
Viral esophagitis in non-human immunodeficiency virus patients: a case-control study
Highlight box
Key findings
• Hematemesis was the predominant symptom in patients with herpes simplex virus (HSV) (33%), while dysphagia was more prevalent in cytomegalovirus (CMV) patients (42%). The most common finding during esophagogastroduodenoscopy was ulceration in HSV patients (67%) and esophagitis in CMV patients (37%).
What is known and what is new?
• Viral esophagitis (VE) has been commonly associated with immunosuppression.
• This study identified 17 patients (34% of cases) without identifiable risk factors.
What is the implication, and what should change now?
• Further research is warranted to explore the rising incidence of VE in immunocompetent individuals.
Introduction
Inflammation of the esophagus, known as esophagitis, arises from different etiologies such as reflux, infections, food allergies, medications, and trauma. Worldwide, infectious esophagitis ranks as the third leading cause of esophagitis, after gastroesophageal reflux disease (GERD) and eosinophilic esophagitis (1-3). The most common causes of infectious esophagitis are candida esophagitis followed by viral esophagitis (VE) secondary to either herpes simplex virus (HSV) or cytomegalovirus (CMV) (1-3).
VE is typically associated with an immunosuppression state and the classical risk factors include malignancy, chemotherapy, organ transplant, and human immunodeficiency virus (HIV) (4). Infectious esophagitis is prevalent in nearly one-third of untreated acquired immunodeficiency syndrome (AIDS) patients during the course of their disease (1,5). However, recent reports suggest a rise in cases of VE in immunocompetent individuals (6-10). This study aims to elucidate risk factors and general patient demographics in non-HIV patients. We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-44/rc).
Methods
This retrospective case-control study identified patients older than 18 years old and diagnosed with either HSV or CMV esophagitis by histopathologic examination or by confirmatory immunohistochemical staining. Cases were obtained through a free text search using pathology reports from MedStar Health Hospitals, which includes five hospitals across the District of Columbia and Maryland between 2009–2022 Patients with a diagnosis of HIV/AIDS or younger than 18 were excluded from the study. Controls were identified through International Classification of Diseases (ICD) codes for esophagogastroduodenoscopy (EGD) with biopsy during the same period and were negative for VE. We conducted a chart review of these patients to collect data on patient demographics, comorbidities, laboratory parameters, endoscopic findings, and potential risk factors.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Medstar Health (No. 00005623) and individual consent for this retrospective analysis was waived.
Immunocompetent patients were identified based on the absence of the following conditions: history of active malignancy, active chemotherapy, diabetes mellitus (DM), liver cirrhosis, end-stage renal disease (ESRD), organ transplant, and the use of immunomodulatory agents or systemic steroids.
Statistical analysis
Data analysis was conducted using Statistical Analysis System (SAS) version 9.4. We presented frequencies and percentages for categorical data and used Pearson’s Chi-square or Fisher’s exact test for inference if the expected value in any cell was less than 5. Continuous variables were summarized as mean (± standard deviation). For comparing VE cases with control cases and HSV with CMV on alcohol, tobacco, comorbidities, and medications, we used logistic regression analysis to calculate odds ratio (OR). A P value of less than 0.05 was considered statistically significant. Missing data were excluded from statistical analysis.
Results
General characteristics
Cases
This study aimed to investigate the clinical features of VE in non-HIV patients. Out of 40,224 esophageal biopsies reports, we identified a total of 50 (0.12%) cases from January 2009 to December 2022 with a histopathological diagnosis of either HSV or CMV esophagitis.
Out of the 50 cases, 19 (38%) had CMV esophagitis, 30 (60%) had HSV esophagitis, and one had CMV and HSV confection (Figure 1). Females represented a higher number of cases than males [31 (62%) vs. 19 (38%), respectively]. The mean age of all patients was 57 years, with 25 cases (50%) being of Black ethnicity and 20 (40%) being of White ethnicity.
