
Role of endoscopy in the diagnosis of coeliac disease: a narrative review
Introduction
Coeliac disease (CD) is the most common autoimmune enteropathy, affecting approximately 1% of the population worldwide (1). CD is characterised by an immune-mediated response to gluten, a protein commonly found in wheat, barley and rye, resulting in intestinal villous atrophy in genetically predisposed individuals (2). This leads to nutritional deficiencies due to the malabsorption of nutrients and a wide array of gastrointestinal and extraintestinal symptoms. The diagnosis of CD typically involves a combination of serological testing with tissue-transglutaminase or endomysial antibodies, followed by endoscopy with duodenal biopsies for those who have positive serology or high clinical suspicion of CD (3).
Increasing awareness about the diverse presentations of patients with CD and the increasing accuracy of serological tests led to a significant rise in the incidence and prevalence of CD over the past two decades (4). Despite this, it is estimated that most people with CD remain undiagnosed or experience substantial delays in diagnosis (5).
In this review, we aim to discuss the role of endoscopy in the diagnosis of CD and the advancement in endoscopic techniques to identify villous atrophy. We present this article in accordance with the Narrative Review reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-122/rc).
Methods
The details of the search strategy for this narrative review are provided in Table 1. We searched PubMed and Google Scholar from their inception to December 2023 for relevant articles on the role of endoscopy in CD. Two authors (M.G.S. & A.Y.) reviewed these references and relevant studies were included in the discussion section of this review.
Table 1
| Items | Specification |
|---|---|
| Date of search | November & December 2023 |
| Databases and other sources searched | PubMed & Google Scholar |
| Search terms used | We used combinations of the following subject heading terms and keywords “celiac disease” OR “coeliac disease” AND “endoscopy” OR “capsule endoscopy” OR “artificial intelligence” |
| Timeframe | From inception to 18 December 2023 |
| Inclusion criteria | All study types, including review articles and systematic reviews published in English |
| Selection process | The literature search was conducted by M.G.S. and A.Y. |
Discussion
Endoscopic markers of CD
The loss of small bowel folds in patients with CD was first recongised in the 1930s. The “moulage sign” by Kantor described the featureless appearance of the dilated jejunal loops following the introduction of contrast media, resembling a tube into which wax has been poured (6). Decades later, the advent of endoscopy allowed the direct visualisation of the small bowel mucosa and the acquisition of duodenal biopsies to confirm the diagnosis of CD. The earliest reported endoscopic features of CD were scalloping and loss of duodenal folds (7,8). Initially routine duodenal biopsies were being taken at endoscopy for all patients with non-specific gastrointestinal symptoms. Bardella et al. reported a low diagnostic yield of routine duodenal biopsies in patients with dyspepsia presenting to endoscopy, with only 3 cases out of 517 (0.5%) found to have villous atrophy (9). A similar low prevalence of CD was found in a large Finnish study of open-access endoscopy, where routine duodenal biopsies confirmed CD in 0.7% of 5,347 patients with dyspepsia and in 0.6% of 2,974 patients with reflux symptoms (10). Therefore, owing to the low diagnostic yield and the high associated costs, routine duodenal biopsies were not recommended for patients with non-specific gastrointestinal symptoms and low pre-test probability of CD. Currently, endoscopic markers of CD such as scalloping, mosaic pattern, loss of duodenal folds, fissuring, nodularity, and erosions, are well described (Figure 1) (11). Obtaining duodenal biopsies during routine endoscopy in patients with endoscopic markers of CD has been shown to increase the diagnostic yield from 0.1% to 0.8% (12). However, the accuracy of these markers in predicting villous atrophy has been disappointing, especially in patients with partial villous atrophy, with a sensitivity ranging between 50–78.4% (9,13-17) (Table 2). Despite this, the recognition of endoscopic markers of CD remains important. In a recent study, almost 1 in 10 patients with newly diagnosed CD had at least one non-diagnostic endoscopy where no duodenal biopsies were taken in the 5 years prior to diagnosis (26). It is likely that some of these patients had endoscopic markers of CD at the index endoscopy, which were not recognised by the endoscopists, leading to significant delays in diagnosis (11). Moreover, documenting the presence of these markers during endoscopy may help with diagnosis in some cases where there are discrepancies between serology and histology results.
