From random to precise: updated colon cancer screening and surveillance for inflammatory bowel disease
Introduction
Drs. Crohn and Rosberg first reported the association of colitis and colorectal cancer (CRC) in 1925, while Warren and Summers reported a similar association for Crohn’s and CRC in 1948 (1). Since then, CRC has been the dreaded complication of inflammatory bowel disease (IBD). The risk of CRC in patients with IBD is one third higher than in the general population. In addition, patients with IBD and advanced stage CRC have poorer survival rates than patients with sporadic CRC (2). Longstanding inflammation has been associated with an increased risk of developing dysplasia and malignancy, and it appears that patients with IBD develop CRC through different mechanisms than those with sporadic CRC (3). Dysplasia is the precursor of cancer and therefore, early detection and management of dysplasia in IBD is crucial to prevent CRC.
Endoscopic surveillance practices and management of dysplasia have evolved over the past two decades and will continue to do so. This is largely due to changes in understanding of disease, newer treatments, and improved technology. In this paper we review current thinking and recommendations for prevention of CRC in IBD, using a chronological historic perspective.
Background, epidemiology and risk factors
In western countries, over the past 30 years, the incidence of CRC related death in the IBD population has been decreasing (4-6). One study showed that the relative risk of CRC in ulcerative colitis (UC) patients decreased from 1.34 to 0.57 (6).
This may be due to the use of biologic therapies, improved surveillance strategies as well as overall awareness of the disease process (7). IBD related CRC only accounts for about 1–2% of all cases of CRC but IBD is one of the 3 major risk factors for CRC. In 2001, Eaden et al., in their meta-analysis estimated that the cumulative risk of CRC in patients with UC was 2% at 10 years, 8% at 20 years and 18% at 30 years (8). However, over a decade later, Lutgens et al., in their meta-analysis reported that the incidence of CRC amongst patients with IBD has declined and was 1%, 2% and 5% after 10, 20 and >20 years of disease duration respectively (9). Although this meta-analysis included diverse and heterogenous studies and so should be interpreted with caution, another study from the longest running UC surveillance program in the world confirmed lowered risk, approximately 10% at 40 years of disease (10).
If IBD patients do develop CRC, it tends to occur at a younger age than the general population, possibly reflecting the importance of inflammation in etiopathogenesis (11). A family history of CRC increases the risk of CRC by two-fold in patients with IBD (12). In Crohn’s specifically, colonic involvement as opposed to ileocecal disease or ileal disease, bears a higher risk for CRC. Furthermore, complications of persistent inflammation such as a foreshortened colon, inflammatory polyps and strictures, increase the risk of malignancy (13). Location of the lesions in the right colon may be another risk factor for dysplasia (14).
Primary sclerosing cholangitis (PSC) is an independent risk factor for colon malignancy in patients with IBD. Patients with PSC have a fivefold risk of developing CRC compared with those that don’t (14). The correlation between PSC and UC is stronger than that with Crohn’s disease (CD). The occurrence of concurrent PSC in UC patients is reported to be as high as 8%, but this varies by the extent of the disease (15). The prevalence is thought to be close to 6% in patients with pancolitis, but 1% in patients with distal colitis (15). In patients with CD the occurrence of concurrent PSC ranges from 1% to 3% in CD (15). The reason for the increased risk is unknown. Individuals with PSC have impaired ability to eliminate bile acids and exhibit a higher concentration of secondary bile acids (16). It is believed that secondary bile acids may have tumor-promoting effects, which is one of the proposed explanations for the elevated risk of CRC in this patient population (16). Furthermore, patients with PSC and IBD have more right sided colon cancers (17). It is important to note that aggressive surveillance should not be ceased after liver transplantation in patients with PSC, as these patients continue to be at high risk for CRC (18).
The risk of CRC also increases with disease severity and duration. In UC, there is a 10–15-fold increased risk of CRC with pancolitis and a two-fold increased risk with left sided UC. This risk increases with duration of disease. We have traditionally used duration from symptom onset in determining risk, but there is an increasing body of literature showing that it is duration of active inflammation that increases risk, cumulative inflammatory burden (CIB) rather than simply duration from symptom onset. In an interesting retrospective study from St Marks, in the UK, 987 patients who had 6,985 colonoscopies between 2003 and 2012, were scored for endoscopic and histologic inflammation and a numerical number was calculated for CIB using a multiplication of inflammation score and surveillance intervals. They showed through statistical analysis that the higher the number for CIB, the lower the chance of remaining free of advanced neoplasia (AN) (19). Disease extent also determines risk, and patients with limited disease such as proctosigmoiditis or proctitis may not be at higher risk for CRC than the general population (20).
While colon removal, such as with a proctocolectomy, may eliminate the risk of malignancy, there is still an increased risk of pouch dysplasia in patients who have undergone an ileal pouch anal anastomosis (IPAA) (20).
Pathogenesis of CRC in IBD
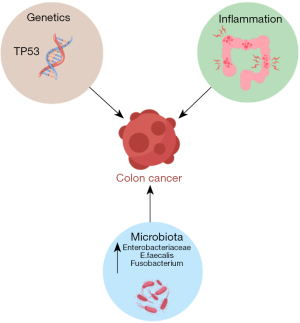
The pathogenesis of CRC in IBD is believed to be multifactorial, involving chronic inflammation, genetic/epigenetic factors and environmental/microbial factors (Figure 1).
IBD appears to be the prototype of inflammation induced cancer. It is postulated that, in IBD, multiple cytokines and pro-inflammatory molecules stimulate and maintain inflammation, inducing oxidative stress, which leads to tumorigenesis (21). Tumor necrosis factor signals oncogenic pathways such as Wnt and nuclear factor kappa B (NF-κB) in epithelial cells. NF-κB stimulates the production of reactive oxygen and nitrogen species which cause DNA damage and mutations of oncogenes and tumor suppressor genes as well as chromosomal instability. This cascade further advances the proliferation of tumor progenitor cells (21).
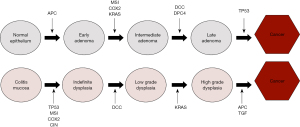
Unlike sporadic CRC which develops from dysplasia in one or two foci of the colon, dysplasia arising in colitis mucosa is usually multifocal and arises from swathes of inflammation that is prone to chromosomal instability (7). This is the principle behind segmental biopsies. Most colitis associated cancer (CAC) follows the traditional intestinal pathway, in terms of crypt architecture and cellular changes. Morphologically it can be tubular, villous or serrated lesions occur. Dysplasia is the precursor of cancer and is described as indefinite for dysplasia (IND) low-grade dysplasia (LGD), and high-grade dysplasia (HGD), depending on various characteristic pathological features such as nuclear stratification, pleomorphism, nucleolar size, mitosis, etc. However, in contrast to sporadic colon cancer, where adenomatous polyposis coli (APC) drives early adenoma formation and tumor suppressor P53 (TP53) is the last gene mutation that drives late adenomas into cancer, early loss of the TP53 gene drives dysplasia formation in CAC and the APC gene is responsible for the later progression of HGD to cancer (22) (Figure 2). TP53 can be found in inflamed tissue and early dysplasia while tumor epithelial tissue in CAC displays a decreased occurrence of APC and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations (21).