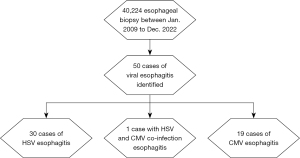
Of the total patients with VE, 12 cases (24%) had a history of GERD. While 17 (34%) had no identifiable immunocompromised status, and 33 (66%) were immunocompromised at the time of diagnosis. Among the immunocompromised patients: 20 (61%) were on immunosuppressive therapy, 17 (52%) were on systemic steroids, 8 (24%) had DM, 5 (15%) had undergone organ transplant, 5 (15%) had ESRD and were on dialysis. Additionally, 13 (39%) had an active malignancy, with colon cancer being the most common, followed by esophageal, breast, and multiple myeloma. Refer to Table 1 for a summary of patients’ demographic comorbidities.
Table 1
| Demographics and risk factors | Cases (VE) (N=50) | Control (N=246) | P value |
|---|---|---|---|
| Gender | 0.33 | ||
| Male | 19 (38.0) | 112 (45.5) | |
| Female | 31 (62.0) | 134 (54.5) | |
| Age (years) | 57 (±18.9) | 63 (±15.9) | 0.02* |
| Race | 0.17 | ||
| White | 20 (40.0) | 126 (51.2) | |
| Black | 25 (50.0) | 109 (44.3) | |
| Asian | 2 (4.0) | 2 (0.8) | |
| Others | 3 (6.0) | 9 (3.7) | |
| BMI (kg/m2) | 26.27 (±9.21) | 27.73 (±7.47) | 0.30 |
| Heavy alcohol use | 5 (11.6) (missing =7) | 42 (17.1) | 0.37 |
| Tobacco use | 12 (27.3) (missing =6) | 122 (49.6) | 0.006* |
| Comorbidities | |||
| GERD | 12 (24.0) | 68 (27.6) | 0.60 |
| DM | 8 (16.0) | 70 (28.5) | 0.07 |
| ESRD | 5 (10.0) | 26 (10.6) | 0.91 |
| Liver cirrhosis | 0 | 7 (2.8) | 0.61 |
| Organ transplant | 5 (10.0) | 5 (2.0) | 0.005* |
| Active malignancy | 13 (26.0) | 32 (13.0) | 0.02* |
| Medications | |||
| PPI | 16 (32.0) | 74 (30.1) | 0.79 |
| H2RB | 5 (10.0) | 30 (12.2) | 0.66 |
| Aspirin | 15 (30.0) | 67 (27.2) | 0.69 |
| NSAIDs | 4 (8.0) | 21 (8.5) | >0.99 |
| Bisphosphonates | 1 (2.0) | 2 (0.8) | 0.43 |
| Antibiotics | 3 (6.0) | 19 (7.7) | >0.99 |
| Antifungals | 1 (2.0) | 5 (2.0) | >0.99 |
| Immunosuppressive therapy | 20 (40.0) | 20 (8.1) | 0.001* |
| Systemic corticosteroids | 17 (34.0) | 42 (17.1) | 0.006* |
Data are presented as n (%) or mean (± standard deviation). *, statistically significant (P value <0.05). N, number of patients; VE, viral esophagitis; BMI, body mass index; GERD, gastroesophageal reflux disease; DM, diabetes mellitus; ESRD, end-stage renal disease; PPI, proton-pump inhibitor; H2RB, histamine-2 receptors blocker; NSAIDs, nonsteroidal anti-inflammatory drugs.
Control
This study included 246 randomly sampled control group with 1:5 ratio, which were predominantly female (45.5%) and white race (51.2%). The average age of the control group was 63 years. When compared to the group of patients with VE, there was no significant difference in gender or race. However, it is worth noting that the cases had a significantly lower mean age of 57 years (P=0.02). Please see Table 1 for further details. The patients with VE were found to have a higher likelihood of a history of immunosuppressive therapy {OR: 7.53 [95% confidence interval (CI): 3.64, 15.59]; P<0.001}, organ transplant [OR: 5.35 (95% CI: 1.49, 19.26); P=0.005], active malignancy [OR: 2.35 (95% CI: 1.13, 4.89); P=0.02], and systemic steroid use [OR: 2.5 (95% CI: 1.28, 4.90); P=0.006]. Cigarette smoking had lower odds of being reported in patients with VE [OR: 0.38 (95% CI: 0.19, 0.77); P=0.006].
