Cost-comparison of robotic versus laparoscopic colorectal resections: a mapped systematic review and meta-analysis of published studies
Highlight box
Key findings
• Laparoscopic colorectal resections (LCR) seem to be more economical as compared to robotic colorectal resections (RCR) in terms of operative cost as well as total cost.
What is known and what is new?
• Debate for the superiority of RCR over LCR in terms of perioperative outcomes
• In the random effects model analysis, LCR was associated with reduced total cost as well as reduced operative cost.
What is the implication, and what should change now?
• Concurrent use of this study along with the suitable cohort of the patients and previously published data on the postoperative outcome comparison, can help surgeons make better decisions considering the cost for the hospital and patient.
Introduction
Colorectal cancers are the third most diagnosed cancer worldwide (1). In the UK alone there are 42,000 new cases diagnosed every year (2). Risk factors for these colon cancers can include male sex, advanced age, familial history and lifestyle preferences (3). These patients undergoing colorectal resections for locally advanced tumours have poor survival rate and is also associated with high rates of recurrence in patients undergoing treatment with a curative intent (4,5).
Colorectal resections involve removing the pathological part of the bowel. Sometimes, it may involve removing the entire colon and/or rectum. Laparoscopic colorectal surgeries have shown to be superior when compared to the open approach, especially in emergency scenarios (6,7). The laparoscopic approach has been shown to be superior to the open approach in terms of post-operative pain, faster recovery, shorter hospital stays and better cosmesis (8,9). The laparoscopic approach is also reported to be a feasible and safe option for patients with underlying colorectal cancer (10). In 2006, Pigazzi et al. described a robotic approach for colorectal resections which showed to have a better 3D vision, wristed instruments offering better manoeuvrability in the pelvis and tremor abolition (11). Since the advent of robotic assistance in colorectal resections, multiple studies have been done comparing post-operative outcomes of robotic colorectal resections (RCR) versus laparoscopic colorectal resections (LCR). Some have shown that RCR is superior to LCR (12,13). A network meta-analysis done by Seow et al. showed that RCR offers better distal resection margin distance and a shorter length of hospital stay (14). But, on the other hand, there have been trials like COLRAR (15), which have shown no significant operative advantage.
In this systematic review, we explored the financial implications of these two approaches. This is important for patients and decreasing the cost of healthcare. This systematic review will compare the total and operative costs of LCR and RCR. We present this article in accordance with the PRISMA reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-73/rc).
Methods
Data sources and literature search technique
The literature review was methodically carried out from electronic databases like MEDLINE, EMBASE, PubMed and Cochrane Library using the MeSH search terms. Boolean operators (AND, OR, NOT) were used for extended search results. The titles were carefully screened for study selection. Moreover, references from shortlisted articles were examined to find additional relevant studies.
Trial selection
The trial selection period was limited to between the year 2010–till date. The primary inclusion criteria for the meta-analysis were the cost comparison of LCR versus RCR. The exclusion criteria for this meta-analysis were the studies without available cost data. The availability of the cost incurred for colorectal resections to the hospital was considered the only endpoint for this meta-analysis.
Data collection and management
Reported data were collected from the included trials by independent investigators on a standard data extraction form. The collected dataset was matched and found to be in satisfactory inter-reviewer agreement. The extracted data consisted of a list of the authors, title of the published study, journal of publication, country and year of the publication, sample size, patients in each group and total/operative cost. Following data extraction, the reviewers went through discussing their respective results and a consensus of mutual agreement was reached on likely discrepancies.
Quality of analysis
The methodological quality of the included randomised control trials was assessed using the published guidelines of Jadad et al., Chalmers et al. and Rangel et al. (16-18). A comprehensive table for the assessment of the quality of the included randomised control trial is given in Table 1. The quality of the comparative trials was assessed by the Scottish Intercollegiate Guidelines Network and Rangel et al. (18), and shown in Table 2.