In a healthy gut, the microbiome serves several functions including regulation of the immune system, production of nutrients and protection against pathogens (24). It is postulated that alterations of inflammatory pathways and barrier dysfunction caused by an abnormality in the microbiome may play a role in CAC. Studies have shown that the gut flora has less diversity in patients with IBD, particularly in Crohn’s. These patients were found to have an increase of Enterobacteriaceae, E faecalis and Fusobacterium compared to the general population (25,26). IBD patients have reduced levels of butyrate-producing bacteria, which is significant because butyrate acid helps to impede signaling pathways of pro-inflammatory cytokines (27). The reduced quantity of short chain fatty acids (SCFAs) in the microbiome of individuals with IBD can compromise the epithelium’s integrity, thus increasing translocation of bacteria. Recent studies have revealed the presence of invasive bacterial biofilms (28). In a normal epithelial surface, the mucus layer is shed with stool, carrying microbes with it. When there are invasive bacteria within the mucus film, the mucus remains in the crypts, allowing bacteria to gain closer access to cells, producing an inflammatory response. It is likely that these biofilms are more important than specific bacteria in the pathogenesis of inflammation and tumorigenesis (29).
Dysplasia in IBD
Dysplasia is defined by the World Health Organization (WHO) as histologically unequivocal neoplastic epithelium without evidence of tissue invasion. It is the precursor of cancer. Most dysplasia in IBD follows intestinal morphology with tubular, villous and serrated patterns in terms of crypt morphology and IND, LGD and HGD at the cellular level. The neoplastic advancement in IBD occurs in a sequential manner. Inflamed mucosa can be difficult to distinguish from IND, underscoring the importance of attaining remission prior to surveillance. IND in normal mucosa can subsequently progress to LGD and HGD (30).
LGD and HGD are defined by the degree of architectural and cytologic atypia. The criteria for distinguishing between the types of dysplasia are subject to interobserver variability. This is particularly so in distinguishing post inflammatory regenerative changes from IND and underscores the importance of achieving remission prior to surveillance and also of having an expert pathologist confirm dysplasia (31,32). Identifying dysplasia allows for timely intervention and implementation of appropriate surveillance strategies. The majority of dysplasia in IBD is of intestinal type or conventional dysplasia. More recently, another type of dysplasia, non-conventional dysplasia, has been described that does not follow the traditional intestinal morphology. Non-conventional dysplasia has been divided into 5–7 subtypes: hypermucinous, goblet cell deficient (GCD), terminal epithelial differentiation (TED), traditional serrated adenoma (TSA), sessile serrated lesion (SSL), serrated lesion, not otherwise specified (NOS) (33). In an interesting retrospective analysis of 207 patients who had a colectomy and had one high-definition (HD) colonoscopy with targeted biopsies within one year prior to surgery, 27 patients had undetected dysplasia, the majority of which were invisible and of the non-conventional type (34). Other studies have also shown that HGD and CRC are more commonly linked to non-conventional dysplasia in patients with IBD and that greater than 40% of non-conventional dysplasia is flat or invisible (35).
Importance of surveillance for dysplasia
The goal of CRC screening and surveillance in patients with IBD is to detect dysplasia as early as possible while it is endoscopically resectable. Although there are no randomized controlled trials comparing surveillance to no surveillance, population-based studies and meta-analyses have shown that patients who undergo surveillance have a lower risk of CRC related death and a higher rate of detection of early CRC detection (36). Given the importance of early detection, it is crucial to understand the appropriate timing for surveillance, to perform high quality endoscopy and to be able to identify lesions that may harbor dysplasia in individuals with IBD.
Surveillance guidelines
Endoscopic evaluation is the gold standard for diagnosing and managing IBD. Patients with IBD usually undergo multiple endoscopic procedures in their lifetime to ensure mucosal healing and to prevent CRC. Colonoscopies carry a low risk of adverse events and are generally safe. The effectiveness of a colonoscopy relies on two key factors: a good bowel prep and the endoscopists ability to visualize the entire colon. Inadequate preparation for the colonoscopy can result in longer procedural durations and a higher likelihood of missed lesions (37).
Traditionally, white light endoscopy (WLE) in conjunction with four quadrant non-targeted biopsies every 10 centimeters were used for surveillance (38). Studies have reported that about 33 biopsies from the colonic mucosa are needed to reach close to a 90% confidence that dysplasia, if present, will be detected (39). This practice is labor intensive for the endoscopist and the pathologist and is costly. In addition, literature shows that random biopsies only sample 1% of the colonic mucosa and lesions can therefore be missed (40).
In terms of description of endoscopically visible lesions, terminologies such as dysplasia associated lesion or mass (DALM), adenoma like mass (ALM) and non-adenoma like mass (NALM) were used to describe visible lesions but produced confusion and heterogeneity of practice amongst gastroenterologists (41). In addition, chromoendoscopy (CE) was introduced as an endoscopic tool to the US by the Japanese, and studies showed that CE was superior to standard techniques using white light with random biopsies (42). Clearly standardization was required. In 2015 an international group of multidisciplinary experts published the “Surveillance for Endoscopic Detection and Management in Inflammatory Bowel Disease: SCENIC guidelines” in both Gastroenterology and Gastrointestinal Endoscopy (43). Key recommendations of SCENIC were that HD-WLE is superior to standard WLE (SD-WLE) for surveillance. They recommended DCE in all patients undergoing surveillance whether with WLE or HD-WLE. They did not recommend virtual CE (VCE). There was no consensus regarding random versus targeted biopsies when using HD-WLE with or without CE.
CE, first introduced in the early 2000s, assists endoscopists to identify neoplastic lesions, by enhancing mucosal contours (44). DCE utilizes a dye, indigo carmine or methylene blue, to stain colonic mucosa. Methylene blue is absorbed by the mucosa, while indigo carmine coats it. The dye enhances visualization of subtle mucosal changes in dysplasia, thus allowing the endoscopist to perform targeted biopsies (45).
When methylene blue is used for CE, 1 vial (50 mg) is diluted in 200–250 cc of water and sprayed onto the antigravity wall of the colon. The mucosa is carefully inspected and targeted biopsies can be taken. Additional biopsies may be taken to determine disease activity and stage disease (Figure 3).
