
Revamping hepatitis C global eradication efforts: towards simplified and enhanced screening, prevention, and treatment
Hepatitis C virus (HCV) remains a significant global health challenge, with considerable morbidity and mortality. The introduction of direct-acting antivirals (DAAs) has been transformative, offering cure rates above 95%. The World Health Organization (WHO) set forth the goal of eliminating viral hepatitis as a major health threat by 2030 (1). While the global challenge of HCV elimination is constrained by limited resources for education, screening, and access to DAAs, it is important to address the situation in the United States, which extends beyond just screening and DAAs access. While the current opioid crisis in the United States is linked to a surge in HCV infection among young individuals (2,3), the coronavirus disease 2019 (COVID-19) pandemic has significantly disrupted the hepatitis C eradication effort, leading to a notable reduction in HCV antibody testing during the pandemic (4).
The new and updated HCV guidance from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Disease Society of America (IDSA) is crucial, highlighting the mission of HCV elimination in the United States through universal HCV screening, a simplified management algorithm, and minimal monitoring (5). Although this manuscript primarily focuses on the AASLD-IDSA guideline, we will also discuss the global effort towards HCV elimination, particularly in low- and middle-income countries.
In the United States, it is recommended to offer at least one time HCV screening for all adults aged 18 years and order. HCV screening testing consists of HCV antibody screening with reflex HCV RNA testing to determine the presence of active infection as compared to spontaneous or treatment-induced viral clearance (5-7). In addition to one-time screening for adult individuals, all pregnant females should be offered HCV screening during each pregnancy unless HCV prevalence is <0.1%. Furthermore, individuals who have human immunodeficiency virus (HIV) infection, people who inject drugs (PWID), men who have sex with men (MSM), and incarcerated persons should be offered HCV screening at least once a year even after they were previously treated for HCV with DAAs. This universal screening strategy with repeat screening at the high-risk population is cost-effective in finding and treating HCV especially as the epidemiology of new HCV infection is shifting toward young adults, PWID, HIV, MSM, and incarcerated individuals (5).
Incomplete medication adherence of HCV DAAs is frequent and is challenging to track down and manage (8). While nonadherence episodes are common, they are mostly short-lived and these are not associated with virological failure, with sustained virologic response (SVR) 12 weeks after the completion of treatment (SVR12) was 94% among both adherent and nonadherent patients where nonadherence was defined as taking <90% of the total prescribed dosage (9).
Recent global data (10) indicates that a minimal monitoring approach during DAA treatment for chronic HCV is safe and effective, yielding a 95% SVR rate comparable to standard monitoring. This approach involves no pre-treatment genotyping, dispensing the full doses for the entire treatment period when initiating the therapy, no scheduled for on-treatment visits or lab tests, and remote check-ins at weeks 4 (for DAA adherence) and 22 (to arrange SVR assessment at week 24).
Simplified HCV treatment guidance from AASLD-IDSA involves a 12-week course of once-daily sofosbuvir (400 mg)/velpatasvir (100 mg) or an 8-week course of glecaprevir (300 mg)/pibrentasvir (120 mg) (Figure 1). This treatment is suitable for acute and chronic HCV mono-infection or HCV/HIV coinfected patients (aged ≥3 years), except those who are pregnant, have uncontrolled hepatitis B virus infection, current or prior decompensated cirrhosis (Child-Pugh score >7), hepatocellular carcinoma (HCC), previous HCV treatment failure, or have undergone liver transplantation. For both treatment naïve and experienced liver or kidney transplant recipients with HCV, either a 12-week course of glecaprevir/pibrentasvir or sofosbuvir/velpatasvir is recommended. For glecaprevir/pibrentasvir regimen, cyclosporine should be kept <100 mg/d due to mild drug-drug interaction between cyclosporine and glecaprevir (5).
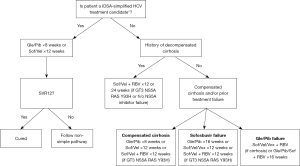
While the simplified HCV treatment guidance from AASLD-IDSA does not specifically reference the sofosbuvir/daclatasvir regimen, it is crucial to note that the sofosbuvir/daclatasvir regimen represents a cost-effective treatment option. It consistently demonstrates a SVR rate exceeding 90%, regardless of the presence of cirrhosis or HCV genotype. Therefore, it should be considered as a primary treatment choice, especially in low- to middle-income countries (11).
It is important to mention that sofosbuvir/velpatasvir, in conjunction with weight-based ribavirin, is advisable for the treatment of compensated cirrhotic patients infected with HCV genotype 3 and exhibiting either baseline HCV nonstructural protein 5A (NS5a) Y93 resistance-associated substitution (RAS) or decompensated HCV cirrhotic patients, irrespective of their HCV genotype or the presence of RAS (5).