Table 1
| Variables | Park 2019, (19) |
|---|---|
| Randomization technique | Computer generated |
| Concealment | Sealed envelope |
| Blinding | Single |
| Intention to treat analysis | Reported |
| Ethical approval | Reported |
| Registration number | NCT01423214 |
| Power calculation | Reported, not achieved |
Table 2
| Quality variables | Al-Mazrou 2018, (20) | Baek 2012, (21) | Ezeokoli 2023, (22) | Hollis 2016, (23) | Gebhardt 2022, (24) | Merola 2020, (25) | Moghadamyeghaneh 2016, (26) | Morelli 2016, (27) | Roskam 2023, (28) | Vasudevan 2016, (29) | Wei 2023, (30) | Wlodarczyk 2023, (31) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion criteria | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Exclusion criteria | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| Demographics comparable | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 |
| Can the number of participating centres be determined | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Can the number of surgeons who participated be determined | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Can the reader determine where the authors are on the learning curve for the reported procedure | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Are diagnostic criteria clearly stated for clinical outcomes if required | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Is the surgical technique adequately described | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Is there any way that they have tried to standardize the operative technique | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Is there any way that they have tried to standardize perioperative care | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| Is the age and range given for patients in the laparoscopic group | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Do authors address whether there is any missing data | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Is the age and range given for patients in the robotic group | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Were patients in each group treated along similar timelines | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| Did all the patients asked to enter the study take part | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dropout rates stated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Outcomes clearly defined | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Blind assessors | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Standardised assessment tools | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Analysis by intention to treat | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Score | 8 | 8 | 9 | 8 | 13 | 8 | 8 | 9 | 11 | 12 | 10 | 10 |
Statistical analysis
Statistical analysis was performed using RevMan 5.4 (Review Manager 5.4, The Nordic Cochrane Centre, Copenhagen, Denmark). The standard mean difference and a confidence interval (CI) of 95% were used for continuous data and a random-effects model (32,33) was used. Heterogeneity was calculated by inspecting the forest plots and by computing the Chi2 test, with significance set at P<0.05 as well as using the I2 test with a maximum value of 30 percent identifying low heterogeneity (34). For the sensitivity analysis, in each cell frequency, 0.5 was added in the studies where no event occurred in either the treatment or control group, as per the guidelines recommended by Deeks et al. (35). The inverse-variance method was used for the calculation of standard mean difference under the random effect model analysis. If the standard deviation was not available, then the risk of bias was calculated according to the guidelines provided by the Cochrane Collaboration (32). This process assumed that both groups had the same variance, which may not have been true, and variance was either estimated from the range or from the P value. The estimate of the difference between both techniques was pooled, depending upon the effect weights in results determined by each trial estimate variance. A forest plot was used for the graphical display of the results. The square around the estimate stood for the accuracy of the estimation (sample size), and the horizontal line represented the 95% CI.
Results
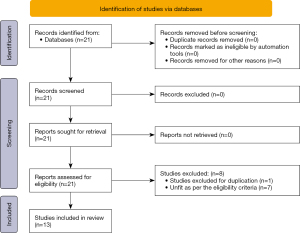
The initial database search for cost comparison between laparoscopic and RCR generated 21 studies. After excluding duplication and undesirable studies we were left with 13 studies, 11 studies were used in total cost comparison and 7 were used in operative cost comparison. One study was used twice as it had separate comparisons for diverticulitis and colon cancers (27) (Figure 1).
Characteristics and demographics of included studies
Twelve comparative trials (20-31) and one randomized controlled trial (RCT) (19) on 16,082 patients were included to conduct this meta-analysis for comparing total cost and operative cost of robotic versus laparoscopic colorectal surgeries, based upon the principles provided by the Cochrane Collaboration. Seven studies were from the USA (20,22,23,26,28,29,31), two were from Korea (19,21), two were from Italy (25,27), one was from Taiwan region (30) and one was from Germany (24). Mean age and gender were also noted in these studies. The PRISMA flow chart for trial selection is given in Figure 1. The main characteristics of the included studies are given in Table 3. The treatment protocol used in each is given in Table 4.