Because of confusion around terminology for visible lesions, and to ensure consistent communication practices amongst endoscopists and pathologists The SCENIC guidelines recommended that the term DALM be discontinued, and a modified Paris classification be used to describe lesions (46,47). They recommended that dysplasia be categorized as visible or invisible. Visible dysplasia is subcategorized as either polypoid or non-polypoid, based on protrusion of more than or less than 2.5 mm (size of closed cup of biopsy forceps) from mucosal surface. Polypoid lesions were further classified as either pedunculated or sessile. Non-polypoid lesions are further characterized as elevated, flat or depressed. SCENIC emphasized the importance of careful delineation of the border of lesions. Invisible dysplasia refers to dysplasia found on random biopsies. This classification was also adopted by other professional societies (46). Proper reporting of lesions is critical for determining whether endoscopic resection can be performed (5).
Another key change from previous recommendations was that the SCENIC guidelines recommended that, rather than surgery, all resectable lesions be endoscopically removed and that after removal of a polypoid lesion, biopsies be performed of the mucosa around the resection site to ensure that the endoscopist has completely resected the polyp and that there is no dysplasia in the base (43). SCENIC did recommend close surveillance after resection of a visible lesion but did not specify intervals.
New developments since SCENIC
CE
Although older studies showed superiority of CE to WLE, there had been some debate about the utility of CE in the setting of HD colonoscopes. However, several studies have shown an advantage of CE even with HD scopes (48). In 2018, Carballal et al. conducted a prospective study and evaluated each colonic segment first with WLE (SD and HD) and then again with CE. The incremental yield of detecting dysplasia with CE was 57.4% (49).
However, in spite of the SCENIC recommendations, CE use during surveillance amongst endoscopists has remained low. Some argue that DCE increases procedural time and that gastroenterologists would benefit from standardized training. DCE relies on the ability of the endoscopist to recognize and correctly identify dysplasia. A 2020 survey was performed to assess the use of DCE amongst gastroenterologists who are members of the Crohn’s & Colitis Foundation. Fifty-seven gastroenterologists responded, sixty-one percent believed that DCE is the preferred method for dysplasia surveillance, however 72% used only WLE for surveillance. Two-thirds of them had used DCE at least once and only 14% (8/57) of them use it consistently. According to the respondents, the main obstacles to the utilization of DCE were identified as extended procedure time (75.4%), absence of standardized training in DCE (73.7%), and lack of reimbursement for DCE (63.2%) (50).
The SCENIC guidelines did not recommend the use of narrow band imaging (NBI) over WLE or DCE based on studies using older technology. A key development since SCENIC has been the improvement in VCE technology. NBI, i-SCAN and flexible spectral imaging color enhancement (FICE) with improved filters, brightness and contrast, have gained considerable attention recently. These technologies enhance mucosal visualization, and theoretically should improve dysplasia detection (51).
A meta-analysis in 2020 compared different surveillance modalities and found DCE to be superior to SD-WLE but noted non-inferiority to HD-WLE and VCE (NBI, i-SCAN and FICE) (52). This was further supported by a multicenter randomized control study in 2021 (51). In order to fill the gaps in knowledge and recommendations since SCENIC, US and European societies have made a number of recommendations; we review US recommendations here.
Most recently, in 2021, the American Gastroenterological Association (AGA) produced a Clinical Practice Update based on expert consensus rather than GRADE methodology. The update was peer reviewed and also reviewed by a Clinical Practice Update Committee.
AGA guidelines suggest DCE in all patients. A key difference from SCENIC was the recommendation that virtual chromo (NBI) is a suitable alternative to DCE. They also state that random biopsies are not necessary with chromo HD-WLE but should be performed if chromo is not being used.
El-Dallal et al. conducted a meta-analysis that encompassed 11 randomized controlled trials including 1,328 patients and found no statistical difference between virtual chromo when compared to DCE. The study used autofluorescence (AF) imaging, FICE, i-SCAN, and NBI, with NBI having the largest number of trials. When NBI was compared with DCE and WLE there was no statistical difference per patient and dysplasia analysis (52). Another study found no significant difference between NBI and DCE for detection of neoplasia, However, it’s worth noting that the NBI group experienced a reduction in the average total procedural time by approximately 7 minutes (53).
Patients who have a personal history of dysplasia, concomitant PSC, a tubular colon or strong family history of CRC should still undergo random biopsies and targeted biopsies even if CE is used (54). Another key difference was the recommendation that, after resection of resectable lesions, base biopsies are not necessary.
Intervals
There are no prospective studies to guide our decision-making regarding timing of the first surveillance colonoscopy in IBD. The risk of CRC increases with duration of disease and therefore the general consensus is that the first screening colonoscopy should take place after 8 years of pancolitis, and after 10 years of left sided colitis. There are certain risk factors that may require commencing screening earlier. Risk factors such as, previous histology, family history, and personal history of dysplasia or strictures, should be taken into consideration when determining the appropriate timing and intervals for surveillance (55). Inflammatory burden is also an important risk factor and identification of this as an independent risk factor may call for earlier commencement of surveillance (19).
Patients with PSC are an exception to the above guidelines and require first surveillance at the time of diagnosis of PSC and annual surveillance thereafter, because the risk of CRC is increased by four-fivefold in patients with PSC and IBD (14,56).
The AGA Clinical Practice Update differs from other guidelines in providing for a longer surveillance interval of 1 to 5 years after a normal initial colonoscopy depending on the presence or absence of risk factors and the CIB. Surveillance interval can be increased to every 3 years if the patient has had two negative exams. High risk patients who have a history of strictures, foreshortened colons, multiple pseudo polyps, family history of CRC, indeterminate or known dysplasia, or ongoing inflammation should undergo a more aggressive surveillance schedule. Individuals with ulcerative proctitis or proctosigmoiditis are not considered at increased risk for IBD related CRC and can have screening and surveillance intervals similar to the general population (57).
The American College of Gastroenterology (ACG) published separate guidelines on management of Crohn’s in 2018 and UC in 2019. Surveillance was recommended for Crohn’s patients who had thirty percent colonic involvement after 8 years of diagnosis. Furthermore, patients with Crohn’s limited to the ileum should undergo age specific screening as per general guidelines (58). Patients with UC who have disease extending beyond the rectum are recommended a 1- to 3-year interval surveillance protocol depending on patient’s risks (59) (Table 1).
Table 1
| US society (methodology) | Initiation after symptom onset (years) | Intervals (years) | Risk factors that determine interval |
|---|---|---|---|
| ACG [2019] (GRADE) | 8–10 | 1–2 | PSC, other risk factors for CRC, previous histology |
| ASGE [2015] (GRADE) | 8 | 1–3 | PSC, inflammation, FDR with CRC |
| AGA 2021: Clinical Practice Update | 8–10 | 1–5 | PSC and other risk factors, prior histology, Inflammatory burden, consecutive examinations without dysplasia |
ACG, American College of Gastroenterology; ASGE, American Society of Gastrointestinal Endoscopy; AGA, American Gastroenterological Association; PSC, primary sclerosing cholangitis; CRC, colorectal cancer; FDR, first degree relative.