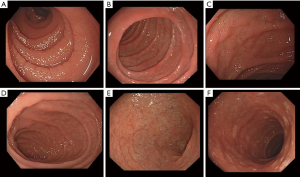
Table 2
| Endoscopic tool | Sensitivity | Specificity | Studies |
|---|---|---|---|
| White-light endoscopy | 50–78.4% | 86.1–99.6% | Bardella et al. (9) |
| Dickey et al. (13) | |||
| Oxentenko et al. (14) | |||
| Barada et al. (15) | |||
| Penny et al. (16) | |||
| Raju et al. (17) | |||
| Water immersion technique | 85–90.9% | 99–99.5% | Gasbarrini et al. (18) |
| Cammarota et al. (19) | |||
| Dye-based chromoendoscopy | 94% | 99% | Niveloni et al. (20) |
| Magnification endoscopy | 86.4–95% | 74.4–99% | Raju et al. (17) |
| Badreldin et al. (21) | |||
| Banerjee et al. (22) | |||
| I-Scan | 75–96% | 63–86.8% | Penny et al. (16) |
| Iacucci et al. (23) | |||
| Narrow band imaging | 93% | 95% | Shiha et al. (24) |
| Capsule endoscopy | 89% | 95% | Rokkas et al. (25) |
The optimal biopsy strategy
The villous atrophy in CD has a patchy distribution, and the severity of histological lesions may vary within the duodenal samples taken from individual patients (27). Villous atrophy may also be only confined to the duodenal bulb, known as ultra-short CD. Therefore, obtaining at least 3 duodenal biopsies, including a duodenal bulb biopsy, is required to ensure no cases of villous atrophy are missed (28). Whereas, a five-biopsy strategy is required for the recognition of the most severe histological lesions (28). A meta-analysis of 17 studies showed that duodenal bulb biopsies increased the diagnostic yield of CD by 5% [95% confidence interval (CI): 3–9%] (29). Current guidelines recommend that at least four duodenal biopsies are taken from the second part of the duodenum, and an extra 1 or 2 biopsies are taken from the duodenal bulb to optimise the diagnosis of CD (30). However, multiple studies have consistently shown that adherence to the biopsy guidelines occurs in less than 40% of cases, which is associated with an increased risk of missed diagnosis (26,31,32). Increasing awareness about the evidence supporting the biopsy guidelines and setting quality benchmarks for endoscopy in CD (Table 3) could lead to a significant improvement in the accuracy of endoscopy in CD and reduce the risk of post-gastroscopy CD.
Table 3
| Clinical domain | Suggested performance measures |
|---|---|
| Indication | Appropriate indication of upper GI endoscopy with available serology results |
| Completeness | Adequate photo-documentation of the duodenal bulb and the second part of the duodenum |
| Diagnosis | Adequate mucosal visualisation of the duodenum and the use of enhanced imaging |
| Accuracy | Obtaining ≥4 biopsies from the duodenal bulb and the second part of the duodenum using a single-bite technique |
| Documentation | Clear documentation of the presence or absence of endoscopic markers of coeliac disease |
GI, gastrointestinal.
Obtaining two biopsy specimens with each pass of the biopsy forceps increases the risk of specimen loss and reduces histological quality (33). A study comparing the single-biopsy and double-biopsy techniques in CD showed that the single-biopsy technique was associated with improved orientation of the duodenal biopsy specimens. However, there was no difference in the final Marsh scoring between the single- and double-bite biopsy techniques (34). These findings have not been replicated in other studies. In fact, a more recent study showed that there was no difference in the quality of the specimens between the single- and double-bite biopsy techniques (35). Further studies are needed to evaluate the optimal biopsy technique for CD. Another important factor to optimise the diagnosis is the correct orientation of the biopsy specimens. Non-oriented biopsies could lead to false-positive diagnosis of CD, even by expert pathologists (36). The correct orientation of biopsies begin in the endoscopy suite, with placing the biopsy specimens on a strip of paper in a straight line, with the luminal surface upwards (36). This technique aids the pathologists in making a more accurate diagnosis.