Table 3
| Study | Country/region | Study type | N | Age (years), mean ± SD | Gender (men) (%) |
|---|---|---|---|---|---|
| Al-Mazrou 2018, (20) | USA | Retrospective study | |||
| Laparoscopic | 2,219 | 63±16.6 | 55 | ||
| Robotic | 2,219 | 64±16.6 | 54.4 | ||
| Baek 2012, (21) | Korea | Prospective study | |||
| Laparoscopic | 150 | 62.3±10.9 | 72.7 | ||
| Robotic | 154 | 59.1±12.2 | 68.2 | ||
| Ezeokoli 2023, (22) | USA | Retrospective study | |||
| Laparoscopic | 258 | 66.4±15.5 | 42 | ||
| Robotic | 240 | 64.9±12.4 | 53 | ||
| Gebhardt 2022, (24) | Germany | Retrospective study | |||
| Laparoscopic | 38 | 37±12 | 52.6 | ||
| Robotic | 29 | 39±15 | 44.8 | ||
| Hollis 2016, (23) | USA | Retrospective study | |||
| Laparoscopic | 67 | 57.9* | 50.8 | ||
| Robotic | 45 | 58.4* | 42.2 | ||
| Merola 2020, (25) | Italy | Retrospective study | |||
| Laparoscopic | 94 | 72.09±9.54 | 64.89 | ||
| Robotic | 94 | 69.41±10.31 | 63.82 | ||
| Moghadamyeghaneh 2016, (26) | USA | Retrospective study | |||
| Laparoscopic | 9,614 | 48±17 | 46.1 | ||
| Robotic | 326 | 46±18 | 51.1 | ||
| Morelli 2016, (27) | Italy | Retrospective study | |||
| Laparoscopic | 25 | 68.9±11.5 | 60 | ||
| Robotic | 50 | 68.8±10.7 | 66 | ||
| Park 2019, (19) | South Korea | Randomized control trial | |||
| Laparoscopic | 36 | 67.2±10.1 | 68.8 | ||
| Robotic | 35 | 65.5±11.4 | 64.2 | ||
| Roskam, (a) 2023, (28) | USA | Retrospective study | |||
| Laparoscopic | 8 | 58.8±5.7 | NR | ||
| Robotic | 14 | 63.4±11.5 | NR | ||
| Roskam, (b) 2023, (28) | USA | Retrospective study | |||
| Laparoscopic | 8 | 60.2±9.8 | NR | ||
| Robotic | 14 | 58.8±5.7 | NR | ||
| Vasudevan 2016, (29) | USA | Retrospective study | |||
| Laparoscopic | 131 | 70.9±13.4 | 49 | ||
| Robotic | 96 | 63.6±12.7 | 51 | ||
| Wei 2023, (30) | Taiwan | Retrospective study | |||
| Laparoscopic | 49 | 66.2±12.5 | 38.77 | ||
| Robotic | 17 | 63.4±12.0 | 41.17 | ||
| Wlodarczyk 2023, (31) | USA | Retrospective study | |||
| Laparoscopic | 33 | 66.5±15.5 | 18.2 | ||
| Robotic | 19 | 66.8±16.0 | 10.5 |
*, average age from the data available using Microsoft Bing AI. N, number; SD, standard deviation; NR, not reported.
Table 4
| Study | Laparoscopic [%] | Robotic [%] |
|---|---|---|
| Al-Mazrou 2018, (20) | Diagnosis—neoplasm [91.3], diverticular disease [6.9] and others [1.8] | Diagnosis—neoplasm [91.5], diverticular disease [6.8] and others [1.7] |
| Procedures—colectomy [52.8], sigmoidectomy [17.9], AR [28.6], APR [13.3], rectal procedures [4.2] and other [1.1] | Procedures—colectomy [53], sigmoidectomy [16.7], AR [28.9], APR [12.5], rectal procedures [4.4] and other [1.2] | |
| Baek 2012, (21) | Diagnosis—neoplasm [100] | Diagnosis—neoplasm [100] |
| Procedures—AR [86.7], APR [2.7] and others [10.6] | Procedures—AR [69.4], APR [7.1] and others [23.5] | |
| Ezeokoli 2023, (22) | Diagnosis—neoplasm [100] | Diagnosis—neoplasm [100] |
| Procedures—colectomy [82], rectal procedures [16] and others [4] | Procedures—colectomy [63], rectal procedures [34] and others [3] | |
| Gebhardt 2022, (24) | Diagnosis—IBD [100] | Diagnosis—IBD [100] |
| Procedures—colectomy [100] | Procedures—colectomy [100] | |
| Hollis 2016, (23) | Diagnosis—neoplasm [52.2], IBD [11.9], diverticular disease [14.9] and others [20.9] | Diagnosis—neoplasm [75.6], IBD [15.6] and diverticular disease [8.9] |
| Procedures—colectomy [79.1], AR [17.9], APR [1.5] and others [1.5] | Procedures—colectomy [17.8], AR [60], APR [15.6] and others [6.6] | |
| Merola 2020, (25) | Diagnosis—neoplasm [100] | Diagnosis—neoplasm [100] |
| Procedures—colectomy [100] | Procedures—colectomy [100] | |
| Moghadamyeghaneh 2016, (26) | Diagnosis—neoplasm [31.2], IBD [38.9], diverticular disease [3.8] and others [26.1] | Diagnosis—neoplasm [39.9], IBD [46.4], and others [13.7] |
| Procedures—colectomy [100] | Procedures—colectomy [100] | |
| Morelli 2016, (27) | Diagnosis—neoplasm [100] | Diagnosis—neoplasm [100] |