Patients’ presentation
The leading presenting symptoms in patients with CMV and HSV combined were dysphagia, reported by 18 patients (36%), followed by odynophagia in 16 patients (32%), and hematemesis in 12 patients (24%). Only a minority of patients (8%) presented with fever, while a small proportion of patients (12%) had oral/perioral herpetic lesions and an even smaller percentage (6%) had genital herpetic lesions at the time of diagnosis. The majority of patients (62%) had normal white blood cell counts on presentation, with a minority of patients exhibiting low (10%) or elevated (28%) counts. Regarding endoscopic findings, ulceration was the most common (50%), followed by severe esophagitis (28%), and mild-to-moderate esophagitis (14%). Refer to Table 2 for further details.
Table 2
| Presenting symptoms and EGD findings | VE (N=50) | CMV (N=19) | HSV (N=30) | Immunocompetent (N=17) | Immunocompromised (N=33) |
|---|---|---|---|---|---|
| Presenting symptoms | |||||
| Dysphagia | 18 [36] | 8 [42] | 9 [30] | 4 [24] | 14 [42] |
| Odynophagia | 16 [32] | 7 [37] | 8 [27] | 5 [29] | 11 [33] |
| Hematemesis | 12 [24] | 2 [11] | 10 [33] | 5 [29] | 7 [21] |
| Vomiting | 9 [18] | 4 [21] | 5 [17] | 2 [12] | 7 [21] |
| Epigastric pain | 7 [14] | 2 [11] | 5 [17] | 3 [18] | 4 [12] |
| Nausea | 5 [10] | 2 [11] | 3 [10] | 1 [6] | 4 [12] |
| Melena | 5 [10] | 1 [5] | 4 [13] | 2 [12] | 3 [9] |
| Fever | 4 [8] | 1 [5] | 3 [10] | 2 [12] | 2 [6] |
| Acid reflux | 3 [6] | 2 [11] | 1 [3] | 2 [12] | 1 [3] |
| Dyspepsia | 2 [4] | 1 [5] | 1 [3] | 0 | 2 [6] |
| Chest pain | 1 [2] | 0 | 1 [3] | 1 [6] | 0 |
| Diarrhea | 1 [2] | 0 | 1 [3] | 1 [6] | 0 |
| EGD findings | |||||
| Esophagitis | 21 [42] | 7 [37] | 14 [47] | 4 [24] | 17 [52] |
| Mild to moderate | 7 [14] | 3 [16] | 4 [13] | 2 [12] | 5 [15] |
| Severe | 14 [28] | 4 [21] | 10 [33] | 2 [12] | 12 [36] |
| Ulceration | 25 [50] | 5 [26] | 20 [67] | 9 [53] | 16 [48] |
| Plaques | 5 [10] | 1 [5] | 4 [13] | 1 [6] | 4 [12] |
| Exudate | 2 [4] | 2 [11] | 0 | 0 | 2 [6] |
| Membranous changes | 2 [4] | 2 [11] | 0 | 1 [6] | 1 [3] |
| Esophageal stricture | 1 [2] | 0 | 1 [3] | 0 | 1 [3] |
| Normal findings | 1 [2] | 1 [5] | 0 | 0 | 1 [3] |
| Others | 3 [6] | 2 [11] | 1 [3] | 2 [12] | 1 [3] |
Data are presented as n [%]. VE, viral esophagitis; N, number of patients; CMV, cytomegalovirus; HSV, herpes simplex virus; EGD, esophagogastroduodenoscopy.
After stratifying presenting symptoms based on the viral etiology, we found that hematemesis was the most common presentation in HSV patients (33% vs. 11%). However, dysphagia was the most presenting symptom in CMV esophagitis. Ulcerations were more commonly seen in patients with HSV (67%) compared to CMV (26%). Refer to Table 2 for further details.
CMV and HSV
The mean age of patients diagnosed with CMV esophagitis was 61 and 54 years in HSV esophagitis (P=0.20). There was no significant difference between the two viral types in terms of gender distribution, with 57.9% of CMV patients and 66.7% of HSV patients being female (P=0.54). Similarly, there was no statistically significant difference in terms of race or BMI. Refer to Table 3 for further information.