Endoscopic tools and techniques
Given the limitations of traditional white-light endoscopy in detecting villous atrophy, different novel endoscopic tools and techniques have been investigated, as summarised in Table 2.
Water immersion
Filling the duodenum with water during endoscopy enhances the visualisation of the intestinal villi (18). This technique, known as water immersion, is easy to perform and safe. It involves aspirating air from the duodenum, followed by the infusion of 100–200 mL of water (Figure 2) (19). A prospective study of 396 patients with dyspepsia, the sensitivity, specificity, positive and negative predictive values of the water immersion technique to detect villous atrophy were 90.9%, 99.5%, 83.3%, and 99.7%, respectively (19). Although this technique appears to be highly accurate and cost-effective, it is rarely used in routine clinical practice, probably due to perceptions of it as time-consuming (19).
Dye-based chromoendoscopy
Dye-based chromoendoscopy enables the detection of subtle mucosal abnormalities and has been shown to improve dysplasia detection in patients with long-standing inflammatory bowel disease (37). Conversely, the benefits of using chromoendoscopy in patients with CD are less clear. In a study by Niveloni et al., dye staining with methylene blue did not provide additional diagnostic information to expert endoscopists, compared with conventional endoscopy (20). Another study by Bonatto et al. proposed an endoscopic classification incorporating chromoendoscopy, using 0.5% indigo carmine, with zoom magnification to confirm the presence of villous atrophy during endoscopy. The authors showed that this classification increased the agreement between endoscopy and histopathology. However, the agreement remained weak in less severe cases (38).
Magnification endoscopy
High-magnification endoscopes have the capacity to optically magnify images up to 150 times, enabling detailed assessment of the intestinal mucosa (39). Few studies evaluated the role of magnification endoscopy in the diagnosis of CD. The first study to report the accuracy of endoscopic magnification for the detection of villous atrophy showed impressive results with a sensitivity of 95% and specificity of 99% (40). However, a larger study by Raju et al. reported a lower sensitivity of 86.4% and a specificity of 74.4% (17). The high cost of the high-magnification endoscopes and the lack of added diagnostic benefit over conventional endoscopy, hindered their routine use in clinical practice.
Narrow-band imaging (NBI)
NBI is a widely available advanced imaging technique that filters specific wavelengths of light to enhance the visualisation of the mucosal surface architecture (Figure 3) (41). NBI is routinely used for the assessment of polyps, Barrett’s oesophagus and early gastric cancers (42). However, it is rarely used for the assessment of duodenal mucosa outside expert centres and clinical studies. In a recent meta-analysis, we showed that NBI has a summary sensitivity of 93% (95% CI: 81–98%), and summary specificity of 95% (95% CI: 92–98%) to detect villous atrophy (24). Combining NBI with water immersion and magnification endoscopy may further improve the diagnostic accuracy (43,44). Using NBI in patients with suspected CD could help endoscopists target more accurate biopsies of potentially abnormal mucosa and reduce the reliance on multiple random biopsies. Furthermore, biopsies could be avoided in patients with low pre-test probability of CD and normal NBI findings (45).

Gulati et al. developed and validated a near-focus (NF)-NBI classification of villous atrophy in patients with suspected CD (46). This simple classification requires minimal training and could help expert and non-expert endoscopists in diagnosing villous atrophy during endoscopy. Yet, clinical validation of the (NF-NBI) in patients with a low- and high pre-test probability of CD is required.
Other endoscopic modalities
Several other endoscopic modalities have been investigated over the years to improve the optical diagnosis of villous atrophy, including I-scan, optimal band imaging, confocal laser endomicroscopy, and optical coherence tomography (16,23,47-49). However, none of these techniques is used in clinical practice due to their high cost, limited availability, and the absence of clear clinical benefits.