| Procedures—AR [84], APR [8] and others [8] | Procedures—AR [64], APR [14] and others [22] | |
| Park 2019, (19) | Diagnosis—neoplasm [100] | Diagnosis—neoplasm [100] |
| Procedures—AR [81.5], APR [2.1] and others [16.4] | Procedures—AR [73.5], APR [3.3] and others [23.2] | |
| Roskam (a) 2023, (28) | Diagnosis—neoplasm [100] | Diagnosis—neoplasm [100] |
| Procedures—sigmoidectomy [100] | Procedures—sigmoidectomy [100] | |
| Roskam (b) 2023, (28) | Diagnosis—diverticular disease [100] | Diagnosis—diverticular disease [100] |
| Procedures—sigmoidectomy [100] | Procedures—sigmoidectomy [100] | |
| Vasudevan 2016, (29) | Diagnosis—neoplasm [61.8] and others [38.2] | Diagnosis—neoplasm [62.5] and others [37.5] |
| Procedures—colectomy [92.4] and others [7.7] | Procedures—colectomy [99] and others [1] | |
| Wei 2023, (30) | Diagnosis—neoplasm [61.8] and others [38.2] | Diagnosis—neoplasm [61.8] and others [38.2] |
| Procedures—colectomy [27.66] and AR [72.34] | Procedures—colectomy [17.64] and AR [82.36] | |
| Wlodarczyk 2023, (31) | Diagnosis—rectal prolapse [100] | Diagnosis—rectal prolapse [100] |
| Procedures—rectal procedures [100] | Procedures—rectal procedures [100] |
AR, anterior resection; APR, abdominoperineal resection; IBD, inflammatory bowel disease.
Methodological quality of included studies
The methodological quality of included trials is summarized in Tables 3,4. The randomization in RCT was performed through a computer-generated random number, and the concealment was ensured with the help of sealed envelopes. The trial was a single-blind RCT. The quality of the 13 comparative studies (retrospective & prospective) was analysed by using the Scottish Intercollegiate Guidelines Network and Rangel et al. (18), and 12 studies were found to have fair quality (20-31).
The outcome of the primary variable
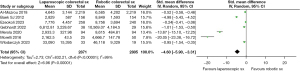
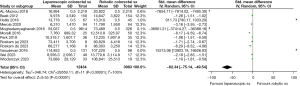
In the analysis, the use of LCR seems to be more economical in terms of total and operative costs. In the random effects model analysis, LCR was associated with the reduced total cost [standardised mean difference −62.34, 95% CI: −75.14 to −49.54, Z=9.55, P<0.001] as well as reduced operative cost [standardised mean difference −4.60, 95% CI: −5.90 to −3.31, Z=6.96, P<0.001] compared to RCR. However, there was significant heterogeneity (Tau2=346.74; Chi2=29,559.11, df=11; (P<0.001; I2=100%) (Tau2=2.73; Chi2=832.21, df=6; (P<0.001; I2=99%) among included studies (Figures 2,3).
Discussion
Key findings
The review of medical databases resulted in thirteen studies (one RCT and 12 retrospective studies) on 16,082 patients undergoing oncological and non-oncological colorectal resections. Eleven studies reported total cost whereas seven studies reported only operative cost. The LCR seems to be more economical compared to RCR in terms of operative cost as well as total cost (operative plus in-patient stay).
Comparison with existing literature
According to the review of the literature, this is the only meta-analysis comparing the costs of laparoscopic versus RCR. There have been multiple studies comparing the perioperative outcomes of LCR versus RCR. Zhang et al. have shown that the safety and efficacy of RCR are comparable to LCR (36). Similarly, Safiejko et al. and Wang et al. have also shown that RCR provides several advantages lower conversion rate, decreased hospital stay, improved overall survival and decreased infection rates (37,38).
Strength and limitations
The RCT used in the analysis was strong in strength, randomization was computer generated, concealment was sealed, ethical approval was taken, it was a blinded trial and intention to treat analysis was reported. The rest of the thirteen comparative trials used in this study were also of adequate strength. Therefore, in total the evidence provided is of high quality.
