
Lower FIB-4 threshold in patients with diabetes improves diagnostic accuracy of the test in a Hispanic population
Highlight box
Key findings
• Authors provide a novel concept of modifying the fibrosis index based on 4 factors (FIB-4) threshold to ≥1.0 in patients with diabetes and ≥1.5 in patients without diabetes to improve the diagnostic performance of the test.
What is known and what is new?
• Non-invasive tests are commonly used to estimate severity of fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). Patients with diabetes are known to have a more progressive course of NAFLD and can have normal aminotransferases which could falsely lower the FIB-4 score. Per society guidelines, a FIB-4 threshold of <1.3 is often used to rule out advanced fibrosis.
• The new finding from the study included a modified FIB-4 threshold of ≥1.0 in patients with diabetes to improve the sensitivity of the test and thus, properly screen patients with diabetes who are at risk of significant or advanced fibrosis. Patients without diabetes can be appropriately risk stratified for significant or advanced fibrosis with a higher FIB-4 threshold of ≥1.5.
What is the implication, and what should change now?
• By lowering the FIB-4 threshold, patients with diabetes, who are at a higher risk of fibrosis progression in NAFLD, will be more appropriately screened.
• Providers should consider modifying FIB-4 threshold in patients with diabetes, especially those with normal aminotransferases, so that fibrosis can be appropriately screened in this population.
Introduction
Staging of liver fibrosis is considered the most important predictor of liver-related morbidity and mortality in patients with nonalcoholic fatty liver disease (NAFLD) (1-5). Non-invasive tests (NITs) to determine fibrosis stage have largely replaced liver biopsy in clinical practice, especially in patients with metabolic associated fatty liver disease (1,6). Imaging elastography and serum biomarkers are the two predominant types of NITs that are currently available (7-9).
As NAFLD is increasing in prevalence, screening for the disease in at-risk populations, such as those with metabolic comorbidities, is warranted in the primary care and endocrinology settings. In fact, the American Association of Clinical Endocrinology and newly published American Association for the Study of Liver Diseases (AASLD) practice guidelines propose an algorithm for screening NAFLD in the non-gastroenterology (GI)/hepatology setting using the fibrosis index based on 4 factors (FIB-4). If FIB-4 is <1.3, the patient is considered low risk and FIB-4 can be monitored periodically. Whereas, if FIB-4 is >2.67 or if secondary risk assessment with elastography or enhanced liver fibrosis (ELF) indicates intermediate or high risk, the patient should be referred to GI/hepatology care (1,10). The widespread use of this NIT in clinical practice relies on its diagnostic abilities.
The diagnostic performance of scores such as FIB-4, NAFLD Fibrosis Score (NFS) and Aspartate aminotransferase-to-platelet ratio index (APRI) is variable and can vary across disease etiologies (11-15). The inconsistent results of NITs and variability between the different scores are certainly a limitation for providers to consider when utilizing in clinical practice (16). Recent studies have demonstrated the poor performance of FIB-4, especially in patients with diabetes (17,18). In fact, one study found a 43% overall false negative rate of FIB-4 using elastography as the reference standard with a higher proportion of false negatives in patients with diabetes than in patients without diabetes (18). Another recent study evaluated the accuracy of FIB-4 in detecting elevated liver stiffness measurement (LSM) and found that diabetes was one variable in which there was a higher proportion of false negative FIB-4 scores (19). This may be due to the fact that patients with diabetes may not have elevated aminotransferases and have a more progressive clinical course than their non-diabetic counterparts (1,10,17,20).
Furthermore, the reliability of NITs is unknown in patients of Hispanic ethnicity and has not been validated in this population. Prevalence of both NAFLD and diabetes is higher in Hispanic patients (1,10,21). If NITs underperform in patients with comorbid conditions such as diabetes, then patients of Hispanic origin may be further disadvantaged by these noninvasive scores. Therefore, we aimed to evaluate diagnostic performance of FIB-4 in NAFLD using LSM as the comparative standard in patients of Hispanic origin and proposed a modified FIB-4 to include diabetes. We present this article in accordance with the STARD reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-62/rc).