Capsule endoscopy (CE) and enteroscopy
Video CE enabled the visualisation of the entire length of the small bowel and revolutionised the diagnosis of small bowel diseases (50). A meta-analysis of 6 early studies showed that CE had a pooled sensitivity of 89% (95% CI: 82–94%) and specificity of 95% (95% CI: 89–98%) to predict villous atrophy (25). Therefore, CE is not recommended for the diagnosis of CD (30). Nonetheless, it plays an important role in the diagnosis of complications, such as ulcerative jejunitis and small bowel malignancy in patients with non-responsive or refractory CD (Figure 4) (51). A sequential approach of CE as a first-line investigation, followed by device-assisted enteroscopy if CE detected complications, has been shown to have a high diagnostic yield in patients with suspected refractory CD (52,53). In a meta-analysis of three studies, the pooled diagnostic yield of push endoscopy and double-balloon enteroscopy for the diagnosis of small bowel malignancy and ulcerative jejunitis in patients with complicated CD was 27% (95% CI: 14.8–42.6%) (51).

Artificial intelligence (AI)
AI has the potential to revolutionise the optical diagnosis of villous atrophy during endoscopy. Scheppach et al. recently developed an AI algorithm to detect villous atrophy from endoscopic still images (54). The AI algorithm significantly outperformed expert and non-expert endoscopists for the detection of villous atrophy, and its performance remained stable even in difficult images with subtle changes. Yet, the overall sensitivity, specificity and accuracy of the AI algorithm to detect villous atrophy were 90%, 76% and 84%, respectively (54). More studies with larger and more diverse training datasets are needed to improve the accuracy of the deep learning algorithms. Given the rapid advancements in this field, real-time computer-aided detection of villous atrophy during endoscopy may be on the horizon.
Role of endoscopy in the no-biopsy era
Although endoscopy and biopsy has been long considered as the gold standard test to diagnose CD, recent evidence suggests that serology-based diagnosis in selected adult patients with markedly high tissue transglutaminase antibody levels (≥10 times the upper limit of normal) is highly accurate (55). This no-biopsy approach has been used in the paediatric population for over a decade (56). Yet, following the same approach to diagnose adults with CD has been a matter of an ongoing debate (57,58). Avoiding unnecessary endoscopy could lead to significant reductions in both the healthcare costs and the carbon footprint of endoscopy (59). However, it is important to recognise that less than a third of patients with suspected CD would fulfill the criteria for a serology-based diagnosis, and that most patients will still need endoscopy and biopsy to confirm the diagnosis. Furthermore, many patients may still want to have a histological confirmation of CD before adhering to a life-long gluten-free diet. Therefore, the decision to pursue endoscopy- versus serology-based diagnosis for CD should be tailored to individual patient preferences, clinical presentation, and risk factors. Future studies on the accuracy of endoscopic tools for the detection of villous atrophy in CD may yield different results if they only included patients with low and intermediate tissue transglutaminase antibody levels.
Conclusions
In conclusion, endoscopy plays a vital role in the diagnosis of CD. Performing high-quality endoscopy and adhering to the biopsy guidelines reduce the risk of missed diagnosis. Integrating enhanced endoscopic imaging and deep learning can further enhance the accuracy of the optical diagnosis of villous atrophy, potentially reducing the need for multiple random biopsies. This approach could improve patient outcomes and reduce healthcare costs.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-122/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-122/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-122/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singh P, Arora A, Strand TA, et al. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:823-836.e2. [Crossref] [PubMed]
- Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet 2018;391:70-81. [Crossref] [PubMed]
- Downey L, Houten R, Murch S, et al. Recognition, assessment, and management of coeliac disease: summary of updated NICE guidance. BMJ 2015;351:h4513. [Crossref] [PubMed]
- Makharia GK, Chauhan A, Singh P, et al. Review article: Epidemiology of coeliac disease. Aliment Pharmacol Ther 2022;56:S3-S17. [Crossref] [PubMed]
- Anderson RP. Review article: Diagnosis of coeliac disease: a perspective on current and future approaches. Aliment Pharmacol Ther 2022;56:S18-37. [Crossref] [PubMed]
- Kantor JL. The roentgen diagnosis of idiopathic steatorrhea and allied conditions; practical value of the “moulage sign”. Am J Roentgenol Radium Ther 1939;41:758-77.