Nonetheless, this meta-analysis also has multiple limitations. There was a statistically significant heterogeneity among included studies and paucity of the RCT, these findings should be taken cautiously. Also, the procedures used in this meta-analysis were of wide range and although most of the studies were centred around colon cancers there were a significant number of patients with other pathology as well.
Implications
Multiple studies have been done comparing the post-operative outcomes of RCR versus LCR. Concurrent use of this study along with the suitable cohort of the patients and previously published data on the post-operative outcome comparison, can help surgeons make better decisions considering the cost for the hospital and patient.
Conclusions
The LCR seems to be more economical compared to RCR in terms of operative cost as well as total cost (operative plus in-patient stay). However, due to statistically significant heterogeneity among included studies and paucity of the RCT, these findings should be taken cautiously. A major multi-centric large-scale RCT comparing the post-operative outcomes along with the cost is imperative to confirm the findings of our meta-analysis.
Acknowledgments
The provisional abstract of this systematic review has been presented at the annual conference of “The Association of Surgeons of Great Britain and Ireland” on 17th–19th May 2023 at Harrogate, United Kingdom and it is now available in the British Journal of Surgery.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-73/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-73/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-73/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Cancer Research UK. Bowel cancer statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer
- Landmann RG, Weiser MR. Surgical management of locally advanced and locally recurrent colon cancer. Clin Colon Rectal Surg 2005;18:182-9. [Crossref] [PubMed]
- Schellenberg AE, Moravan V, Christian F. A competing risk analysis of colorectal cancer recurrence after curative surgery. BMC Gastroenterol 2022;22:95. [Crossref] [PubMed]
- van der Stok EP, Spaander MCW, Grünhagen DJ, et al. Surveillance after curative treatment for colorectal cancer. Nat Rev Clin Oncol 2017;14:297-315. [Crossref] [PubMed]
- Kudou K, Kusumoto T, Hasuda H, et al. Comparison of Laparoscopic and Open Emergency Surgery for Colorectal Perforation: A Retrospective Study. J Laparoendosc Adv Surg Tech A 2023;33:464-70. [Crossref] [PubMed]
- Chen YC, Tsai YY, Chang SC, et al. Laparoscopic versus open emergent colectomy for ischemic colitis: a propensity score-matched comparison. World J Emerg Surg 2022;17:53. [Crossref] [PubMed]
- Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 2013;100:75-82. [Crossref] [PubMed]
- Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637-45. [Crossref] [PubMed]
- Ustuner MA, Deniz A, Simsek A. Laparoscopic versus Open Surgery in Colorectal Cancer: Is Laparoscopy Safe Enough? J Coll Physicians Surg Pak 2022;32:1170-4. [Crossref] [PubMed]
- Pigazzi A, Ellenhorn JD, Ballantyne GH, et al. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 2006;20:1521-5. [Crossref] [PubMed]
- Emile SH, Horesh N, Garoufalia Z, et al. Robotic and laparoscopic colectomy: propensity score-matched outcomes from a national cancer database. Br J Surg 2023;110:717-26. [Crossref] [PubMed]
- Juang SE, Chung KC, Cheng KC, et al. Outcomes of robot-assisted versus laparoscopic surgery for colorectal cancer in morbidly obese patients: A propensity score-matched analysis of the US Nationwide Inpatient Sample. J Gastroenterol Hepatol 2023;38:1510-9. [Crossref] [PubMed]
- Seow W, Dudi-Venkata NN, Bedrikovetski S, et al. Outcomes of open vs laparoscopic vs robotic vs transanal total mesorectal excision (TME) for rectal cancer: a network meta-analysis. Tech Coloproctol 2023;27:345-60. [Crossref] [PubMed]
- Park JS, Lee SM, Choi GS, et al. Comparison of Laparoscopic Versus Robot-Assisted Surgery for Rectal Cancers: The COLRAR Randomized Controlled Trial. Ann Surg 2023;278:31-8. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Chalmers TC, Smith H Jr, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49. [Crossref] [PubMed]