Are the guidelines for dysplasia screening in IBD followed?
It has been found that adherence to screening programs is usually low even in high-risk patients. Recently, a retrospective Spanish study in which 25 hospitals participated and a total of 1,031 patients included, shows that 90% of target patients are included in screening programs, although in the end only 27% follow the guidelines adequately, adherence much lower than that recommended for CRC screening in the general medium-risk population, 40–45%. Furthermore, adherence was even worse in the high CRC risk groups. Patients with adequate follow-up had a greater number of advanced lesions detected earlier than the rest (60).
Management of dysplasia
Shared decision making: the importance of a “personalized approach”
Having a detailed discussion with patients regarding the potential advantages and risks associated with endoscopic resection versus surgery is crucial. These risks include missing lesions, synchronous or metachronous lesions or the possibility of incomplete resection leading to the need for surgery and have to be weighed against the inherent risks of surgery (5). If patients do opt to undergo endoscopic resection, they must be committed to undergoing aggressive endoscopic surveillance. In an interesting study, Siegel et al. found that patients usually prefer colectomy over intensive surveillance only when the risk of synchronous colon cancer reaches 73% (61).
Individuals who have a higher likelihood of developing CRC, such as those with a personal history of CRC or strictures, severe pseudo polyposis (which indicates a higher preexisting inflammatory burden and also makes surveillance difficult/impossible), or strong family history of CRC, should be referred to surgery. When there is a lack of certainty regarding the most appropriate management strategy, clinicians should refer their patients to a specialized IBD center (54).
The rates at which individuals with IBD develop neoplasia from LGD vary significantly across different studies, with estimates ranging from 0% to 54% (62). Both continued surveillance and surgery are associated with risk and determining the best course of action can be challenging. The risk of progression to neoplasia also depends on many different variables as outlined elsewhere.
A group from St Marks in the UK developed an AN risk prediction calculator in patients with UC with LGD. In a multicenter retrospective analysis, patients from four centers in the UK with index LGD were followed until they developed AN. Prediction models were used to determine the variables most associated with progression to AN. They found that large lesions, active inflammation, multifocal LGD and unresectable or invisible dysplasia was most likely to progress to AN. They developed a statistical calculator, available online, which allows physicians to input specific patient LGD variables from which a percent likelihood of developing CRC over a period of time is calculated. The calculator also produces a Paling chart for ease of patient understanding. This calculator has been validated in four UK centers. Utilizing risk calculators such as this can help healthcare professionals in discussions with patients about their individual risks, thus aiding in the decision-making process between surveillance strategies and surgery (www.uc-care.uk) (63).
Management of visible dysplasia
Visible dysplasia is managed therapeutically with either endoscopic or surgical resection. Current guidelines suggest that all endoscopically resectable lesions be removed endoscopically (5). Endoscopic resection techniques include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). EMR is favored in lesions that are <2 cm that have clear borders without evidence of submucosal invasion, large ulceration, depression or fibrosis, particularly in regions of normal mucosa ESD is favored in lesions >2 cm, particularly in regions of inflammation due to the possibility of submucosal fibrosis (54). It is important to remove lesions en bloc with adequate lateral and vertical margins. If this cannot be achieved, then surgery should be performed. A tattoo should also be placed distal to the resection site (54).
If the visible lesion has indistinct borders, is multifocal or appears in other ways to be unresectable endoscopically, the patient should be referred for surgery.
Although there is not a lot of clear evidence, the 2021 Clinical Practice Update from the AGA has made clear recommendations regarding surveillance intervals after complete removal of dysplastic lesions. Summarizing the recommendations: if a lesion is <2 cm with good histology, surveillance interval can be from 12–24 months, while if there is HGD, a 3–6 month-follow-up is indicated. If the lesion is >2 cm, 3–6 month-follow-up is recommended, and surgery if it is unresectable or recurs (54).
Management of invisible dysplasia (dysplasia found on random biopsy alone)
HD-WLE endoscopy allows for better visualization of dysplasia. Less than one-third of dysplasia, initially deemed “invisible”, remains invisible and the majority may be suitable for endoscopic resection (5). In cases where dysplasia is found by random biopsies, the slides should be reviewed by a second pathologist. If the biopsy is confirmed to have dysplasia, then current guidelines suggest that a colonoscopy should be repeated with dye spray CE with particular attention to the region where dysplasia was found, by a gastroenterologist with experience in CE in order to try and unmask a lesion. During this examination, targeted biopsies as well as random every 10 cm, should be taken if there are no concerning lesions observed. If there is confirmation of LGD, patients should undergo surveillance with DCE every 3–6 months (5). It is important for patients to be aware that they face an elevated risk of progressing to HGD and cancer. Additionally, there is a possibility that HGD may be present even if it has not been detected in previous biopsies. If there is any evidence of HGD or multifocal dysplasia, a colectomy should be considered. HGD should also be confirmed by a second pathologist with expertise in IBD. If no histologic dysplasia is identified, continued intensive surveillance should be performed until two high quality negative examinations, after which the interval can be lengthened (54).
Outcomes after resection of dysplasia
Since resection of dysplastic lesions as opposed to surgery is a relatively recent practice, high quality studies on outcomes after resection are lacking. Mohan et al. conducted a retrospective review and meta-analysis on studies that reported on incidence or recurrence of neoplasia after resection of dysplasia in multiple databases from inception to 2019. Eighteen studies identified 1,037 patients with 1,428 lesions resected during a follow-up of 6–82 months. Pooled risk of cancer per 1,000 person years of follow-up was two for CRC and HGD. Interestingly the risk was not dependent upon the histology of the originally resected lesion. Although this is a comforting statistic, there was considerable heterogeneity in the studies, particularly in the size and modes of resection of lesions (64).
ESD is used for larger lesions particularly in areas of inflammation. There are Asian studies following outcomes after ESD. In the first US multicenter study reported in 2022 from nine centers in the US between 2015–2019, 49 dysplastic lesions were removed using ESD, median size 30 mm, non-polypoid, majority LGD. En bloc resection with few complications was achieved in the majority despite 73% submucosal fibrosis. Over a median follow-up of 18 months, metachronous lesions occurred in 11 patients: nine were treated endoscopically and two surgically. This higher incidence of recurrent lesions may be due to the higher risk profile of the initially resected lesion.
Although overall, the outcomes after resection of dysplastic lesions appears to be favorable, further fine tuning of risk factors will enable personalized models for post resection surveillance and cancer prevention. and surveillance.