- Jabbari M, Wild G, Goresky CA, et al. Scalloped valvulae conniventes: an endoscopic marker of celiac sprue. Gastroenterology 1988;95:1518-22. [Crossref] [PubMed]
- Brocchi E, Corazza GR, Caletti G, et al. Endoscopic demonstration of loss of duodenal folds in the diagnosis of celiac disease. N Engl J Med 1988;319:741-4. [Crossref] [PubMed]
- Bardella MT, Minoli G, Radaelli F, et al. Reevaluation of duodenal endoscopic markers in the diagnosis of celiac disease. Gastrointest Endosc 2000;51:714-6. [Crossref] [PubMed]
- Collin P, Rasmussen M, Kyrönpalo S, et al. The hunt for coeliac disease in primary care. QJM 2002;95:75-7. [Crossref] [PubMed]
- Dickey W. Endoscopic markers for celiac disease. Nat Clin Pract Gastroenterol Hepatol 2006;3:546-51. [Crossref] [PubMed]
- Vjero K, Martucci S, Alvisi C, et al. Defining a proper setting for endoscopy in coeliac disease. Eur J Gastroenterol Hepatol 2003;15:675-8. [Crossref] [PubMed]
- Dickey W, Hughes D. Disappointing sensitivity of endoscopic markers for villous atrophy in a high-risk population: implications for celiac disease diagnosis during routine endoscopy. Am J Gastroenterol 2001;96:2126-8. [Crossref] [PubMed]
- Oxentenko AS, Grisolano SW, Murray JA, et al. The insensitivity of endoscopic markers in celiac disease. Am J Gastroenterol 2002;97:933-8. [Crossref] [PubMed]
- Barada K, Habib RH, Malli A, et al. Prediction of celiac disease at endoscopy. Endoscopy 2014;46:110-9. [Crossref] [PubMed]
- Penny HA, Mooney PD, Burden M, et al. High definition endoscopy with or without I-Scan increases the detection of celiac disease during routine endoscopy. Dig Liver Dis 2016;48:644-9. [Crossref] [PubMed]
- Raju SA, White WL, Lau MS, et al. A comparison study between Magniview and high definition white light endoscopy in detecting villous atrophy and coeliac disease: A single centre pilot study. Dig Liver Dis 2018;50:920-4. [Crossref] [PubMed]
- Gasbarrini A, Ojetti V, Cuoco L, et al. Lack of endoscopic visualization of intestinal villi with the "immersion technique" in overt atrophic celiac disease. Gastrointest Endosc 2003;57:348-51. [Crossref] [PubMed]
- Cammarota G, Pirozzi GA, Martino A, et al. Reliability of the "immersion technique" during routine upper endoscopy for detection of abnormalities of duodenal villi in patients with dyspepsia. Gastrointest Endosc 2004;60:223-8. [Crossref] [PubMed]
- Niveloni S, Fiorini A, Dezi R, et al. Usefulness of videoduodenoscopy and vital dye staining as indicators of mucosal atrophy of celiac disease: assessment of interobserver agreement. Gastrointest Endosc 1998;47:223-9. [Crossref] [PubMed]
- Badreldin R, Barrett P, Wooff DA, et al. How good is zoom endoscopy for assessment of villous atrophy in coeliac disease? Endoscopy 2005;37:994-8. [Crossref] [PubMed]
- Banerjee R, Shekharan A, Ramji C, et al. Role of magnification endoscopy in the diagnosis and evaluation of suspected celiac disease: correlation with histology. Indian J Gastroenterol 2007;26:67-9. [PubMed]
- Iacucci M, Poon T, Gui XS, et al. High definition i-SCAN endoscopy with water immersion technique accurately reflects histological severity of celiac disease. Endosc Int Open 2016;4:E540-6. [Crossref] [PubMed]
- Shiha MG, Nandi N, Oka P, et al. Narrow-band imaging for optical diagnosis of duodenal villous atrophy in patients with suspected coeliac disease: A systematic review and meta-analysis. Dig Liver Dis 2023; Epub ahead of print. [Crossref] [PubMed]
