Family history as a major prerequisite for microsatellite instability screening in colorectal cancer is a poor selection tool
Highlight box
Key findings
• Family history is a weak selection criterion for patients with high degree of microsatellite instable colorectal cancer (CRC).
• High degree microsatellite instability (MSI-H) cancer is common in the right colon.
• Germline testing may be warranted.
What is known and what is new?
• CRC may develop due to genetic predisposition.
• MSI-H CRC is usually found in families with mismatch repair deficiencies.
• Clinical guidelines have been used to select patients needing screening for MSI-H tumors.
What is the implication, and what should change now?
• MSI screening in patients with CRC should be routinely performed independent of family history.
Introduction
Colorectal cancer (CRC) is one of the most common solid malignant tumors and is responsible for a relevant portion of cancer-related death worldwide (1,2). CRC is thought to develop along one of three common pathways namely chromosomal instability (CIN), CpG island methylator phenotype (CIMP) and microsatellite instability (MSI) (3). The CIN pathway begins with acquired mutations in the adenomatous polyposis coli (APC) gene, followed by mutagenic activation of RAS oncogene and the inactivation of the tumor suppressor gene TP53 (4). This pathway has been shown to be responsible for over 80% of sporadic CRC. The CIMP pathway is characterized by promotor methylation resulting in mutations in the BRAF gene (5,6). The MSI pathway is characterized by mutations in at least one of the common DNA—mismatch repair (MMR) genes, MLH1, MSH2, MSH6 and PMS2. Such mutations may occur secondary to loss of heterozygosity in the tumor as described in the second hit theory by Knudson et al. (7) or due to germline mutations in Lynch syndrome (8). A high degree of MSI (MSI-H) is a common aspect of both the MSI and CIMP pathways (9,10). Traditionally, clinic criteria have been implemented to select patients with CRC who may require further investigation with regard to MSI (11-13). In the current German guidelines for the diagnosis and management of CRC for example (14), MSI screening is recommended in CRC patients who fulfill either the Amsterdam (13) or revised Bethesda Criteria (12). A family history of lynch-like (MSI-H) tumors is a pivotal aspect of these clinical criteria, which may be associated with low sensitivity and specificity in identifying mutation carriers. This implies that the risk of false selection is high and thus some patients with MSI-H CRC may go unselected. The aim of this study was to investigate the incidence of MSI-H tumors in an unselected population of patients undergoing radical surgery for CRC and compare the frequency of MSI-H CRC amongst patients with and without any family history of MSI-H cancer based on the Amsterdam and revised Bethesda criteria. We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-71/rc).
Methods
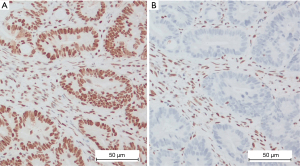
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients managed in our Department of Surgery II of the Witten/Herdecke University consented to the use of their data for research purposes and ethical approval was received from the ethics commission of the Witten/Herdecke University (approval No. 65/2017). All patients with CRC completed a questionnaire with items from both the Amsterdam and revised Bethesda criteria. Thus information concerning cancer in the family was systematically collected from all CRC patients. A search of our prospectively maintained institutional database for CRC was performed as previously published elsewhere (15). Data of all consecutive patients undergoing radical resection for CRC were retracted. All patients diagnosed with CRC in our institution were staged and discussed in the multidisciplinary tumor board before and after surgery per institutional standards independent of disease extent. The decision to perform surgery on patients with stage IV disease was based on individual cases with the aim of achieving complete resection in a multimodal treatment setting. Neoadjuvant chemoradiation therapy was performed in cases with locally advanced cancers of the mid and lower rectum prior to surgery. Radical surgery was performed in accordance with oncologic standards, i.e., central mesocolic excision (CME) for colon cancer (16), partial mesorectal excision for cancers of the rectosigmoid junction and total mesorectal excision (TME) for mid and low rectal cancer (17). Histopathology was performed to enable a TNM staging in all cases. Furthermore, immunohistochemistry (IHC) was performed on tumor slides to determine the presence or absence of gene products (proteins) of the four clinically relevant MMR genes, MLH1, MSH2, MSH6 and PMS2 as published elsewhere (18) (Figure 1A,1B). In order to address our main question, only cases with available MSI data were included in the study. Systematic MSI screening via IHC, independent of family history of cancer, was initiated in our department in 2015. Patients with family history of any MSI-related cancer were included in the study group, while those without family history of MSI-related tumors constituted the control group. Both groups were compared with respect to MSI findings following IHC of tumor samples.