Interval cancer
Interval cancer is the dreaded outcome for both endoscopists and patients. Patients with IBD are at a higher risk of developing interval CRC than the general population. Factors that contribute to interval cancers are missed surveillance colonoscopies, lesions that are not detected by the endoscopist (invisible dysplasia) as well as incomplete resection of lesions (65). Flat, non-polypoid lesions are more frequently missed or not fully resected (65). In an interesting single center retrospective analysis from Helsinki between 2000–2019, 27 patients with UC associated cancer, who had a colonoscopy performed at a mean of 14.5 months prior to cancer diagnosis, were evaluated. Colonoscopic images were carefully evaluated with regard to cancer location. Of the 27 interval cancers, seven were visible as neoplasia on the prior colonoscopy while 20 were invisible. Of the seven that were visible, three were not diagnosed as neoplasia on biopsy, two were diagnosed as adenoma and two were unrecognized in a field of inflammation. This underscores the importance of attaining remission prior to surveillance and of repeating a colonoscopy in short order if inflammation is found.
Pouch surveillance
An IPAA is a surgical procedure during which the entire colon and rectum are removed, and a pouch is created from the small intestine and sewn or stapled into the rectum. Although this procedure allows for bowel continuity, there may be residual rectal mucosa left at the anal transition zone, also called a rectal cuff (66). This presents a concern for the development of dysplasia. The incidence of dysplasia and cancer in ileal pouches varies, with some studies reporting these occurrences to be rare (66). A retrospective cohort study by Kariv et al. examined over 3,000 IBD-IPAA patients and showed cumulative incidences of neoplasia of 0.9%, 1.3%, 1.9%, 4.2%, and 5.1% at 5, 10, 15, 20, and 25 years after pouch creation, respectively (67).
There is no consensus on when and how to surveil an ileal pouch. A survey conducted by Gu et al., aimed to evaluate the opinions and practice patterns of experts on pouch surveillance in academic medical centers in the US (68). Most experts (79%) believed that surveillance pouchoscopy was necessary, and 69% believed that pouchoscopy with biopsy was effective for detecting neoplasia (68). However, there was great variation in the frequency of surveillance pouchoscopy.
The British Society of Gastroenterology (BSG) and European Crohn’s and Colitis Organization (ECCO) recommend that gastroenterologists consider pouch surveillance yearly in patients who have PSC, a history of dysplasia, CRC or a family history of CRC (68). The BSG suggests a 5-year surveillance interval for low-risk patients, whereas ECCO does not provide surveillance recommendations for this group of patients (69,70).
Colon cancer prevention
In IBD related CRC, it is clear that inflammation plays a vital role in carcinogenesis. A key factor in prevention of CRC in patients with IBD therefore, is to reduce and ultimately eliminate inflammation. The goal of treatment is to obtain endoscopic and histologic remission. Patients who exhibit inflammation should be followed closely and a rapid step-up approach should be implemented (71). Clinicians should ensure that their patients adhere to a surveillance program to prevent missing dysplasia and interval cancers.
Inflammation may be triggered by environmental factors, one of them being diet. It appears that nutrition may play a role in both the onset and management of IBD. The western diet is largely composed of fast food, refined sugars, high calories, large portions and lacks fruits and vegetables. The western diet has been hypothesized to trigger pro-inflammatory cytokines that alter the gut microbiome (72). On the other hand, the Mediterranean diet, which includes fruits, vegetables, olive oil, fish, wholes grains and nuts is thought to have protective properties (73). It is more suitable for patients who are in remission and should not be recommended to those who are in an active flare (72). A meta-analysis from 2022 showed that adherence to a Mediterranean diet resulted in improved symptoms and disease activity in CD patients (74).
Chemoprevention is another strategy that has been studied to prevent CRC in patients with IBD. 5-aminosalicylic acid (5-ASA) has been used to treat IBD, particularly UC, for over five decades (75). 5-ASA compounds can decrease epithelial cell turnover and promote apoptosis through COX-2 dependent pathways. They have also been shown to interfere with Wnt/β-catenin signaling pathways by activating the peroxisome proliferator activated receptor. 5-ASAs can reduce DNA oxidative stress and microsatellite instability (76). Although studies in the 1990s and early 2000s showed a possible chemoprotective effect for CAC, subsequent large meta-analyses have not confirmed this. Therefore, US gastrointestinal (GI) societies do not recommend 5-ASA use for pure chemoprotection against CRC in IBD. Nonsteroidal anti-inflammatory drugs (NSAIDs), thiopurines, statins, ursodeoxycholic acid and biologics have all been studied and may have chemo preventative properties, but this is likely secondary to control of inflammation rather than a true anti-tumor effect (77).
Future trends
Primary prevention
As treatments for IBD improve, the focus will be on early reduction of the burden of inflammation, thus reducing the risk of dysplasia and cancer. NF-κB is an important inducer of oxidative stress and molecular DNA instability at the cellular level, and its inhibition appears promising as an anti-cancer strategy in mouse studies (78).
Secondary prevention
Future trends in CRC surveillance among patients with IBD are focused on improving detection and managing the disease. Despite aggressive surveillance programs that have been proposed, interval CRC in IBD patients is not uncommon (79,80).
Therefore, noninvasive testing would be an attractive tool to detect precancerous lesions and CRC. The multitarget stool DNA (s-DNA) test involves the use of quantitative molecular assays to measure β-actin, along with a hemoglobin immunoassay. KRAS and the hemoglobin immunoassay are used to detect advanced adenomas and CRC. NDRG4 and BMP3 can detect SSLs (80). According to a study published in 2014, the sensitivity of s-DNA testing for detecting both precancerous lesions and CRC was greater than that of the standard fit testing (81). In a different study, the analysis of DNA methylation patterns in stool samples revealed that the levels of methylation in certain promoter regions of genes were able to identify CRC and HGD in patients with IBD with a sensitivity of 92% and specificity of 90% (82).
Confocal laser endomicroscopy (CLE) is an endoscopic assisted technique that allows endoscopists to obtain images of “virtual” histology (83,84). There are two options for utilizing CLE: either by using a microprobe that is inserted through the endoscope, or by using an endoscope that has a built-in CLE function (85). CLE can be used to diagnose IBD, assess disease extent and activity as well as detect intraepithelial neoplasia in real time (86). A study performed by Kiesslich et al. investigated the effectiveness of combining CLE with CE to detect dysplasia in patients with UC and found that this approach led to the detection of 4.75 times more neoplasia than conventional colonoscopy (87). However, this approach would rely heavily on the education of endoscopists in detection and identification of lesions.