Methods
Study population
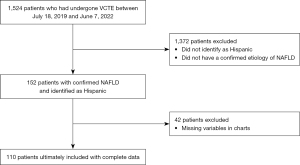
We conducted a retrospective, single-center review of patients who underwent vibration-controlled transient elastography (VCTE) for suspected NAFLD at University of California, Los Angeles from July 18, 2019 to June 7, 2022. A total of 1,524 patients completed VCTE in this time period. Patients were then identified as “Hispanic” origin based on ethnicity/race in electronic medical record (EMR). Disease etiology was confirmed as NAFLD in this group of patients based on EMR and steatosis score on VCTE. Data on co-morbid conditions were identified via chart extraction. Ultimately, 110 patients were included in the study. A flow chart of patients included in the study is depicted in Figure 1. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was certified exempt from the Institutional Review Board (IRB) at UCLA per 45 CFR 46.104 category 4(iii). Informed consent was waived due to the IRB exempt status of the study.
Data collection
Basic demographic information including age, sex, weight, height, body mass index (BMI), household income, insurance type, co-morbid conditions, lab values, VCTE scores and imaging results were obtained from the EMR. Laboratory values were collected within 1 year of the date of VCTE. FIB-4 was calculated based on the following equation: [age (years) × AST (U/L)]/[(platelets (109/L)] × [ALT (U/L)1/2] (22). LSM values and steatosis scores were the average of 10 values that were obtained. Patients were designated into the F0–F1 fibrosis group and F2–F4 fibrosis group based on the LSMs. Values of LSM ranging from 1.5 kilopascals (kPa) to 8.2 kPa were categorized as F0–F1, 8.3–9.7 as F2, 9.8–13.6 as F3 and 13.6 and above as F4 (23).
Outcomes
We evaluated the sensitivity, specificity, positive predictive value and negative predictive value of FIB-4 in 110 patients of Hispanic origin. We defined a positive test using a threshold of ≥1.3, which is based on the algorithm for fibrosis assessment developed by the AASLD (1). We also evaluated the performance of a score including both FIB-4 and presence or absence of diabetes. Using the enhanced diagnostic accuracy of this new score, we proposed new thresholds for FIB-4 to predict significant fibrosis in patients with diabetes and without diabetes.
Statistical analysis
Differences in demographic and clinical characteristics between fibrosis groups (F0–F1 vs. F2–F4) were assessed using t-tests for continuous variables and exact tests for categorical. These tests were also used to assess for bivariate differences in imaging characteristics between those with FIB-4 values < 1.3 vs. ≥1.3. Because diabetes is a known confounder of fibrosis, we attempted to identify diabetes status specific FIB-4 thresholds. Logistic regression models were the used with quadratic terms for continuous FIB-4 in order to determine the discriminatory ability of FIB-4 to differentiate between fibrosis group membership within groups with diabetes.
Models were fit using clustered robust standard error for patient to account for the correlation within patients across repeated observations. Area under the receiver operating curve (AUROC) was computed as the primary measure of discriminatory ability and sensitivity, specificity, positive predictive value, and negative predictive value were computed at the various thresholds of FIB-4. Youden’s index was used to select an optimal threshold for FIB-4 which maximized both sensitivity and specificity (24). Conducted in Stata version 17.0, StataCorp LLC (College Station, Texas, USA). Descriptive statistics were utilized.
Results
A total of 110 Hispanic patients were included in the study. Demographic characteristics comparing patients with F0–F1 fibrosis and F2–F4 fibrosis based on LSM are shown in Table 1. Sixty-five percent were female. Mean BMI in the cohort was 32.4±5.9 kg/m2 with a significantly higher mean BMI of 35.1±6.0 kg/m2 in the F2–F4 group compared to a mean BMI of 31.3±5.5 kg/m2 in the F0–F1 group (P=0.003). Household income was evenly distributed between the categories of <$75,000, $75,000–100,000 and $100,000-150,000 with a minority in the >$150,000 group and was similar between patients with F0–F1 and F2–F4 fibrosis (P=0.380). The majority of patients had private insurance. Almost half (48%) of patients had diabetes and there was a significantly higher incidence of diabetes in the group with F2–F4 fibrosis as compared to the group with F0–F1 fibrosis (76% vs. 36%, P<0.001). Many patients also had other comorbid conditions such as hypertension, dyslipidemia and obesity. Laboratory values were also collected and are included in Table 1. Notably, the mean ALT was elevated at 57.1±37.7 but was not significantly different between patients with F0–F1 and F2–F4 fibrosis (55.1±37.7 and 61.5±38.0 respectively, P=0.419). Mean liver stiffness was 7.63±6.30 kPa overall, with a mean LSM of 5.00±1.17 kPa in the F0–F1 group and 13.77±8.75 kPa in the F2–F4 group (P<0.001). The majority of patients overall had S3 steatosis (56%). Additional patient characteristics are shown in Table 1. All patients had imaging with either computed tomography (CT), ultrasound or magnetic resonance imaging (MRI). Eighty-two percent of patients overall had steatosis on imaging, 29% with hepatomegaly, 7% with splenomegaly and 7% with a nodular contour.