- Rangel SJ, Kelsey J, Colby CE, et al. Development of a quality assessment scale for retrospective clinical studies in pediatric surgery. J Pediatr Surg 2003;38:390-6. [Crossref] [PubMed]
- Park JS, Kang H, Park SY, et al. Long-term oncologic after robotic versus laparoscopic right colectomy: a prospective randomized study. Surg Endosc 2019;33:2975-81. [Crossref] [PubMed]
- Al-Mazrou AM, Baser O, Kiran RP. Propensity Score-Matched Analysis of Clinical and Financial Outcomes After Robotic and Laparoscopic Colorectal Resection. J Gastrointest Surg 2018;22:1043-51. [Crossref] [PubMed]
- Baek SJ, Kim SH, Cho JS, et al. Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg 2012;36:2722-9. [Crossref] [PubMed]
- Ezeokoli EU, Hilli R, Wasvary HJ. Index cost comparison of laparoscopic vs robotic surgery in colon and rectal cancer resection: a retrospective financial investigation of surgical methodology innovation at a single institution. Tech Coloproctol 2023;27:63-8. [Crossref] [PubMed]
- Hollis RH, Cannon JA, Singletary BA, et al. Understanding the Value of Both Laparoscopic and Robotic Approaches Compared to the Open Approach in Colorectal Surgery. J Laparoendosc Adv Surg Tech A 2016;26:850-6. [Crossref] [PubMed]
- Gebhardt JM, Werner N, Stroux A, et al. Robotic-Assisted versus Laparoscopic Proctectomy with Ileal Pouch-Anal Anastomosis for Ulcerative Colitis: An Analysis of Clinical and Financial Outcomes from a Tertiary Referral Center. J Clin Med 2022;11:6561. [Crossref] [PubMed]
- Merola G, Sciuto A, Pirozzi F, et al. Is robotic right colectomy economically sustainable? a multicentre retrospective comparative study and cost analysis. Surg Endosc 2020;34:4041-7. [Crossref] [PubMed]
- Moghadamyeghaneh Z, Hanna MH, Carmichael JC, et al. Comparison of open, laparoscopic, and robotic approaches for total abdominal colectomy. Surg Endosc 2016;30:2792-8. [Crossref] [PubMed]
- Morelli L, Guadagni S, Lorenzoni V, et al. Robot-assisted versus laparoscopic rectal resection for cancer in a single surgeon's experience: a cost analysis covering the initial 50 robotic cases with the da Vinci Si. Int J Colorectal Dis 2016;31:1639-48. [Crossref] [PubMed]
- Roskam JS, Soliman SS, Chang GC, et al. A Single-Institution Comparison of Robot-Assisted and Conventional Laparoscopic Colorectal Surgeries. Am Surg 2023;89:4952-4. [Crossref] [PubMed]
- Vasudevan V, Reusche R, Wallace H, et al. Clinical outcomes and cost-benefit analysis comparing laparoscopic and robotic colorectal surgeries. Surg Endosc 2016;30:5490-3. [Crossref] [PubMed]
- Wei PL, Huang YJ, Wang W, et al. Comparison of robotic reduced-port and laparoscopic approaches for left-sided colorectal cancer surgery. Asian J Surg 2023;46:698-704. [Crossref] [PubMed]
- Wlodarczyk J, Brabender D, Gupta A, et al. Increased cost burden associated with robot-assisted rectopexy: do patient outcomes justify increased expenditure? Surg Endosc 2023;37:2119-26. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341-50. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta-Analysis. In: Egger M, Smith GD, Altman DG. editors. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd ed. London: John Wiley & Sons, Ltd.; 2001:285-312.
- Zhang X, Wei Z, Bie M, et al. Robot-assisted versus laparoscopic-assisted surgery for colorectal cancer: a meta-analysis. Surg Endosc 2016;30:5601-14. [Crossref] [PubMed]
- Safiejko K, Tarkowski R, Koselak M, et al. Robotic-Assisted vs. Standard Laparoscopic Surgery for Rectal Cancer Resection: A Systematic Review and Meta-Analysis of 19,731 Patients. Cancers (Basel) 2021;14:180. [Crossref] [PubMed]
- Wang X, Cao G, Mao W, et al. Robot-assisted versus laparoscopic surgery for rectal cancer: A systematic review and meta-analysis. J Cancer Res Ther 2020;16:979-89. [Crossref] [PubMed]
Cite this article as: Singh A, Kaur M, Baig MK, Swaminathan C, Subramanian A, Sajid MS. Cost-comparison of robotic versus laparoscopic colorectal resections: a mapped systematic review and meta-analysis of published studies. Transl Gastroenterol Hepatol 2024;9:21.