the past few decades, South Asia exhibited the lowest prevalence of NAFLD and NASH in both males and females. Conversely, High-income Western countries demonstrated the highest prevalence of NAFLD and NASH in males. However, for females, the prevalence of NAFLD and NASH in the countries of Central Asia, Middle East, and North Africa surpassed that observed in the countries of Central and Eastern Europe after 1995. From 1995 onwards, the prevalence of NAFLD/NASH among females in Central Asia, the Middle East, and North Africa was the highest, as depicted in Figure 4 (https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 106-147). Except in Sub-Saharan Africa and Oceania, where the EAPC of NAFLD prevalence in females exceeded that of males, most regions exhibited a higher EAPC of NAFLD prevalence in males compared to females. However, males consistently displayed a higher EAPC of NASH prevalence than females. The regional annual change in NAFLD prevalence among females varied from 0.24% (95% CI: 0.23–0.25%) in Central and Eastern Europe to 0.96% (95% CI: 0.96–0.97%) in Sub-Saharan Africa. The prevalence of NASH among males varied across regions, with rates ranging from 0.66% (95% CI: 0.65–0.66%) in Central and Eastern Europe to 1.17% (95% CI: 1.14–1.20%) in East and South East Asia (https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 136). The EAPC in NASH prevalence among males also varied, ranging from 1.00% (95% CI: 1.00–1.01%) in Central and Eastern Europe to 1.58% (95% CI: 1.53–1.62%) in East and South East Asia. In contrast, the EAPC of NASH prevalence among females ranged from 0.30% (95% CI: 0.29–0.31%) in Central and Eastern Europe to 1.01% (95% CI: 1.00–1.01%) in Sub-Saharan Africa (https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 137).
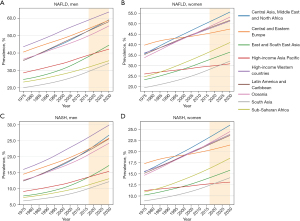
In 2016, the prevalence of NAFLD/NASH was found to be highest among males in high-income Western countries, while South Asia had the lowest prevalence. This pattern is projected to persist until 2030. Among females, Central and Eastern European countries exhibited the highest prevalence of NAFLD, which was later surpassed by Central Asia, Middle East, and North Africa in 1995. The changing trend in NASH prevalence mirrored that described in https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf on pages 106-135. From 2016 to 2030, males from South Asia consistently exhibited the lowest prevalence of NAFLD and NASH. Conversely, during this same time frame, the prevalence of NAFLD and NASH was projected to steadily increase for both males and females in most regions. Specifically, it was estimated that by 2030, males from high-income Western countries could experience NAFLD prevalence as high as 63.58% and NASH prevalence as high as 29.87%. Similarly, females from Central Asia, the Middle East, and North Africa could have NAFLD prevalence as high as 55.69% and NASH prevalence as high as 25.93% by 2030 (see https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 138-147).
Assessment of NAFLD/NASH prevalence at national or regional level
In 2016, there was a significant variation in the prevalence of NAFLD among different countries or regions. Among males, Nauru, American Samoa, and Cook Islands in the Pacific region exhibited the highest estimated burden of NAFLD. Specifically, Nauru had the highest prevalence of NAFLD among males at 73.89%, while Ethiopia had the lowest prevalence at 27.28%, followed by Eritrea and Burundi (Figure 5A). Similarly, among females in 2016, American Samoa (66.43%), Nauru, and Cook Islands had the highest prevalence of NAFLD, whereas Viet Nam (27.14%), India, and Bangladesh had the lowest prevalence (Figure 5B; https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 148-471). Unsurprisingly, the prevalence of NASH in specific countries or regions was found to be similar to that of NAFLD (Figure 5C,5D; https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 472-795). From 1975 to 2016, a significant increase in the EAPC of NAFLD/NASH prevalence was observed in most countries or regions. Among males, Malaysia (1.61%, 95% CI: 1.60–1.63%) and Haiti (1.47%, 95% CI: 1.44–1.50%) exhibited the highest increasing EAPC of NAFLD prevalence, while Nauru and Andorra had the lowest increasing EAPC of NAFLD prevalence. For males, Malaysia (2.26%, 95% CI: 2.24–2.29%) and Haiti (2.16%, 95% CI: 2.10–2.21%) exhibited the highest increasing EAPC in non-alcoholic steatohepatitis (NASH) prevalence, while Nauru (0.66%, 95% CI: 0.65–0.67%) and Andorra had the lowest increasing EAPC. In females, Haiti (1.29%, 95% CI: 1.28–1.30%) had the highest increasing EAPC of NAFLD prevalence, followed by Malaysia (1.25%, 95% CI: 1.23–1.27%), whereas Singapore, Estonia, and the Russian Federation had the lowest increasing EAPC of NAFLD prevalence. In terms of female populations, the country with the highest estimated increase in EAPC of NASH prevalence was Haiti (1.45%, 95% CI: 1.44–1.46%), followed by Malaysia. Conversely, Singapore and Estonia had the lowest estimated increase in EAPC of NASH prevalence (https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 796-811).