Statistical analysis
The data generated was analyzed using the SPSS, version 25 (IBM, Armonk, NY, USA). Continuous variables are reported using absolute numbers and percentages, while central tendencies are reported using median and ranges. Sensitivity calculations were performed using a 4×4 cross table and analytic statistics was done using chi-square test. All calculations were done with a 95% confidence interval with statistical significance set at P<0.05.
Results
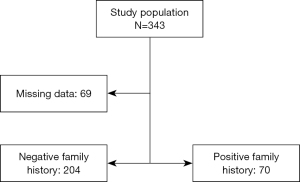
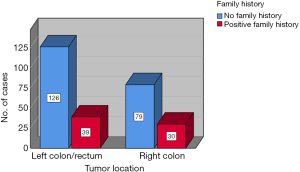
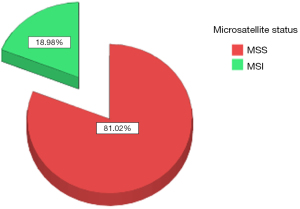
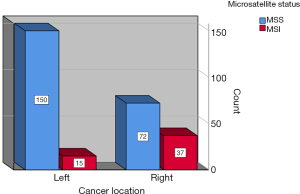
The datasets of 496 cases of CRC managed within a 5-year period from 2015 to 2020 were reviewed and the data of 343 cases with available findings from MSI IHC were retrieved. Complete datasets of 274 patients, 118 female and 156 male, with a median age of 68.5 years (range, 21–90 years) were available for analysis. The study population was divided into two groups, with or without family history of MSI-H cancer as defined in the revised Bethesda criteria (Figure 2). There was no statistically significant differences amongst both groups with regard to age (study group: 66 vs. control: 70 years; range, 21–90 years) at the time of surgery and sex distribution (study group 59% male vs. control 59% male). Equally, both groups were comparable with regard to cancer distribution (Figure 3). Family history was positive for lynch-like tumors in 25.1% of cases in this study, while the incidence of MSI-H CRC in the entire collective was 18.98% (Figure 4). Significantly more MSI-H tumors were found in the right colon in comparison to the left colon/rectum (Figure 5). Equally, significantly more cases of MSI-H CRC were found in the group with family history of MSI tumors compared to the group without any family history of MSI tumors (27.1% vs. 16.5%, P=0.04). The sensitivity and specificity of family history with regard to the presence of an MSI-H tumor in this collective was 36.5% and 77.5% respectively.
Discussion
MSI is present in over 15% of CRC, some of which may be associated with a hereditary predisposition for cancer. Screening for MSI in tumors has traditionally been guided by the presence of clinical characteristics including a positive family history. Our aim was to investigate the prevalence of MSI-H CRC in an unselected population of patients undergoing oncologic surgery for CRC. The sensitivity and specificity of family history based on Amsterdam and revised Bethesda criteria in our study low. Although the rate of MSI-H CRC was significantly higher in patients with a family history, numerically more cases of MSI-H CRC were found in the group without any family history of MSI-related tumors.