Table 1
| Variable | Total (n=110) | F0–F1 (n=77) | F2–F4 (n=33) | P |
|---|---|---|---|---|
| Age (years) | 53.2±13.5 | 51.0±13.8 | 59.3±11.4 | 0.005 |
| Sex | 0.189 | |||
| Male | 38 (35%) | 30 (39%) | 8 (24%) | |
| Female | 72 (65%) | 47 (61%) | 25 (76%) | |
| Weight (kg) | 87.2±17.5 | 84.7±16.5 | 92.9±18.7 | 0.035 |
| Height (cm) | 164±11 | 165±11 | 163±11 | 0.373 |
| BMI (kg/m2) | 32.4±5.9 | 31.3±5.5 | 35.1±6.0 | 0.003 |
| Household income | 0.380 | |||
| <$75,000 | 30 (27%) | 19 (25%) | 11 (33%) | |
| $75,000–100,000 | 29 (26%) | 23 (30%) | 6 (18%) | |
| $100,000–150,000 | 36 (33%) | 23 (30%) | 13 (39%) | |
| >$150,000 | 15 (14%) | 12 (16%) | 3 (9%) | |
| Insurance | 0.584 | |||
| Private | 78 (71%) | 55 (71%) | 23 (70%) | |
| Public | 29 (26%) | 19 (25%) | 10 (30%) | |
| Other | 3 (3%) | 3 (4%) | 0 | |
| Diabetes | 53 (48%) | 28 (36%) | 25 (76%) | <0.001 |
| Essential hypertension | 51 (46%) | 32 (42%) | 19 (58%) | 0.146 |
| Dyslipidemia | 53 (48%) | 38 (49%) | 15 (45%) | 0.835 |
| Obesity (BMI >30 kg/m2) | 71 (65%) | 43 (56%) | 28 (85%) | 0.004 |
| White blood cell count (×109) | 6.84±2.19 | 6.83±2.18 | 6.86±2.23 | 0.941 |
| Hemoglobin (g/dL) | 13.9±1.8 | 14.1±1.9 | 13.6±1.6 | 0.140 |
| Platelet count (×109) | 251±65 | 258±64 | 235±68 | 0.111 |
| Glucose (mg/dL) | 116±39 | 110±34 | 128±47 | 0.049 |
| AST (IU/L) | 47.0±43.7 | 43.0±46.3 | 56.5±35.6 | 0.101 |
| ALT (IU/L) | 57.1±37.7 | 55.1±37.7 | 61.5±38.0 | 0.419 |
| Elevated ALT* | 83 (75%) | 56 (73%) | 27 (82%) | 0.345 |
| AST/ALT ratio | 0.907±0.495 | 0.875±0.536 | 0.983±0.382 | 0.235 |
| Alkaline phosphatase (IU/L) | 92.7±31.6 | 89.3±31.9 | 100.6±29.9 | 0.081 |
| Total bilirubin (mg/dL) | 0.545±0.431 | 0.532±0.414 | 0.576±0.473 | 0.650 |
| Albumin (g/dL) | 4.43±0.39 | 4.50±0.40 | 4.29±0.30 | 0.005 |
| Total cholesterol (mg/dL) | 185±49 | 186±48 | 182±51 | 0.729 |
| Hemoglobin A1c (%) | 6.56±1.38 | 6.29±1.07 | 7.15±1.78 | 0.019 |
| TSH (mIU/L) | 2.52±3.96 | 2.70±4.63 | 2.10±1.31 | 0.347 |
| CAP score | 329±46 | 328±43 | 330±52 | 0.815 |
| IQR CAP | 29.3±14.5 | 30.8±15.3 | 25.8±11.9 | 0.072 |
| Liver stiffness (kPa) | 7.63±6.30 | 5.00±1.17 | 13.77±8.75 | <0.001 |
| IQR liver stiffness | 0.871±0.920 | 0.506±0.279 | 1.721±1.277 | <0.001 |
| Steatosis | 0.057 | |||
| S1 | 20 (18%) | 12 (16%) | 8 (24%) | |
| S2 | 28 (25%) | 16 (21%) | 12 (36%) | |
| S3 | 62 (56%) | 49 (64%) | 13 (39%) | |
| FIB-4 >1.3 | 0.001 | |||
| No | 63 (57%) | 52 (68%) | 11 (33%) | |
| Yes | 47 (43%) | 25 (32%) | 22 (67%) | |
| FIB-4, mean ± SD | 1.29±1.29 | 1.99±1.31 | 0.013 | |
| FIB-4, median [IQR] | 1.01 [0.70, 1.49] | 1.57 [1.16, 2.77] | <0.001 |
Values, where applicable, reported as mean ± SD, n (%), or median [IQR]. *, defined as >33 IU/L for men and >25 IU/L for women. Equation: FIB-4 = age (years) × AST (U/L)/[PLT (109/L) × ALT (U/L)1/2]. BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase; TSH, thyroid stimulating hormone; CAP, controlled attenuation parameter; IQR, interquartile range; FIB-4, fibrosis-4; SD, standard deviation; PLT, platelet count.