- Rokkas T, Niv Y. The role of video capsule endoscopy in the diagnosis of celiac disease: a meta-analysis. Eur J Gastroenterol Hepatol 2012;24:303-8. [Crossref] [PubMed]
- Taylor MA, Blanshard RJ, Naylor G, et al. Do gastroenterologists have medical inertia towards coeliac disease? A UK multicentre secondary care study. BMJ Open Gastroenterol 2021;8:e000544. [Crossref] [PubMed]
- Ravelli A, Villanacci V, Monfredini C, et al. How patchy is patchy villous atrophy?: distribution pattern of histological lesions in the duodenum of children with celiac disease. Am J Gastroenterol 2010;105:2103-10. [Crossref] [PubMed]
- Hopper AD, Cross SS, Sanders DS. Patchy villous atrophy in adult patients with suspected gluten-sensitive enteropathy: is a multiple duodenal biopsy strategy appropriate? Endoscopy 2008;40:219-24. [Crossref] [PubMed]
- McCarty TR, O'Brien CR, Gremida A, et al. Efficacy of duodenal bulb biopsy for diagnosis of celiac disease: a systematic review and meta-analysis. Endosc Int Open 2018;6:E1369-78. [Crossref] [PubMed]
- Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019;7:583-613. [Crossref] [PubMed]
- Lebwohl B, Kapel RC, Neugut AI, et al. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc 2011;74:103-9. [Crossref] [PubMed]
- Wallach T, Genta RM, Lebwohl B, et al. Adherence to Celiac Disease and Eosinophilic Esophagitis Biopsy Guidelines Is Poor in Children. J Pediatr Gastroenterol Nutr 2017;65:64-8. [Crossref] [PubMed]
- Hookey LC, Hurlbut DJ, Day AG, et al. One bite or two? A prospective trial comparing colonoscopy biopsy technique in patients with chronic ulcerative colitis. Can J Gastroenterol 2007;21:164-8. [Crossref] [PubMed]
- Latorre M, Lagana SM, Freedberg DE, et al. Endoscopic biopsy technique in the diagnosis of celiac disease: one bite or two? Gastrointest Endosc 2015;81:1228-33. [Crossref] [PubMed]
- Rettally C. Duodenal Biopsies: Individual or Multiple Samples? Am J Gastroenterol 2023;118:911-2. [Crossref] [PubMed]
- Villanacci V, Del Sordo R, Casella G, et al. The correct methodological approach to the diagnosis of celiac disease: the point of view of the pathologist. Gastroenterol Hepatol Bed Bench 2023;16:129-35. [PubMed]
- Feuerstein JD, Rakowsky S, Sattler L, et al. Meta-analysis of dye-based chromoendoscopy compared with standard- and high-definition white-light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer. Gastrointest Endosc 2019;90:186-195.e1. [Crossref] [PubMed]
- Bonatto MW, Kotze L, Orlandoski M, et al. Endoscopic evaluation of celiac disease severity and its correlation with histopathological aspects of the duodenal mucosa. Endosc Int Open 2016;4:E767-77. [Crossref] [PubMed]
- High-definition and high-magnification endoscopes. Gastrointest Endosc 2014;80:919-27. [Crossref] [PubMed]
- Cammarota G, Martino A, Pirozzi GA, et al. Direct visualization of intestinal villi by high-resolution magnifying upper endoscopy: a validation study. Gastrointest Endosc 2004;60:732-8. [Crossref] [PubMed]
- ASGE TECHNOLOGY COMMITTEE. Narrow band imaging and multiband imaging. Gastrointest Endosc 2008;67:581-9. [Crossref] [PubMed]
- Sugimoto M, Koyama Y, Itoi T, et al. Using texture and colour enhancement imaging to evaluate gastrointestinal diseases in clinical practice: a review. Ann Med 2022;54:3315-32. [Crossref] [PubMed]