On a national scale, Pacific Island countries exhibited high prevalence rates of NAFLD, with males in Nauru reaching an astonishing 76.47% and females in American Samoa reaching 67.88% by 2030. Among males, the highest estimated NASH prevalence in 2030 was for Nauru (41.45%), followed by American Samoa and Palau. In terms of NASH prevalence in females, American Samoa (34.00%), Nauru, and Palau are projected to have the highest estimates in 2030. Conversely, Ethiopia (30.52%), Uganda, and Burundi are expected to have the lowest NAFLD prevalence among males, while Japan (29.46%), India, and Viet Nam, are projected to have the lowest NAFLD prevalence among females in the same year. Additionally, Ethiopia (10.23%), Uganda, and Burundi are estimated to have the lowest NASH prevalence among males in 2030, while Japan (12.68%), Viet Nam, and India, are projected to have the lowest NASH prevalence among females (see https://cdn.amegroups.cn/static/public/tgh-23-118-1.pdf, p 812-1027 for further details). Interactive web pages for national or regional NAFLD/NASH prevalence were shown in Figure S1.
Discussion
Utilizing sigmoidal fitting models and the BMI data sourced from the NCD-RisC dataset, an extensive analysis has been conducted to provide a comprehensive overview of the prevalence of NAFLD/NASH among adults worldwide. This approach has facilitated the examination of various factors such as gender, geographical regions, countries, and temporal trends. The key findings of this study are as follows: (I) a notable association has been observed between BMI and susceptibility to NAFLD/NASH; (II) the application of sigmoidal fitting models has yielded accurate representations of the correlation between BMI and the prevalence of NAFLD/NASH in the adult population; (III) the prevalence of NAFLD and NASH among adults worldwide has exhibited an upward trajectory from 1975 to 2016, and this trend is projected to persist from 2017 to 2030; (IV) males have demonstrated a higher prevalence of NAFLD/NASH compared to females; (V) among males, high-income Western countries have consistently exhibited the highest prevalence of NAFLD/NASH from 1975 to 2016, and this pattern is expected to continue until 2030. In contrast, among females, Central and Eastern European countries held the highest prevalence from 1975 to 1995, but this was surpassed by Central Asia, the Middle East, and North Africa after 1995. In contrast, South Asia demonstrated a comparatively lower prevalence of adult NAFLD/NASH in both genders.
Considering the substantial negative effects of NAFLD/NASH on intrahepatic and extrahepatic health conditions, including benign and malignant diseases, there is a growing interest in understanding the burden of NAFLD/NASH. It is widely acknowledged that the global burden of NAFLD/NASH is progressively increasing, as evidenced by various systematic reviews (16,18,31). Based on recent systematic reviews, there has been a significant increase in the global prevalence of NAFLD over the past three decades, with a reported prevalence of 38.0% in 2016 (16,18). This data closely aligns with the average global prevalence of NAFLD in 2016 estimated by our model, which stands at 39.2%. Furthermore, a comprehensive cross-sectional study conducted in China, involving 15 million eligible participants, reveals a prevalence of fatty liver disease of 34.9% in 2019 (32). This finding is consistent with the projected prevalence of NAFLD in China for the same year, as determined by our model, which stands at 37.0%. Consequently, these results suggest that our model demonstrates satisfactory accuracy.
Meta-analysis is a commonly employed methodology for synthesizing existing evidence; however, it is not without limitations, primarily stemming from the heterogeneity of the included studies. Furthermore, meta-analysis may not always yield the desired granularity of information, such as country-specific or year-specific prevalence rates of NAFLD. Consequently, alternative approaches are warranted to estimate NAFLD prevalence. Numerous investigations have demonstrated a dose-dependent association between BMI and the likelihood of developing NAFLD (32-34). This discovery prompted us to consider the feasibility of estimating the prevalence of NAFLD by examining the prevalence of different BMI intervals. Our investigation revealed that the correlation between various BMI levels and the risk of NAFLD can be accurately simulated using an S-shaped curve fitting method, as evidenced by the near-perfect R2 value (Figure 2). The sigmoidal curve fitting technique, introduced by Venegas et al. (35), offers a means to implement this approach. Furthermore, recent studies have highlighted the significance of NAFLD in lean individuals, emphasizing the importance of not overlooking this population (36,37). It should be noted that the inclusion of our estimation model does not necessarily exclude the possibility of lean NAFLD. The probability of developing NAFLD/NASH is reduced in individuals with a low BMI, aligning with established clinical practices.