Table 3
| Demographics and risk factors | CMV esophagitis (N=19) | HSV esophagitis (N=30) | P value |
|---|---|---|---|
| Gender | 0.54 | ||
| Male | 8 (42.1) | 10 (33.3) | |
| Female | 11 (57.9) | 20 (66.7) | |
| Age (years) | 61 (±17.30) | 54 (±19.86) | 0.20 |
| Race | 0.48 | ||
| White | 10 (52.6) | 10 (33.3) | |
| Black | 7 (36.8) | 18 (60.0) | |
| Asian | 1 (5.3) | 1 (3.3) | |
| Others | 1 (5.3) | 1 (3.3) | |
| BMI (kg/m2) | 28.65 (±9.67) | 24.86 (±8.91) | 0.17 |
| Heavy alcohol use | 2 (11.1) (missing =1) | 3 (12.5) (missing =6) | >0.99 |
| Tobacco use | 5 (27.8) (missing =1) | 7 (28.0) (missing =5) | >0.99 |
| Comorbidities | |||
| GERD | 5 (26.3) | 7 (23.3) | >0.99 |
| DM | 4 (21.1) | 4 (13.3) | 0.69 |
| ESRD | 2 (10.5) | 3 (10.0) | >0.99 |
| Liver cirrhosis | 0 | 0 | – |
| Organ transplant | 2 (10.5) | 2 (6.7) | 0.64 |
| Active malignancy | 7 (36.8) | 6 (20.0) | 0.19 |
| Medications | |||
| PPI | 7 (36.8) | 9 (30.0) | 0.62 |
| H2RB | 1 (5.3) | 4 (13.3) | 0.64 |
| Aspirin | 7 (36.8) | 7 (23.3) | 0.31 |
| NSAIDs | 2 (10.5) | 2 (6.7) | 0.64 |
| Bisphosphonates | 0 | 1 (3.3) | >0.99 |
| Antibiotics | 1 (5.3) | 2 (6.7) | >0.99 |
| Antifungals | 1 (5.3) | 0 | 0.39 |
| Immunosuppressive therapy | 11 (57.9) | 8 (26.7) | 0.03* |
| Systemic corticosteroids | 10 (52.6) | 7 (23.3) | 0.04* |
Data are presented as n (%) or mean (± standard deviation). *, statistically significant (P value <0.05). CMV, cytomegalovirus; HSV, herpes simplex virus; N, number of patients; BMI, body mass index; GERD, gastroesophageal reflux disease; DM, diabetes mellitus; ESRD, end-stage renal disease; PPI, proton-pump inhibitor; H2RB, histamine-2 receptors blocker; NSAIDs, nonsteroidal anti-inflammatory drugs.
Compared to patients with CMV esophagitis, those with HSV esophagitis had lower odds of having a history of immunosuppressive therapy [OR: 0.26 (95% CI: 0.08, 0.89); P=0.03] and systemic steroid use at the time of diagnosis [OR: 0.27 (95% CI: 0.08, 0.94); P=0.04]. However, there was no significant difference between the two groups in terms of other comorbidities such as active malignancy, organ transplantation, DM, ESRD, and GERD. Refer to Table 3 for further details.
Immunocompetent and immunocompromised
The mean age of immunocompetent patients was 46 years, which was significantly lower than that of immunocompromised patients (63 years, P=0.002). However, there was no significant difference in gender or race between the two groups. Notably, immunocompromised patients were more likely to report using aspirin, with a statistically significant difference between the two groups (42.4% vs. 5.9% in immunocompromised and immunocompetent patients, respectively, P=0.008. Refer to Table 4 for further details.