Computer-aided polyp detection (CADe) systems have been developed to aid endoscopists in identifying lesions, thus making artificial intelligence (AI) an alternative approach to enhance dysplasia detection (88). The use of CADe has been reported to increase the adenoma detection rate (ADR) in the general population by 30% (89). While there are many AI tools to detect colorectal polyps, these technologies have not been adopted to identify polypoid and flat lesions in individuals with IBD. To further address this issue, a group from the Mayo Clinic trained a CADe with images of colon polyps from patients without IBD and tested it on a dataset of unlabeled IBD polypoid lesions. The original CADe model’s performance was highest for dysplastic polyps and lowest for serrated lesions and pseudopolyps. They re-trained the system with IBD polypoid lesions and the sensitivity for all polyp types were improved (89). This study highlights the need for additional research into the use of AI for dysplasia detection in patients with IBD.
With AI deep learning of dysplasia morphology, and widespread use of AI during endoscopy, CE and random biopsies may become obsolete in the near future.
Conclusions
Colon cancer screening and surveillance in IBD patients has evolved over the past two decades and continues to evolve. A personalized approach is necessary in order to determine individual risk, optimal surveillance intervals, and best options for management of dysplasia. Shared decision-making, possibly using modelling tools, regarding options and risks of potential interventions, is essential to ensure effective screening and management of dysplasia and CRC in patients with IBD (Table 2).
Table 2
| Begin surveillance after 8–10 years after symptom onset |
| PSC and IBD patients should begin surveillance at diagnosis of PSC and continue yearly thereafter |
| Duration of active inflammation (cumulative inflammatory burden) is probably more important than total duration of disease |
| Surveillance intervals can be determined based on histology and known risk factors |
| Chromoendoscopy (dye spray vs. NBI) with targeted biopsies is recommended for all patients |
| Random biopsies are not required with chromoendoscopy, in average risk patients |
| If chromoendoscopy is not performed, random biopsies should be performed (>30) |
| Chromoendoscopy with random and targeted biopsies should be chosen for high-risk groups (PSC, personal h/o CRC, h/o dysplasia, family h/o CRC) |
| When dysplasia is encountered shared decision making is important |
| Endoscopically resectable lesions can be safely resected with continued surveillance: <2 cm in normal mucosa with standard techniques, >2 cm +/− in field of inflammation, consider ESD by expert |
| Resection should be performed “en bloc” |
| Endoscopically unresectable lesions should be referred for surgery |
| Low-grade dysplasia can be surveilled closely: modelling tools can assist in decision making |
| High-grade dysplasia/multifocal dysplasia, consider surgery |
| Invisible dysplasia (found on random biopsy), confirmed by expert pathologist, repeat colonoscopy with dye spray, with targeted and random biopsies by an expert IBD endoscopist. Follow-up depends on findings (refer to AGA practice update) |
PSC, primary sclerosing cholangitis; IBD, inflammatory bowel disease; NBI, narrow band imaging; h/o, history of; CRC, colorectal cancer; ESD, endoscopic submucosal dissection; AGA, American Gastroenterological Association.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Keith Sultan) for the series “Controversies and Updates in Inflammatory Bowel Disease” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-36/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-36/coif). The series “Controversies and Updates in Inflammatory Bowel Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Warren S, Sommers SC. Cicatrizing enteritis as a pathologic entity; analysis of 120 cases. Am J Pathol 1948;24:475-501. [PubMed]
- Watanabe T, Ajioka Y, Mitsuyama K, et al. Comparison of Targeted vs Random Biopsies for Surveillance of Ulcerative Colitis-Associated Colorectal Cancer. Gastroenterology 2016;151:1122-30. [Crossref] [PubMed]
- Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol 2008;14:3937-47. [Crossref] [PubMed]
- Lauricella S, Fabris S, Sylla P. Colorectal cancer risk of flat low-grade dysplasia in inflammatory bowel disease: a systematic review and proportion meta-analysis. Surg Endosc 2023;37:48-61. [Crossref] [PubMed]
- Shah SC, Itzkowitz SH. Management of Inflammatory Bowel Disease-Associated Dysplasia in the Modern Era. Gastrointest Endosc Clin N Am 2019;29:531-48. [Crossref] [PubMed]
- Jess T, Simonsen J, Jørgensen KT, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology 2012;143:375-81.e1; quiz e13-4. [Crossref] [PubMed]
- Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol 2014;20:9872-81. [Crossref] [PubMed]
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526-35. [Crossref] [PubMed]
- Lutgens MW, van Oijen MG, van der Heijden GJ, et al. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 2013;19:789-99. [Crossref] [PubMed]
- Choi CH, Rutter MD, Askari A, et al. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am J Gastroenterol 2015;110:1022-34. [Crossref] [PubMed]
- Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J Gastroenterol 2019;25:4148-57. [Crossref] [PubMed]
- Askling J, Dickman PW, Karlén P, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology 2001;120:1356-62. [Crossref] [PubMed]
- Dulai PS, Sandborn WJ, Gupta S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev Res (Phila) 2016;9:887-94. [Crossref] [PubMed]
- López-Serrano A, Suárez MJ, Besó P, et al. Virtual chromoendoscopy with iSCAN as an alternative method to dye-spray chromoendoscopy for dysplasia detection in long-standing colonic inflammatory bowel disease: a case-control study. Scand J Gastroenterol 2021;56:820-8. [Crossref] [PubMed]
- Wang R, Leong RW. Primary sclerosing cholangitis as an independent risk factor for colorectal cancer in the context of inflammatory bowel disease: a review of the literature. World J Gastroenterol 2014;20:8783-9. [Crossref] [PubMed]
- Nagengast FM, Grubben MJ, van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer 1995;31A:1067-70. [Crossref] [PubMed]
- Claessen MM, Lutgens MW, van Buuren HR, et al. More right-sided IBD-associated colorectal cancer in patients with primary sclerosing cholangitis. Inflamm Bowel Dis 2009;15:1331-6. [Crossref] [PubMed]
- Higashi H, Yanaga K, Marsh JW, et al. Development of colon cancer after liver transplantation for primary sclerosing cholangitis associated with ulcerative colitis. Hepatology 1990;11:477-80. [Crossref] [PubMed]
- Choi CR, Al Bakir I, Ding NJ, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut 2019;68:414-22. [Crossref] [PubMed]
- Siddique O, Vaziri H, Anderson JC. Colon Cancer Screening and Surveillance in the IBD Patient. In: Rajapakse R. (editor). Inflammatory Bowel Disease. Clinical Gastroenterology. Humana, Cham; 2021. Available online:
10.1007/978-3-030-81780-0_10 10.1007/978-3-030-81780-0_10 - Lucafò M, Curci D, Franzin M, et al. Inflammatory Bowel Disease and Risk of Colorectal Cancer: An Overview From Pathophysiology to Pharmacological Prevention. Front Pharmacol 2021;12:772101. [Crossref] [PubMed]
- Fantini MC, Guadagni I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Dig Liver Dis 2021;53:558-65. [Crossref] [PubMed]
- Kameyama H, Nagahashi M, Shimada Y, et al. Genomic characterization of colitis-associated colorectal cancer. World J Surg Oncol 2018;16:121. [Crossref] [PubMed]
- Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012;3:4-14. [Crossref] [PubMed]
- Khan I, Ullah N, Zha L, et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019;8:126. [Crossref] [PubMed]
- Zhou Y, Chen H, He H, et al. Increased Enterococcus faecalis infection is associated with clinically active Crohn disease. Medicine (Baltimore) 2016;95:e5019. [Crossref] [PubMed]
- Xu P, Lv T, Dong S, et al. Association between intestinal microbiome and inflammatory bowel disease: Insights from bibliometric analysis. Comput Struct Biotechnol J 2022;20:1716-25. [Crossref] [PubMed]
- Mirzaei R, Mirzaei H, Alikhani MY, et al. Bacterial biofilm in colorectal cancer: What is the real mechanism of action? Microb Pathog 2020; Epub ahead of print. [Crossref] [PubMed]
- Ellermann M, Sartor RB. Intestinal bacterial biofilms modulate mucosal immune responses. J Immunol Sci 2018;2:13-8. [Crossref] [PubMed]
- Zisman TL, Bronner MP, Rulyak S, et al. Prospective study of the progression of low-grade dysplasia in ulcerative colitis using current cancer surveillance guidelines. Inflamm Bowel Dis 2012;18:2240-6. [Crossref] [PubMed]
- Genere JR, Deepak P. Managing Risk of Dysplasia and Colorectal Cancer in Inflammatory Bowel Disease. Tech Innov Gastrointest Endosc 2023;25:372-84. [Crossref]
- Odze, Riddel et al. WHO Classification of tumours of the digestive system. 5th ed. Lyon: IARC Press; 2019.