As a result of ongoing endeavors to prevent and manage hepatitis B and C, NAFLD has emerged as the primary contributor to chronic liver disease, with NASH projected to become the primary cause of cirrhosis. Considering the potential for severe outcomes associated with NAFLD/NASH, the utilization of prevalence modeling can effectively disseminate information to both the general public and professionals regarding the impending disease burden linked to NAFLD and NASH. This, in turn, facilitates the implementation of targeted preventive measures. Our estimation models indicate that excessive BMI represents a significant yet modifiable risk factor for NAFLD. Consequently, this underscores the potential for reducing excessive BMI through the adoption of effective strategies, such as dietary modifications and exercise regimens, to prevent and manage NAFLD/NASH.
One limitation of our estimation model is the utilization of liver transient elastography as the consistent method for determining the presence of NAFLD/NASH, instead of the more commonly employed ultrasound measurement in population studies or more accurate pathological diagnosis. Nevertheless, the employment of liver transient elastography offers certain advantages, such as the provision of more objective measurement values and the mitigation of inter-observer bias to a relatively minor extent. Another constraint of our study is the assumption of a consistent correlation between BMI and the risk of NAFLD/NASH, disregarding potential variations influenced by physical characteristics such as race and skin color. Given that our analysis is derived from NHANES 2017 to March 2020, which predominantly comprises Caucasian individuals, it is plausible that our estimation model may exhibit greater accuracy when applied to Caucasian populations. Furthermore, numerous studies have demonstrated a higher prevalence of NAFLD/NASH in the elderly population (31,32). However, the NCD-RisC database lacks information on age-specific BMI prevalence, thereby hindering our ability to estimate the global or national prevalence of NAFLD/NASH in an age-stratified manner. In recent years, scholars proposed that metabolic dysfunction-associated steatohepatitis (MASH)/metabolic dysfunction-associated steatotic liver disease (MASLD) should replace the NASH/NAFLD. Most of individuals with NAFLD meet MASLD criteria (38). Although this study was conducted in NASH/NAFLD patients, we will also pay more attention to MASH/MASLD in future.
Conclusions
The prevalence of adult NAFLD/NASH has been observed to increase annually, the burdens escalate as BMI, age, and elapsed time increase. Our research also indicates that the bunder of NAFLD/NASH was higher in High-income countries.
Acknowledgments
Funding: The study was funded by t
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-118/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-118/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-118/coif). M.Z.H. reports the funding from the Natural Science Foundation of Fujian Province of China (No. 2020J011218). J.S.P. reports the funding from the National Natural Science Foundation of China (No. 82372318), Natural Science Foundation of Fujian Province of China (No. 2022J02030), and Major Research Project for Young and Middle-aged People of the Health Commission of Fujian Province (No. 2022ZQNZD004). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The data used in this study was sourced from public databases, ethical approval was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xiao J, Wang F, Wong NK, et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J Hepatol 2019;71:212-21. [Crossref] [PubMed]
- Younossi ZM, Wong G, Anstee QM, et al. The Global Burden of Liver Disease. Clin Gastroenterol Hepatol 2023;21:1978-91. [Crossref] [PubMed]
- Younossi Z, Tacke F, Arrese M, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019;69:2672-82. [Crossref] [PubMed]
- Wong VW, Ekstedt M, Wong GL, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol 2023;79:842-52. [Crossref] [PubMed]
- Alon L, Corica B, Raparelli V, et al. Risk of cardiovascular events in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Prev Cardiol 2022;29:938-46. [Crossref] [PubMed]
- Chung GE, Cho EJ, Yoo JJ, et al. Young adults with nonalcoholic fatty liver disease, defined using the fatty liver index, can be at increased risk of myocardial infarction or stroke. Diabetes Obes Metab 2022;24:465-72. [Crossref] [PubMed]
- Simon TG, Roelstraete B, Hagström H, et al. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: results from a nationwide histology cohort. Gut 2022;71:1867-75. [Crossref] [PubMed]
- Wang W, Hu M, Liu H, et al. Global Burden of Disease Study 2019 suggests that metabolic risk factors are the leading drivers of the burden of ischemic heart disease. Cell Metab 2021;33:1943-1956.e2. [Crossref] [PubMed]