Table 4
| Demographics and risk factors | Immunocompetent (N=17) | Immunocompromised (N=33) | P value |
|---|---|---|---|
| Gender | 0.74 | ||
| Male | 7 (41.2) | 12 (36.4) | |
| Females | 10 (58.8) | 21 (63.6) | |
| Age (years) | 46 (±20.26) | 63 (±15.58) | 0.002* |
| Race | 0.78 | ||
| White | 7 (41.2) | 13 (39.4) | |
| Black | 9 (52.9) | 16 (48.5) | |
| Asian | 0 | 2 (6.1) | |
| Others | 1 (5.9) | 2 (6.1) | |
| BMI (kg/m2) | 25.88 (±10.27) | 26.48 (±8.78) | 0.83 |
| Heavy alcohol use | 2 (18.2) (missing =6) | 3 (9.4) (missing =1) | 0.59 |
| Tobacco use | 4 (30.8) (missing =4) | 8 (25.8) (missing =2) | 0.73 |
| GERD | 5 (29.4) | 7 (21.2) | 0.52 |
| Medications | |||
| PPI | 3 (17.7) | 13 (39.4) | 0.19 |
| H2RB | 1 (5.9) | 4 (12.1) | 0.65 |
| Aspirin | 1 (5.9) | 14 (42.4) | 0.008* |
| NSAIDs | 3 (17.7) | 1 (3.0) | 0.11 |
| Bisphosphonates | 1 (5.9) | 0 | 0.34 |
| Antibiotics | 0 | 3 (9.1) | 0.54 |
| Antifungals | 0 | 1 (3.0) | >0.99 |
Data are presented as n (%) or mean (± standard deviation). *, statistically significant (P value <0.05). N, number of patients; BMI, body mass index; GERD, gastroesophageal reflux disease; PPI, proton-pump inhibitor; H2RB, histamine-2 receptors blocker; NSAIDs, nonsteroidal anti-inflammatory drugs.
Discussion
To the best of our knowledge, this is the first study comparing HSV esophagitis with CMV esophagitis, in non-HIV patients.
In this study, we observed no gender-based difference comparing HSV and CMV; the number of cases was slightly higher in females in both CMV and HSV esophagitis. This finding contrasts with other studies that have reported higher occurrences of HSV esophagitis in males compared to females (6-10). However, for CMV esophagitis, studies have shown inconsistent results (10,11,12).
The average age of patients with VE was 63 years old, which aligns with earlier research (3,9). However, it is noteworthy that among patients who were not immunocompromised, the mean age was lower (46 years). Some studies reported even a lower mean age for immunocompetent patients with HSV esophagitis (35 years). This contradicts previous findings indicating a higher average age in immunocompetent patients, attributing this to a decline in immunity associated with aging (13).
Lee et al. were the first researchers to directly compare HSV esophagitis and CMV esophagitis. They observed that CMV esophagitis was more prevalent in patients with a history of solid organ transplants (10). In contrast, this study found that CMV esophagitis was more frequently diagnosed in patients who were taking immunosuppressive medication and steroids
In this study, the risk factors associated with VE were found to be linked to an immunosuppressed state. A potential selection bias might influence this observation, as immunocompromised patients are more likely to undergo EGD with biopsy compared to immunocompetent patients. Similarly, we found that smoking was more frequently reported in the control group, which could be related to avoidance or cessation of smoking in the case group as they are more likely to have other comorbidities.
Odynophagia has been reported as the most common presentation in HSV esophagitis with a frequency ranging between 34–70% followed by dysphagia reported at a frequency of 30.4% (6,7,9). This study showed that the most common presenting symptom in patients with HSV esophagitis was hematemesis (33%), which is more common than what is previously reported (3–13%); this observed difference could be due to the fact that MedStar Health Hospital includes two tertiary hospitals. Orolabial herpetic lesions were present in 10% of HSV esophagitis compared to 25% reported by Canalejo et al. (6).
Ulceration was the most common finding during EGD in patients with HSV esophagitis (67%), this is consistent with previous studies that showed similar frequencies ranging between 50–90% (6,7).
In regard to patients with CMV esophagitis, fever, and epigastric pain were reported only in 5% and 10%, respectively, which contrasts what is reported in previous studies where fever and epigastric pain were reported at a higher frequency (28%–36%, 31–41%) respectively (11,12,14). This study showed that the most common presenting symptom was dysphagia (42%) followed by odynophagia (36%).
CMV should be suspected in patients with severe esophagitis as this study highlights it as the most common presentation (37%) compared to the previously more commonly reported ulceration in 88% of patients with CMV esophagitis (11,12). Ulceration was the second most common EGD finding with a frequency of 26%.