- Choi WT, Yozu M, Miller GC, et al. Nonconventional dysplasia in patients with inflammatory bowel disease and colorectal carcinoma: a multicenter clinicopathologic study. Mod Pathol 2020;33:933-43. [Crossref] [PubMed]
- Bahceci D, Lauwers GY, Choi WT. Clinicopathologic features of undetected dysplasia found in total colectomy or proctocolectomy specimens of patients with inflammatory bowel disease. Histopathology 2022;81:183-91. [Crossref] [PubMed]
- Lee H, Rabinovitch PS, Mattis AN, et al. Non-conventional dysplasia in inflammatory bowel disease is more frequently associated with advanced neoplasia and aneuploidy than conventional dysplasia. Histopathology 2021;78:814-30. [Crossref] [PubMed]
- Bye WA, Ma C, Nguyen TM, et al. Strategies for Detecting Colorectal Cancer in Patients with Inflammatory Bowel Disease: A Cochrane Systematic Review and Meta-Analysis. Am J Gastroenterol 2018;113:1801-9. [Crossref] [PubMed]
- Negreanu L, Voiosu T, State M, et al. Quality of colonoscopy preparation in patients with inflammatory bowel disease: retrospective analysis of 348 colonoscopies. J Int Med Res 2020;48:300060520903654. [Crossref] [PubMed]
- Kiesslich R, Neurath MF. Chromoendoscopy: an evolving standard in surveillance for ulcerative colitis. Inflamm Bowel Dis 2004;10:695-6. [Crossref] [PubMed]
- Bopanna S, Roy M, Das P, et al. Role of random biopsies in surveillance of dysplasia in ulcerative colitis patients with high risk of colorectal cancer. Intest Res 2016;14:264-9. [Crossref] [PubMed]
- van den Broek FJ, Stokkers PC, Reitsma JB, et al. Random biopsies taken during colonoscopic surveillance of patients with longstanding ulcerative colitis: low yield and absence of clinical consequences. Am J Gastroenterol 2014;109:715-22. [Crossref] [PubMed]
- Farraye FA, Waye JD, Moscandrew M, et al. Variability in the diagnosis and management of adenoma-like and non-adenoma-like dysplasia-associated lesions or masses in inflammatory bowel disease: an Internet-based study. Gastrointest Endosc 2007;66:519-29. [Crossref] [PubMed]
- Marion JF, Waye JD, Present DH, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol 2008;103:2342-9. [Crossref] [PubMed]
- Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148:639-651.e28. [Crossref] [PubMed]
- Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003;124:880-8. [Crossref] [PubMed]
- Guagnozzi D, Lucendo AJ. Colorectal cancer surveillance in patients with inflammatory bowel disease: What is new? World J Gastrointest Endosc 2012;4:108-16. [Crossref] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Chiu K, Riddell RH, Schaeffer DF. DALM, rest in peace: a pathologist's perspective on dysplasia in inflammatory bowel disease in the post-DALM era. Mod Pathol 2018;31:1180-90. [Crossref] [PubMed]
- Alexandersson B, Hamad Y, Andreasson A, et al. High-Definition Chromoendoscopy Superior to High-Definition White-Light Endoscopy in Surveillance of Inflammatory Bowel Diseases in a Randomized Trial. Clin Gastroenterol Hepatol 2020;18:2101-7. [Crossref] [PubMed]
- Carballal S, Maisterra S, López-Serrano A, et al. Real-life chromoendoscopy for neoplasia detection and characterisation in long-standing IBD. Gut 2018;67:70-8. [Crossref] [PubMed]
- Grossberg LB, Farraye FA, Papamichael K, et al. A Survey Study of Gastroenterologists' Views on Dysplasia Surveillance and Chromoendoscopy in IBD. Inflamm Bowel Dis 2020;26:e59-61. [Crossref] [PubMed]
- Kandiah K, Subramaniam S, Thayalasekaran S, et al. Multicentre randomised controlled trial on virtual chromoendoscopy in the detection of neoplasia during colitis surveillance high-definition colonoscopy (the VIRTUOSO trial). Gut 2021;70:1684-90. [Crossref] [PubMed]
- El-Dallal M, Chen Y, Lin Q, et al. Meta-analysis of Virtual-based Chromoendoscopy Compared With Dye-spraying Chromoendoscopy Standard and High-definition White Light Endoscopy in Patients With Inflammatory Bowel Disease at Increased Risk of Colon Cancer. Inflamm Bowel Dis 2020;26:1319-29. [Crossref] [PubMed]
- Bisschops R, Bessissow T, Joseph JA, et al. Chromoendoscopy versus narrow band imaging in UC: a prospective randomised controlled trial. Gut 2018;67:1087-94. [Crossref] [PubMed]
- Murthy SK, Feuerstein JD, Nguyen GC, et al. AGA Clinical Practice Update on Endoscopic Surveillance and Management of Colorectal Dysplasia in Inflammatory Bowel Diseases: Expert Review. Gastroenterology 2021;161:1043-1051.e4. [Crossref] [PubMed]
- Huguet JM, Suárez P, Ferrer-Barceló L, et al. Endoscopic recommendations for colorectal cancer screening and surveillance in patients with inflammatory bowel disease: Review of general recommendations. World J Gastrointest Endosc 2017;9:255-62. [Crossref] [PubMed]
- Trivedi PJ, Crothers H, Mytton J, et al. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People With Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology 2020;159:915-28. [Crossref] [PubMed]
- Farraye FA, Odze RD, Eaden J, et al. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:746-74, 774.e1-4; quiz e12-3.
- Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol 2018;113:481-517. [Crossref] [PubMed]
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol 2019;114:384-413. [Crossref] [PubMed]
- Ballester MP, Mesonero F, Flórez-Diez P, et al. Adherence to endoscopic surveillance for advanced lesions and colorectal cancer in inflammatory bowel disease: an AEG and GETECCU collaborative cohort study. Aliment Pharmacol Ther 2022;55:1402-13. [Crossref] [PubMed]
- Siegel CA, Schwartz LM, Woloshin S, et al. When should ulcerative colitis patients undergo colectomy for dysplasia? Mismatch between patient preferences and physician recommendations. Inflamm Bowel Dis 2010;16:1658-62. [Crossref] [PubMed]
- De Jong ME, Van Tilburg SB, Nissen LHC, et al. Long-term Risk of Advanced Neoplasia After Colonic Low-grade Dysplasia in Patients With Inflammatory Bowel Disease: A Nationwide Cohort Study. J Crohns Colitis 2019;13:1485-91. [Crossref] [PubMed]
- Curtius K, Kabir M, Al Bakir I, et al. Multicentre derivation and validation of a colitis-associated colorectal cancer risk prediction web tool. Gut 2022;71:705-15. [Crossref] [PubMed]
- Mohan BP, Khan SR, Chandan S, et al. Endoscopic resection of colon dysplasia in patients with inflammatory bowel disease: a systematic review and meta-analysis. Gastrointest Endosc 2021;93:59-67.e10. [Crossref] [PubMed]
- Sanduleanu S, Rutter MD. Interval colorectal cancers in inflammatory bowel disease: the grim statistics and true stories. Gastrointest Endosc Clin N Am 2014;24:337-48. [Crossref] [PubMed]
- Samaan MA, Forsyth K, Segal JP, et al. Current Practices in Ileal Pouch Surveillance for Patients With Ulcerative Colitis: A Multinational, Retrospective Cohort Study. J Crohns Colitis 2019;13:735-43. [Crossref] [PubMed]
- Kariv R, Remzi FH, Lian L, et al. Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy. Gastroenterology 2010;139:806-12, 812.e1-2.
- Gu J, Remzi FH, Lian L, et al. Practice pattern of ileal pouch surveillance in academic medical centers in the United States. Gastroenterol Rep (Oxf) 2016;4:119-24. [Crossref] [PubMed]
- Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. [Crossref] [PubMed]
- Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013;7:982-1018. [Crossref] [PubMed]
- Lopez A, Pouillon L, Beaugerie L, et al. Colorectal cancer prevention in patients with ulcerative colitis. Best Pract Res Clin Gastroenterol 2018;32-33:103-9. [Crossref] [PubMed]
- Reddavide R, Rotolo O, Caruso MG, et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed 2018;89:60-75. [PubMed]
- Fiorindi C, Dinu M, Gavazzi E, et al. Adherence to mediterranean diet in patients with inflammatory bowel disease. Clin Nutr ESPEN 2021;46:416-23. [Crossref] [PubMed]
- Jaber M, Altamimi M, Altamimi A, et al. Mediterranean diet diminishes the effects of Crohn's disease and improves its parameters: A systematic review. Nutr Health 2022; Epub ahead of print. [Crossref] [PubMed]
- Cheng Y, Desreumaux P. 5-aminosalicylic acid is an attractive candidate agent for chemoprevention of colon cancer in patients with inflammatory bowel disease. World J Gastroenterol 2005;11:309-14. [Crossref] [PubMed]
- Subramanian V, Logan RF. Chemoprevention of colorectal cancer in inflammatory bowel disease. Best Pract Res Clin Gastroenterol 2011;25:593-606. [Crossref] [PubMed]
- Hsiao SW, Yen HH, Chen YY. Chemoprevention of Colitis-Associated Dysplasia or Cancer in Inflammatory Bowel Disease. Gut Liver 2022;16:840-8. [Crossref] [PubMed]
- Jin BR, Kim HJ, Sim SA, et al. Anti-Obesity Drug Orlistat Alleviates Western-Diet-Driven Colitis-Associated Colon Cancer via Inhibition of STAT3 and NF-κB-Mediated Signaling. Cells 2021;10:2060. [Crossref] [PubMed]
- Vaziri H, Anderson JC. Stool DNA for the Detection of High-Grade Dysplasia and Adenocarcinoma in Patients with Inflammatory Bowel Disease. Gastroenterology 2020;158:789-90. [Crossref] [PubMed]
- Kisiel JB, Yab TC, Nazer Hussain FT, et al. Stool DNA testing for the detection of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013;37:546-54. [Crossref] [PubMed]
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370:1287-97. [Crossref] [PubMed]
- Kisiel JB, Klepp P, Allawi HT, et al. Analysis of DNA Methylation at Specific Loci in Stool Samples Detects Colorectal Cancer and High-Grade Dysplasia in Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol 2019;17:914-921.e5. [Crossref] [PubMed]
- De Palma GD, Rispo A. Confocal laser endomicroscopy in inflammatory bowel diseases: dream or reality? World J Gastroenterol 2013;19:5593-7. [Crossref] [PubMed]
- Fugazza A, Gaiani F, Carra MC, et al. Confocal Laser Endomicroscopy in Gastrointestinal and Pancreatobiliary Diseases: A Systematic Review and Meta-Analysis. Biomed Res Int 2016;2016:4638683. [Crossref] [PubMed]
- Buchner AM. Confocal Laser Endomicroscopy in the Evaluation of Inflammatory Bowel Disease. Inflamm Bowel Dis 2019;25:1302-12. [Crossref] [PubMed]
- Li CQ, Liu J, Ji R, et al. Use of confocal laser endomicroscopy to predict relapse of ulcerative colitis. BMC Gastroenterol 2014;14:45. [Crossref] [PubMed]
- Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology 2007;132:874-82. [Crossref] [PubMed]
- Repici A, Badalamenti M, Maselli R, et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020;159:512-520.e7. [Crossref] [PubMed]
- Guerrero Vinsard D, Fetzer J, Agrawal U, et al. S26 Development of an Artificial Intelligence Tool for Detection of Polypoid Lesions in Inflammatory Bowel Disease (IBD-CADe). The American Journal of Gastroenterology 2022;117:S7. [Crossref]
Cite this article as: Fatakhova K, Rajapakse R. From random to precise: updated colon cancer screening and surveillance for inflammatory bowel disease. Transl Gastroenterol Hepatol 2024;9:27.