- Wu Z, Wang W, Zhang K, et al. Trends in the incidence of cirrhosis in global from 1990 to 2019: A joinpoint and age-period-cohort analysis. J Med Virol 2023;95:e28858. [Crossref] [PubMed]
- Zhai M, Long J, Liu S, et al. The burden of liver cirrhosis and underlying etiologies: results from the global burden of disease study 2017. Aging (Albany NY) 2021;13:279-300. [Crossref] [PubMed]
- Xing QQ, Li JM, Chen ZJ, et al. Global burden of common cancers attributable to metabolic risks from 1990 to 2019. Med 2023;4:168-181.e3. [Crossref] [PubMed]
- Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-analysis, and Meta-regression. Clin Gastroenterol Hepatol 2022;20:283-292.e10. [Crossref] [PubMed]
- Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut 2022;71:778-88. [Crossref] [PubMed]
- Kim GA, Lee HC, Choe J, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol 2017; Epub ahead of print. [Crossref] [PubMed]
- Le MH, Le DM, Baez TC, et al. Global incidence of non-alcoholic fatty liver disease: A systematic review and meta-analysis of 63 studies and 1,201,807 persons. J Hepatol 2023;79:287-95. [Crossref] [PubMed]
- Younossi ZM, Golabi P, Paik JM, et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023;77:1335-47. [Crossref] [PubMed]
- Liu J, Tian Y, Fu X, et al. Estimating global prevalence, incidence, and outcomes of non-alcoholic fatty liver disease from 2000 to 2021: systematic review and meta-analysis. Chin Med J (Engl) 2022;135:1682-91. [Crossref] [PubMed]
- Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022;7:851-61. [Crossref] [PubMed]
- Quek J, Chan KE, Wong ZY, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2023;8:20-30. [Crossref] [PubMed]
- Li J, Ha A, Rui F, et al. Meta-analysis: global prevalence, trend and forecasting of non-alcoholic fatty liver disease in children and adolescents, 2000-2021. Aliment Pharmacol Ther 2022;56:396-406. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57. [Crossref] [PubMed]
- Miyake T, Kumagi T, Hirooka M, et al. Body mass index is the most useful predictive factor for the onset of nonalcoholic fatty liver disease: a community-based retrospective longitudinal cohort study. J Gastroenterol 2013;48:413-22. [Crossref] [PubMed]
- Vusirikala A, Thomas T, Bhala N, et al. Impact of obesity and metabolic health status in the development of non-alcoholic fatty liver disease (NAFLD): A United Kingdom population-based cohort study using the health improvement network (THIN). BMC Endocr Disord 2020;20:96. [Crossref] [PubMed]
- Li L, Liu DW, Yan HY, et al. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev 2016;17:510-9. [Crossref] [PubMed]
- Abeysekera KWM, Fernandes GS, Hammerton G, et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol Hepatol 2020;5:295-305. [Crossref] [PubMed]
- Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010;36:1825-35. [Crossref] [PubMed]
- Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol 2017;112:18-35. [Crossref] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627-42. [Crossref] [PubMed]
- Box GEP, Pierce DA. Distribution of Residual Autocorrelations in Autoregressive-Integrated Moving Average Time Series Models. J Am Stat Assoc. 1970;65:1509-26. [Crossref]
- Hankey BF, Ries LA, Kosary CL, et al. Partitioning linear trends in age-adjusted rates. Cancer Causes Control 2000;11:31-5. [Crossref] [PubMed]
- Le MH, Yeo YH, Li X, et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2022;20:2809-2817.e28. [Crossref] [PubMed]
- Chen K, Shen Z, Gu W, et al. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes Metab 2023;25:3390-9. [Crossref] [PubMed]
- Liu J, Ayada I, Zhang X, et al. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults. Clin Gastroenterol Hepatol 2022;20:e573-82. [Crossref] [PubMed]
- Loomis AK, Kabadi S, Preiss D, et al. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J Clin Endocrinol Metab 2016;101:945-52. [Crossref] [PubMed]
- Venegas JG, Harris RS, Simon BA. A comprehensive equation for the pulmonary pressure-volume curve. J Appl Physiol (1985) 1998;84:389-95. [Crossref] [PubMed]
- Zou B, Yeo YH, Nguyen VH, et al. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999-2016. J Intern Med 2020;288:139-51. [Crossref] [PubMed]
- Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:739-52. [Crossref] [PubMed]
- Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024;73:691-702. [Crossref] [PubMed]
Cite this article as: Dong X, Li JM, Lu XL, Lin XY, Hong MZ, Weng S, Pan JS. Global burden of adult non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) has been steadily increasing over the past decades and is expected to persist in the future. Transl Gastroenterol Hepatol 2024;9:33.