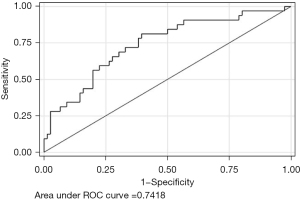
We calculated the diagnostic accuracy of FIB-4, with LSM as the reference standard, using ≥1.3 as the threshold for a positive test. Sensitivity was 68.8% and specificity was calculated to be 67.1%. The positive and negative predictive value were 46.8% and 83.6% respectively. The odds ratio for predicting significant fibrosis (F2–F4 on LSM) was 4.49 [95% confidence interval (CI): 1.88–10.66; P<0.001] with an AUROC of 0.74, depicted in Figure 2.
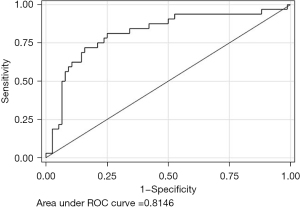
Since diabetes was found to be a significant predictor of F2–F4 fibrosis, we opted to evaluate the diagnostic performance of FIB-4 by adding the presence or absence of diabetes. In patients with diabetes, Youden’s index indicates a FIB-4 threshold of 1.247 to maximally optimize the sensitivity and specificity which would be 70.8% and 71.4% respectively. However, to enhance sensitivity, a FIB-4 threshold of 1.000 with a sensitivity of 87.5% and specificity 46.4% would optimally identify patients at risk of significant fibrosis without foregoing specificity (Table 2). In patients without diabetes, Youden’s index for FIB-4 is 1.522 which corresponds to a sensitivity of 75% and specificity of 75% (Table 3). Clinically, the threshold of 1.522 also reflects the number that optimizes sensitivity in this population. Modifying FIB-4 to include the presence or absence of diabetes increases the AUROC to 0.81, shown in Figure 3.
Table 2
| FIB-4 cutoff | Youden’s index | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| 0.887 | – | 95.8 | 28.6 | 53.5 | 88.9 |
| 1.000* | – | 87.5 | 46.4 | 58.3 | 81.2 |
| 1.247 | Yes | 70.8 | 71.4 | 68.0 | 74.1 |
*, a threshold of 1.000 corresponds to the clinically significant value that optimizes sensitivity. FIB-4, fibrosis index based on 4 factors.
Table 3
| FIB-4 cutoff | Youden’s index | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| 1.441 | – | 75.0 | 68.8 | 28.6 | 94.2 |
| 1.522* | Yes | 75.0 | 75.0 | 33.3 | 94.7 |
| 1.739 | – | 50.0 | 87.5 | 40.0 | 91.3 |
*, a threshold of 1.522 corresponds to the clinically significant value that maximizes sensitivity and specificity. FIB-4, fibrosis index based on 4 factors.
Discussion
In this study, we demonstrated the suboptimal sensitivity and specificity of a FIB-4 threshold of 1.3 to predict significant fibrosis in patients of Hispanic origin with NAFLD, with an AUROC 0.74. By including the presence or absence of diabetes, the AUROC improved to 0.81, using a threshold of 1.0 for patients with diabetes and a threshold of 1.5 for patients without diabetes to predict significant fibrosis. By modifying the thresholds using diabetes as a factor in the FIB-4 model, we increase the sensitivity of the noninvasive test, thereby capturing more patients who are at risk of significant fibrosis.