- De Luca L, Ricciardiello L, Rocchi MB, et al. Narrow band imaging with magnification endoscopy for celiac disease: results from a prospective, single-center study. Diagn Ther Endosc 2013;2013:580526. [Crossref] [PubMed]
- Koay DSC, Ghumman A, Pu LZCT, et al. Narrow-band imaging with magnification and the water immersion technique: a case-finding, cost-effective approach to diagnose villous atrophy. Singapore Med J 2019;60:522-5. [Crossref] [PubMed]
- Tabibian JH, Murray JA. Near-focus narrow-band imaging for endoscopic assessment of duodenal villi: Making the case more than ever? Gastrointest Endosc 2021;94:1082-4. [Crossref] [PubMed]
- Gulati S, Emmanuel A, Ong M, et al. Near-focus narrow-band imaging classification of villous atrophy in suspected celiac disease: development and international validation. Gastrointest Endosc 2021;94:1071-81. [Crossref] [PubMed]
- Cammarota G, Cesaro P, Cazzato A, et al. Optimal band imaging system: a new tool for enhancing the duodenal villous pattern in celiac disease. Gastrointest Endosc 2008;68:352-7. [Crossref] [PubMed]
- Venkatesh K, Abou-Taleb A, Cohen M, et al. Role of confocal endomicroscopy in the diagnosis of celiac disease. J Pediatr Gastroenterol Nutr 2010;51:274-9. [Crossref] [PubMed]
- Masci E, Mangiavillano B, Albarello L, et al. Optical coherence tomography in the diagnosis of coeliac disease: a preliminary report. Gut 2006;55:579. [Crossref] [PubMed]
- Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature 2000;405:417. [Crossref] [PubMed]
- Elli L, Casazza G, Locatelli M, et al. Use of enteroscopy for the detection of malignant and premalignant lesions of the small bowel in complicated celiac disease: a meta-analysis. Gastrointest Endosc 2017;86:264-273.e1. [Crossref] [PubMed]
- Tomba C, Sidhu R, Sanders DS, et al. Celiac Disease and Double-Balloon Enteroscopy: What Can We Achieve?: The Experience of 2 European Tertiary Referral Centers. J Clin Gastroenterol 2016;50:313-7. [Crossref] [PubMed]
- Ferretti F, Branchi F, Orlando S, et al. Effectiveness of Capsule Endoscopy and Double-Balloon Enteroscopy in Suspected Complicated Celiac Disease. Clin Gastroenterol Hepatol 2022;20:941-949.e3. [Crossref] [PubMed]
- Scheppach MW, Rauber D, Stallhofer J, et al. Detection of duodenal villous atrophy on endoscopic images using a deep learning algorithm. Gastrointest Endosc 2023;97:911-6. [Crossref] [PubMed]
- Shiha MG, Nandi N, Raju SA, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology 2024;166:620-30. [Crossref] [PubMed]
- Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136-60. [Crossref] [PubMed]
- Shiha MG, Raju SA, Sidhu R, et al. The debate in the diagnosis of coeliac disease - time to go 'no-biopsy'? Curr Opin Gastroenterol 2023;39:192-9. [Crossref] [PubMed]
- Robert ME, Ciacci C, Lebwohl B. Opportunities for Improving Biopsy and Non-Biopsy-Based Diagnosis of Celiac Disease. Gastroenterology 2024; Epub ahead of print. [Crossref] [PubMed]
- Shiha MG, Nandi N, Hutchinson AJ, et al. Cost-benefits and environmental impact of the no-biopsy approach for the diagnosis of coeliac disease in adults. Frontline Gastroenterol 2024;15:95-8. [Crossref] [PubMed]
Cite this article as: Shiha MG, Yusuf A, Sanders DS. Role of endoscopy in the diagnosis of coeliac disease: a narrative review. Transl Gastroenterol Hepatol 2024;9:51.