The importance of genetic alterations in the development of CRC is gaining more focus in clinical practice (19). Generally, hereditary syndromes with increased risk of CRC can be classified into three groups (20). The first group includes the adenomatous polyposis groups with entities like familial adenomatous polyposis (FAP) (21), MUTYH-associated polyposis (MAP) (22) and polymerase proof reading polyposis (PPAP) (23). In this group, CRC develops along a typical adenoma-carcinoma sequence. The second group includes the hamartomatous polyposis phenotype with entities like Peutz-Jeghers syndrome (PJS), juvenile polyposis and Cowden syndrome with cancer developing via the hamartoma-carcinoma sequence (24), and the third group includes non-polyposis phenotype like Lynch syndrome and Lynch-like syndrome [formerly hereditary nonpolyposis colorectal cancer (HNPCC)] (25). While the first two groups are readily identified on colonoscopy, the third group may be difficult to identify based on morphological aspects alone.
Historically, a familiar cancer predisposition was identified using clinical features mostly based on family history and patient’s age at the time of diagnosis of CRC. The Amsterdam criteria for example were established to identify patients who may harbor MSI-H tumors to initiate analysis of MSI status (13). Due to the stringent nature of the Amsterdam criteria, many potential MSI-H tumor carriers were unidentified, so these criterial were revised to generate the less rigorous Bethesda criteria with even more features (12).
The weak performance of these clinical screening features has remained a major drawback in the daily practice. The sensitivity of family history in identifying carriers of MSI-H tumors in this study was 36.5% with a specificity of 77.5%. Syngal et al. reported a sensitivity and specificity of about 61–94% and 25–67% respectively in a population with known mutations (26). The differences in these findings probably lay in the fact that our study included an unselected population, while the study by Syngal et al. (26) included only patients with known mutations. The data presented by Syngal et al. on the other hand indicate the poor performance of family history alone in identifying patients with MSI-H CRC, even in patients with known mutations.
Current evidence suggests that 15–30% of CRC may develop along the MSI pathway (27). The incidence of MSI-H CRC in our study was 20.4% and therefore in line with the current literature. The statistically significantly high rate of MSI-H CRC in patients with family history of MSI-related tumors seen in this study must be interpreted with caution due to the small sample size keeping in mind that numerically more cases of MSI-H CRC were found in the group without any family history of MSI- related cancer. Nonetheless, this finding does indicate that family history should not be completely neglected. Most of the MSI-H tumors in this study were located in the right colon. Similarly, most of the cases with positive family history of MSI-H tumors had CRC in the right colon. While the right sided predominance of MSI-H CRC seen in this study is in accordance with current literature (28), the tight association seen between right colonic predominance and positive family history represents an interesting finding. This finding may be a subject of future investigation.
The retrospective study design and the small sample size represent two relevant limitations in this study. The study design resulted in the exclusion of a relevant number of cases due to incomplete data with respect to family history and findings from MSI IHC. It is therefore possible that the findings generated in this study may be different in a larger prospective population. Therefore the trends reported in this study should be further investigated in a prospective setting with larger case numbers.
Despite the above limitation, the findings from this study indicated that the current practice with regard to MSI screening in cases with CRC as stated in the current German guidelines needs to be revisited. A systematic screening of all patients with CRC is probably the best and most practical option.
Conclusions
A relevant number of cases with MSI-H CRC may be missed secondary to screening based on clinical criteria like family history alone. Thus, systematic screening independent of clinical characteristics, especially family history of cancer should be recommended in all cases with CRC.
Acknowledgments
Many thanks to all the colleagues of the CRC database for their continued dedication and hard work.