Fibrosis is the single most important predictor of liver-related morbidity and mortality and thus, screening modalities to estimate fibrosis need to be accurate (1-5). Patients with diabetes are at higher risk of advanced fibrosis and the need to minimize false negatives in this population is critical (1,10,20). In fact, patients with diabetes and NAFLD with or without steatohepatitis can have normal aminotransferases and thus, a FIB-4 score of <1.3 may not appropriately capture patients at risk of fibrosis (25). The prevalence of diabetes is also higher in certain minority groups, such as those of Hispanic origin, who would then be disadvantaged by the current FIB-4 model and thresholds (1,10,21).
Furthermore, patients of Hispanic origin have a higher prevalence of NAFLD, which is likely the result of a combination of genetic, environmental, socioeconomic and cultural factors (26). Metabolic disease and in particular diabetes are prevalent in Hispanic patients which is a known risk factor for progression of NAFLD. The I148M allele of the patatin-like phospholipase domain containing 3 (PNPLA3) gene is known to be associated with increased hepatic fat content and associated inflammation and is found more commonly in Hispanic patients than other ethnicities (27). Diet and cultural factors may also play a role in propagation of NAFLD within the Hispanic population. One study by Heredia et al. demonstrated that in a population of Hispanic/Latino adults, high diet quality based on the Healthy Eating Index and higher levels of physical activity were associated with a 40% lower risk of NAFLD (28). These data suggest that lifestyle can have an impact on NAFLD in the Hispanic population. The development of NAFLD in the Hispanic population is evidently multifactorial, contributing to the progressively fibrotic nature of the disease.
The relationship between diabetes and NAFLD in the Hispanic population is critical to understand in order to appropriately risk stratify patients who may have clinically significant fibrosis. As non-invasive testing is over taking liver biopsies for risk assessment, it is crucial to employ NITs with high sensitivity when used as a screening tool. Studies have shown limitations with FIB-4 and poor diagnostic performance in various populations and false negative rates as high as 43% (17,18). Although NFS incorporates diabetes into its algorithm, it also includes BMI which may not necessarily be reflective of metabolic syndrome (1). Thus, we suggest adding diabetes status to FIB-4, which is the most validated NIT in NAFLD. By proposing to lower the cutoff of FIB-4 in patients with diabetes, we will capture more patients with clinically significant fibrosis and even higher stages of fibrosis.
To our knowledge, this is the first study of its kind to evaluate the diagnostic performance of FIB-4 in Hispanic patients with NAFLD and propose a new FIB-4 cutoff by incorporating diabetic status to benefit this population which identifies patients at risk for significant fibrosis with enhanced accuracy. A limitation of this study is that it represents a small population at a single center, in patients of Hispanic origin and may not be generalizable. However, the data is granular, demonstrating that diabetes is a significant factor in fibrosis and can impact the performance of FIB-4. Another limitation is that the reference standard was transient elastography and not liver biopsies. VCTE though is now widely used in lieu of liver biopsies so this study reflects real-world practice. Future studies with larger populations are warranted to investigate lower thresholds of FIB-4 in patients at risk of clinically significant fibrosis.
Conclusions
To optimize sensitivity and thereby minimizing false negatives in patients with diabetes, we propose a new FIB-4 threshold of 1 to identify this patient population that is at risk for significant fibrosis. On the converse, a higher FIB-4 cutoff of 1.5 may be employed in patients without diabetes who may not be as high risk as their diabetic counterparts. By incorporating diabetes into the FIB-4 model, we can better identify patients at higher risk of significant fibrosis and provide enhanced care to a disadvantaged population.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-62/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-62/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-62/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-62/coif). S.S. reports receiving consulting fee from Gilead, Madrigal, Mallinckrodt, and speakers bureaus from AbbVie, Gilead, Intercept, Salix, Eisai, Exelisis, Mallinckrodt, Takeda. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was certified exempt from the Institutional Review Board (IRB) at UCLA per 45 CFR 46.104 category 4(iii). Informed consent was waived due to the IRB exempt status of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023;77:1797-835. [Crossref] [PubMed]