Although, the NS5A RAS test is readily available in high-income countries but may not be easily accessible in other regions across the globe. Hence, in pursuit of the global HCV elimination goal, we recommend treating the HCV-infected patients with sofosbuvir/velpatasvir or glecaprevir/pibrentasvir without the necessity of RAS testing, reserving the use of a salvage regimen for cases where SVR is not attained.
Glecaprevir/pibrentasvir is recommended for 8 weeks of duration for treatment-naïve with or without compensated cirrhosis. Glecaprevir/pibrentasvir is contraindicated to use in prior and present history of decompensated cirrhosis due to its protease inhibitor (5). Patients who fail sofosbuvir-based treatments should undergo 12 weeks of sofosbuvir/velpatasvir/voxilaprevir (Figure 1). However, those with genotype 3 and compensated cirrhosis should add weight-based ribavirin to this regimen. For previous failures with glecaprevir/pibrentasvir treatment, suggested options are either retreatment with glecaprevir/pibrentasvir, ribavirin, and sofosbuvir, or a 12-week course of sofosbuvir/velpatasvir/voxilaprevir. In cases of compensated cirrhosis, considering the addition of weight-based ribavirin is advisable, as indicated by past DAA failure studies (5).
For prior sofosbuvir/velpatasvir/voxilaprevir treatment failures, recommended are 16 weeks of glecaprevir/pibrentasvir with sofosbuvir and weight-based ribavirin; a 24-week course of sofosbuvir/velpatasvir/voxilaprevir with weight-based ribavirin; or a 24-week course of sofosbuvir/velpatasvir/voxilaprevir with weight-based ribavirin. For patients with decompensated HCV cirrhosis and a history of DAA failure, a 24-week retreatment with either sofosbuvir/velpatasvir or ledipasvir/sofosbuvir, both supplemented with weight-based ribavirin, is recommended (5).
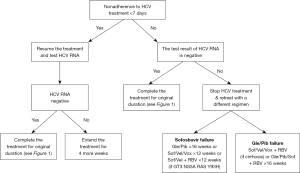
The updated AASLD-IDSA guideline recommends if the HCV treatment nonadherence is <7 days before receiving 28 days of DAA, DAA therapy can resume and complete for original planned duration (8 or 12 weeks) (Figure 2). If nonadherence is only for <7 days regardless of the prior duration of the treatment, DAA therapy can resume and complete for original planned duration (8 or 12 weeks) if the HCV RNA is found to be negative (Figure 2). If HCV RNA is positive, Bhattacharya et al. recommends extending DAA treatment for an additional 4 weeks (5). If nonadherence is for >8 days after receiving >28 days of DAA therapy and HCV RNA is positive, the guideline recommends stop treatment and retreat according to the retreatment recommendation (Figure 2).
Non-cirrhotic patients who have successfully achieved SVR typically do not require ongoing follow-up unless they fall into high-risk categories for HCV reinfection. Individuals who are at an increased risk, such as PWID, MSM, a history of incarceration, or cirrhosis, should undergo lifelong monitoring for HCV reinfection and regular screening for HCC (5).
In the simplified treatment algorithm, it is imperative to assess the presence of cirrhosis, which can be determined by one of various options, including a liver stiffness measurement exceeding 12.5 kPa by FibroScan, a fibrosis-4 (FIB-4) score surpassing 3.25, liver biopsy results, a platelet count lower than 150,000/mm3, or the identification of liver nodularity or splenomegaly through abdominal imaging (5).
The indications and duration of HCC screening following HCV cure remain a contentious and unsettled issue, necessitating long-term data on patients with pre-treatment advanced fibrosis or cirrhosis. Disease burden modeling data (12) suggests that, for patients who successfully achieve SVR, it is cost-effective to implement biannual surveillance for HCC screening. This practice is recommended until the age of 70 years for individuals with cirrhosis and until the age of 60 years for those with stable advanced fibrosis (12).
We recognize that the AASLD-IDSA simplified HCV treatment guidance is primarily designed for high-income countries, emphasizing the need for a comprehensive and global approach. The complexity and associated costs of resistance testing, especially for re-treatment, present substantial challenges in low- to middle-income countries. Furthermore, the high cost and limited accessibility of DAAs in certain regions hinder universal treatment, resulting in health disparities worldwide. This underscores the urgent need for global health equity and the implementation of comprehensive preventive measures, particularly in high-risk populations.
To achieve the elimination of hepatitis C, fostering collaboration among policy experts, healthcare providers, pharmaceutical companies, and public health organizations dedicated to harm reduction is imperative. Initially, public health policy experts and healthcare providers can collaborate to enhance access to treatment options while addressing the strong link between hepatitis C infection and the opioid epidemic in affected countries such as the United States. Harm reduction approaches are crucial in this context, as they connect people who use drugs with the services and resources they need, including protection against overdoses, and prevention and treatment of hepatitis C, as well as addressing associated health problems. Additionally, alliances between governments, hospitals, and pharmaceutical companies can facilitate screening and access to DAAs at both state and international levels.