Funding: Open Access funding of this study was enabled and organized by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-71/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-71/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-71/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was received for this study from the ethics commission of the Witten/Herdecke University (approval No. 65/2017). All patients gave a written consent for participation in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021; Epub ahead of print. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Mundade R, Imperiale TF, Prabhu L, et al. Genetic pathways, prevention, and treatment of sporadic colorectal cancer. Oncoscience 2014;1:400-6. [Crossref] [PubMed]
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010;138:2059-72. [Crossref] [PubMed]
- Esteller M, Corn PG, Baylin SB, et al. A gene hypermethylation profile of human cancer. Cancer Res 2001;61:3225-9. [PubMed]
- Weisenberger DJ, Levine AJ, Long TI, et al. Association of the colorectal CpG island methylator phenotype with molecular features, risk factors, and family history. Cancer Epidemiol Biomarkers Prev 2015;24:512-9. [Crossref] [PubMed]
- Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer 2001;1:157-62. [Crossref] [PubMed]
- Lynch HT, Smyrk T, Lynch J. An update of HNPCC (Lynch syndrome). Cancer Genet Cytogenet 1997;93:84-99. [Crossref] [PubMed]
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073-2087.e3. [Crossref] [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609-18. [Crossref] [PubMed]
- Vasen HF, Mecklin JP, Khan PM, et al. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991;34:424-5. [Crossref] [PubMed]
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. [Crossref] [PubMed]
- Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. [Crossref] [PubMed]
- Leitlinienprogramm Onkologie. S3-Leitlinie Kolorektales Karzinom. Version 2.1. AWEF; 2019.
- Ambe PC, Jansen S, Zirngibl H. New trend in colorectal cancer in Germany: are young patients at increased risk for advanced colorectal cancer? World J Surg Oncol 2017;15:159. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis 2009;11:354-64; discussion 364-5. [Crossref] [PubMed]
- Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg 1998;133:894-9. [Crossref] [PubMed]
- Shia J, Tang LH, Vakiani E, et al. Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: a 2-antibody panel may be as predictive as a 4-antibody panel. Am J Surg Pathol 2009;33:1639-45. [Crossref] [PubMed]
- Ambe PC, Möslein G. Surgical management of hereditary colorectal cancer. Mini-invasive Surg 2018;2:37. [Crossref]
- Valle L. Recent Discoveries in the Genetics of Familial Colorectal Cancer and Polyposis. Clin Gastroenterol Hepatol 2017;15:809-19. [Crossref] [PubMed]
- Bisgaard ML, Fenger K, Bülow S, et al. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat 1994;3:121-5. [Crossref] [PubMed]
- Nielsen M, Morreau H, Vasen HF, et al. MUTYH-associated polyposis (MAP). Crit Rev Oncol Hematol 2011;79:1-16. [Crossref] [PubMed]
- Palles C, Martin L, Domingo E, et al. The clinical features of polymerase proof-reading associated polyposis (PPAP) and recommendations for patient management. Fam Cancer 2022;21:197-209. [Crossref] [PubMed]
- Ambe PC, Möslein G. Management of Hamartomatous Polyps. In: Guillem J, Friedmann G, editors. Management of Hereditary Colorectal Cancer. Cham: Springer; 2020:11-39.
- Carethers JM. Differentiating Lynch-like from Lynch syndrome. Gastroenterology 2014;146:602-4. [Crossref] [PubMed]
- Syngal S, Fox EA, Eng C, et al. Sensitivity and specificity of clinical criteria for hereditary non-polyposis colorectal cancer associated mutations in MSH2 and MLH1. J Med Genet 2000;37:641-5. [Crossref] [PubMed]
- Gatalica Z, Vranic S, Xiu J, et al. High microsatellite instability (MSI-H) colorectal carcinoma: a brief review of predictive biomarkers in the era of personalized medicine. Fam Cancer 2016;15:405-12. [Crossref] [PubMed]
- Carethers JM. Microsatellite Instability Pathway and EMAST in Colorectal Cancer. Curr Colorectal Cancer Rep 2017;13:73-80. [Crossref] [PubMed]
Cite this article as: Jakob D, Orth V, Gödde D, Zirngibl H, Ambe PC. Family history as a major prerequisite for microsatellite instability screening in colorectal cancer is a poor selection tool. Transl Gastroenterol Hepatol 2024;9:17.