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149:389-97.e10. [Crossref] [PubMed]
- Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547-54. [Crossref] [PubMed]
- Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265-73. [Crossref] [PubMed]
- European Association for the Study of the Liver (EASL). European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. [Crossref] [PubMed]
- Rinella ME, Lominadze Z, Loomba R, et al. Practice patterns in NAFLD and NASH: real life differs from published guidelines. Therap Adv Gastroenterol 2016;9:4-12. [Crossref] [PubMed]
- Balakrishnan M, Loomba R. The Role of Noninvasive Tests for Differentiating NASH From NAFL and Diagnosing Advanced Fibrosis Among Patients With NAFLD. J Clin Gastroenterol 2020;54:107-13. [Crossref] [PubMed]
- Younossi ZM, Noureddin M, Bernstein D, et al. Role of Noninvasive Tests in Clinical Gastroenterology Practices to Identify Patients With Nonalcoholic Steatohepatitis at High Risk of Adverse Outcomes: Expert Panel Recommendations. Am J Gastroenterol 2021;116:254-62. [Crossref] [PubMed]
- European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237-64. [Crossref] [PubMed]
- Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract 2022;28:528-62. [Crossref] [PubMed]
- Ito T, Nguyen VH, Tanaka T, et al. Poor Diagnostic Efficacy of Noninvasive Tests for Advanced Fibrosis in Obese or Younger Than 60 Diabetic NAFLD patients. Clin Gastroenterol Hepatol 2023;21:1013-1022.e6. [Crossref] [PubMed]
- Wong VW, Tak WY, Goh GBB, et al. Performance of Noninvasive Tests of Fibrosis Among Asians, Hispanic, and non-Hispanic Whites in the STELLAR Trials. Clin Gastroenterol Hepatol 2023;21:90-102.e6. [Crossref] [PubMed]
- Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep 2020;2:100067. [Crossref] [PubMed]
- Anstee QM, Lawitz EJ, Alkhouri N, et al. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology 2019;70:1521-30. [Crossref] [PubMed]
- McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265-9. [Crossref] [PubMed]
- Schreiner AD, Livingston S, Zhang J, et al. Identifying Patients at Risk for Fibrosis in a Primary Care NAFLD Cohort. J Clin Gastroenterol 2023;57:89-96. [Crossref] [PubMed]
- Kim RG, Deng J, Reaso JN, et al. Noninvasive Fibrosis Screening in Fatty Liver Disease Among Vulnerable Populations: Impact of Diabetes and Obesity on FIB-4 Score Accuracy. Diabetes Care 2022;45:2449-51. [Crossref] [PubMed]
- Graupera I, Thiele M, Serra-Burriel M, et al. Low Accuracy of FIB-4 and NAFLD Fibrosis Scores for Screening for Liver Fibrosis in the Population. Clin Gastroenterol Hepatol 2022;20:2567-2576.e6. [Crossref] [PubMed]
- Viganò M, Pugliese N, Cerini F, et al. Accuracy of FIB-4 to Detect Elevated Liver Stiffness Measurements in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Referral Centers. Int J Mol Sci 2022;23:12489. [Crossref] [PubMed]
- Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol 2019;71:793-801. [Crossref] [PubMed]
- Statistics about diabetes. Statistics About Diabetes | ADA. (n.d.). Retrieved November 22, 2022. Available online: https://diabetes.org/about-us/statistics/about-diabetes
- Sterling RK, Lissen E, Clumeck NAPRICOT Clinical Investigators, et al. Development of a simple noninvasive index to predict significant fibrosis inpatients with HIV/HCV coinfection. Hepatology 2006;43:1317-25. [Crossref] [PubMed]
- Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019;156:1717-30. [Crossref] [PubMed]
- YOUDEN WJ. Index for rating diagnostic tests. Cancer 1950;3:32-5. [Crossref] [PubMed]
- Portillo-Sanchez P, Bril F, Maximos M, et al. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab 2015;100:2231-8. [Crossref] [PubMed]
- Saab S, Manne V, Nieto J, et al. Nonalcoholic Fatty Liver Disease in Latinos. Clin Gastroenterol Hepatol 2016;14:5-12; quiz e9-10. [Crossref] [PubMed]
- Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461-5. [Crossref] [PubMed]
- Heredia NI, Zhang X, Balakrishnan M, et al. Association of lifestyle behaviors with non-alcoholic fatty liver disease and advanced fibrosis detected by transient elastography among Hispanic/Latinos adults in the U.S. Ethn Health 2023;28:299-312. [Crossref] [PubMed]
Cite this article as: Singh J, Ibrahim B, Jackson NJ, Khalil H, Valenzuela J, Yanny B, Saab S. Lower FIB-4 threshold in patients with diabetes improves diagnostic accuracy of the test in a Hispanic population. Transl Gastroenterol Hepatol 2024;9:16.