In 2019, the state of Louisiana demonstrated this approach by establishing a Payer License Agreement (PLA) with a single pharmaceutical company (13). This agreement provided all HCV treatments required by the state for a 5-year period, capping the total annual payment. The adoption of Netflix-like payment models in high-income countries represents an innovative strategy to advance HCV elimination efforts (13). In the quest for worldwide hepatitis C eradication, it is crucial for pharmaceutical companies to consider significant reductions in fixed payments and cost components for low-income countries. Egypt’s national free HCV screening and treatment program (14), available to all its citizens, serves as a promising model for achieving global HCV elimination.
In summary, the updated AASLD-IDSA guideline highlights a simplified HCV management algorithm with minimal monitoring (13). Achieving HCV elimination necessitates tailored approaches at the state, country, and global levels. In the United States, this strategy includes not only ensuring affordable access to DAAs but also implementing harm reduction measures to address opioid addiction and dependence. Additionally, the integration of harm reduction strategies into public health policies is critical to mitigate the spread of HCV among vulnerable populations, especially those impacted by the opioid crisis, by connecting them with essential health services (13,14). In low and middle-income countries, emphasis should be placed on facilitating access to screening and providing low-cost DAAs. The importance of prevention, care linkage, and policy reform to broaden treatment accessibility at an affordable cost is paramount. Such efforts are key in facilitating the cascades of care and meeting the diverse challenges faced in different regions. This comprehensive approach is vital for effectively combating HCV on a global scale.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-104/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-104/coif). C.Q.P. received the institutional research grant from Gilead Sciences, Inc., and he is the Chair of the Hepatitis B Special Interest Group and the AASLD Hepatitis B Practice Guideline Writing Group for 2024. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Global health sector strategy on viral hepatitis, 2016-2021. 2016. Accessed October 30th, 2023. Available online: https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf
- Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis 2014;59:1411-9. [Crossref] [PubMed]
- Akyar E, Seneca KH, Akyar S, et al. Linkage to Care for Suburban Heroin Users with Hepatitis C Virus Infection, New Jersey, USA. Emerg Infect Dis 2016;22:907-9. [Crossref] [PubMed]
- Kaufman HW, Bull-Otterson L, Meyer WA 3rd, et al. Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic. Am J Prev Med 2021;61:369-76. [Crossref] [PubMed]
- Bhattacharya D, Aronsohn A, Price J, et al. Hepatitis C Guidance 2023 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis 2023;ciad319. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2020;323:970-5. [Crossref] [PubMed]
- Schillie S, Wester C, Osborne M, et al. CDC Recommendations for Hepatitis C Screening Among Adults - United States, 2020. MMWR Recomm Rep 2020;69:1-17. [Crossref] [PubMed]
- Serper M, Evon DM, Stewart PW, et al. Medication Non-adherence in a Prospective, Multi-center Cohort Treated with Hepatitis C Direct-Acting Antivirals. J Gen Intern Med 2020;35:1011-20. [Crossref] [PubMed]
- Cunningham EB, Amin J, Feld JJ, et al. Adherence to sofosbuvir and velpatasvir among people with chronic HCV infection and recent injection drug use: The SIMPLIFY study. Int J Drug Policy 2018;62:14-23. [Crossref] [PubMed]
- Solomon SS, Wagner-Cardoso S, Smeaton L, et al. A minimal monitoring approach for the treatment of hepatitis C virus infection (ACTG A5360 [MINMON]): a phase 4, open-label, single-arm trial. Lancet Gastroenterol Hepatol 2022;7:307-17. [Crossref] [PubMed]
- Kurniawan J, Gani RA, Hasan I, et al. Comparative efficacy of sofosbuvir-ribavirin versus sofosbuvir-daclatasvir for treatment of chronic hepatitis C in an area with limited NS5A inhibitor availability. Indian J Gastroenterol 2018;37:520-5. [Crossref] [PubMed]
- Mueller PP, Chen Q, Ayer T, et al. Duration and cost-effectiveness of hepatocellular carcinoma surveillance in hepatitis C patients after viral eradication. J Hepatol 2022;77:55-62. [Crossref] [PubMed]
- Matthews DW, Coleman S, Razavi H, et al. The Payer License Agreement, or "Netflix model," for hepatitis C virus therapies enables universal treatment access, lowers costs and incentivizes innovation and competition. Liver Int 2022;42:1503-16. [Crossref] [PubMed]
- Waked I, Esmat G, Elsharkawy A, et al. Screening and Treatment Program to Eliminate Hepatitis C in Egypt. N Engl J Med 2020;382:1166-74. [Crossref] [PubMed]
Cite this article as: Pan CQ, Park JS. Revamping hepatitis C global eradication efforts: towards simplified and enhanced screening, prevention, and treatment. Transl Gastroenterol Hepatol 2024;9:30.