Conclusions
Immunosuppression, including immunosuppressive therapy and systemic steroids, was a significant risk factor for VE. Other risk factors included organ transplant, active malignancy, and systemic steroid use. Surprisingly, VE was increasingly observed in immunocompetent individuals, challenging the perception that it primarily affects immunocompromised patients. Dysphagia was the prominent symptom in CMV esophagitis, while hematemesis was more common in HSV esophagitis. Ulceration was prevalent in HSV esophagitis, whereas severe esophagitis was frequent in CMV esophagitis. These findings improve our understanding of VE in non-HIV patients, aiding in early detection, management, and targeted interventions. Further research is needed to address the rising incidence in immunocompetent individuals and optimize prevention and treatment strategies.
Acknowledgments
We thank Reshmi Nair Ph.D. for her support in statistical analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-44/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-44/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-44/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-44/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Medstar Health (No. 00005623) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilcox CM. Overview of infectious esophagitis. Gastroenterol Hepatol (N Y) 2013;9:517-9. [PubMed]
- Demir D, Doğanavşargil B, Sarsık B, et al. Is it possible to diagnose infectious oesophagitis without seeing the causative organism? A histopathological study. Turk J Gastroenterol 2014;25:481-7. [Crossref] [PubMed]
- Hoversten P, Kamboj AK, Katzka DA. Infections of the esophagus: an update on risk factors, diagnosis, and management. Dis Esophagus 2018; [Crossref] [PubMed]
- McDonald GB, Sharma P, Hackman RC, et al. Esophageal infections in immunosuppressed patients after marrow transplantation. Gastroenterology 1985;88:1111-7. [Crossref] [PubMed]
- Ahuja NK, Clarke JO. Evaluation and Management of Infectious Esophagitis in Immunocompromised and Immunocompetent Individuals. Curr Treat Options Gastroenterol 2016;14:28-38. [Crossref] [PubMed]
- Canalejo E, García Durán F, Cabello N, et al. Herpes Esophagitis in Healthy Adults and Adolescents: Report of 3 Cases and Review of the Literature. Medicine 2010;89:204-10. [Crossref] [PubMed]
- Ramanathan J, Rammouni M, Baran J Jr, et al. Herpes simplex virus esophagitis in the immunocompetent host: an overview. Am J Gastroenterol 2000;95:2171-6. [Crossref] [PubMed]
- Wilcox CM. Infectious Esophagitis. Gastroenterol Hepatol (N Y) 2006;2:567-8. [PubMed]
- Hoversten P, Kamboj AK, Wu TT, et al. Variations in the Clinical Course of Patients with Herpes Simplex Virus Esophagitis Based on Immunocompetence and Presence of Underlying Esophageal Disease. Dig Dis Sci 2019;64:1893-900. [Crossref] [PubMed]
- Lee JS, Yun J, Ham S, et al. Machine learning approach for differentiating cytomegalovirus esophagitis from herpes simplex virus esophagitis. Sci Rep 2021;11:3672. [Crossref] [PubMed]
- Wang HW, Kuo CJ, Lin WR, et al. The clinical characteristics and manifestations of cytomegalovirus esophagitis. Dis Esophagus 2016;29:392-9. [Crossref] [PubMed]
- Yeh PJ, Wu RC, Chen CM, et al. Risk Factors, Clinical and Endoscopic Features, and Clinical Outcomes in Patients with Cytomegalovirus Esophagitis. J Clin Med 2022;11:1583. [Crossref] [PubMed]
- Wetwittayakhlang P, Rujeerapaiboon N, Wetwittayakhlung P, et al. Clinical Features, Endoscopic Findings, and Predictive Factors for Mortality in Tissue-Invasive Gastrointestinal Cytomegalovirus Disease between Immunocompetent and Immunocompromised Patients. Gastroenterol Res Pract 2021;2021:8886525. [Crossref] [PubMed]
- Hoversten P, Kamboj AK, Wu TT, et al. Risk Factors, Endoscopic Features, and Clinical Outcomes of Cytomegalovirus Esophagitis Based on a 10-year Analysis at a Single Center. Clin Gastroenterol Hepatol 2020;18:736-8. [Crossref] [PubMed]
Cite this article as: Al-Dwairy A, Azar L, Bakain T, Ahmad A, Woo S, Nithagon P, Chalhoub W. Viral esophagitis in non-human immunodeficiency virus patients: a case-control study. Transl Gastroenterol Hepatol 2024;9:19.


