
Crohn’s disease treatment and memory T-cell subset changes: insights from a case series
Highlight box
Key findings
• Treatments with anti-inflammatory agents and/or immunosuppressive drugs affect the frequency and function of memory T (Tm)-cell subsets.
What is known and what is new?
• Inhibiting circulating colitogenic CD4+ effector Tm cells is widely used in the clinic to treat Crohn’s disease (CD) and inhibit intestinal inflammation.
• The frequencies and functions of Tm-cell subsets in the mucosal immune system from patients with CD are changed after treatment.
What is the implication, and what should change now?
• A better understanding of the effect of anti-inflammatory agents and/or immunosuppressive drugs on Tm cells will offer new strategies for the treatment of human CD.
Introduction
Crohn’s disease (CD) is characterized by chronic, uncontrolled inflammation of the intestinal mucosa (1,2). The pathology of intestinal inflammation is mainly induced by an abnormal CD4+ T-cell response. Studies have demonstrated compromised tolerance of intestinal bacteria, which results in a dysregulated mucosal immune response (3,4). Naïve T cells are activated by bacterial antigens presented by antigen-presenting cells (APCs) and proliferate and differentiate into effector T cells. Effector T cells can be subdivided into functional subsets based on the expression of characterized cytokines. T helper (Th)1 cells produce interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin (IL)-12 to eradicate intracellular pathogens. Bacterial signals have been shown to induce a strong Th1 cell response in the lamina propria of germ-free mice (5,6). Th1 cytokines also stimulate innate cells to produce the cytokines IL-6, transforming growth factor-β (TGF-β), and IL-23, which in turn promotes the development of Th17 cells that produce the IL-17 family cytokines, such as IL-17A and IL-17F (7,8). Upregulated IFN-γ and IL-17 have been implicated in potentiating the pathogenesis of CD. The intractability and persistence of CD may be related to the persistence of colitogenic memory CD4+ T cells.
Memory T (Tm) cells mediate long-lasting protective immune responses. Historically, circulating Tm cells were known to be subdivided based on beta7 integrin expression, with the beta7+ population containing cells primed in the intestine, capable of homing back to the gut. Such cells, especially the beta7hi subset, displayed an increased propensity for cytokine production, and variations in their expression and function were implicated in the pathogenesis of CD (9). Building on this foundational understanding, recent studies have provided a more refined classification of Tm cells. They can now be categorized into central Tm (Tcm) cells, effector Tm (Tem) cells, stem cell-like Tm (Tscm) cells, and tissue-resident Tm (Trm) cells based on their cell surface markers and function (10-13). Tscm and Trm cells are newly discovered T-cell subsets. In tumor microenvironment of gastric cancer, tumor flora was significantly correlated with CD8+ Trm depletion (14). The role of Tscm cells in mucosal immunity is limited. CD8+ Trm cells have been identified in the small intestinal mucosa tissue and play an important defense role in mucosal tissue. They have local adaptive immune surveillance function. A targeted adaptive immune response can be initiated rapidly when the antigen is re-exposed. They are also a targetable hallmark after immune checkpoint therapy (15,16). Studies have demonstrated that actively circulating colitogenic CD4+ Tem cells play a key role in the maintenance of colitis (14-17), and suppressing the function of these cells constitutes a feasible treatment for CD. Study has detected the T-cell subsets differences between healthy people and CD patients (18). In CD patients, the characters of CD103+CD69+CD8+ T cells in lamina propria are similar to classical Trm cell. Meanwhile, CD103+CD69+CD8+ T cells in intraepithelial have innate-like cytotoxic profile (19). Amphiregulin secreted by Th17 could induce intestinal fibrosis (20). Further, mucosal and peripheral blood immune cell phenotypes could indicate the treatment efficiency of Vedolizumab. However, the T-cell subsets profile after standard treatment has not been reported yet.
Currently, this strategy is widely used in the clinic to treat CD and inhibit intestinal inflammation in different ways. The administration of nonsteroidal anti-inflammatory agents (e.g., sulfasalazine enteric-coated tablets and slow-release mesalazine) can block the synthesis of prostaglandins by inhibiting cyclooxygenase. Corticosteroid anti-inflammatory agents (e.g., methylprednisolone tablets) inhibit the production of pro-inflammatory cytokines and decrease the number of circulating lymphocytes. Immunosuppressive agents, such as thiopurines, inhibit T-lymphocyte proliferation. However, a systematic analysis of how these different types of drugs affect the compartmentalization of Tm-cell subsets, especially Tscm and Trm cells in distinct anatomical sites, has not been conducted.
In this study, we focused on the frequencies and functions of different subsets of Tm cells from the mesenteric lymph nodes (MLNs), peripheral blood, and small intestinal mucosa of patients with CD after treatment. Observing a higher fraction of CD4+ Tscm cells in the MLNs from treated CD patients compared to healthy donors indicates potential implications for treatment response. Notably, a substantial number of Trm cells were found in the small intestinal mucosa, with CD8+ Trm cells outnumbering CD4+ Trm cells in both the MLN and small intestinal mucosa of CD patients. Additionally, the observed decrease in IFN-γ and TNF-α production in the Tm cells from treated CD patient’s blood, and the increased IFN-γ production in CD8+ Tscm cells suggests nuanced immunological shifts post-treatment. A deeper understanding of how drugs affect human Tm-cell subsets in CD patients can guide more personalized therapeutic strategies, optimize treatment outcomes, and reduce potential side effects. This knowledge is paramount to elevating the standard of care in managing CD. We present this article in accordance with the STROBE and AME Case Series reporting checklists (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-21/rc).
Methods
Study participants
This was a case-control study. A total of eight CD patients from The First Affiliated Hospital of Sun Yat-sen University were recruited for the study. Patients infected with human immunodeficiency virus (HIV), hepatitis B virus (HBV), or hepatitis C virus (HCV) were excluded from the study. The eight patients had been diagnosed with CD and treated with anti-inflammatory agents and/or the immunosuppressive drugs sulfasalazine enteric-coated tablets, methylprednisolone tablets, slow-release mesalazine, and azathioprine tablets. Three donated peripheral blood samples were recruited for this study. Healthy and diseased MLNs as well as small intestinal mucosa samples were taken from the 6 patients with CD. The patients who participated in this study did not exhibit any other sign of inflammation. These patients were all male, with ages ranging from 14 to 33 years. The 6 healthy donors who were recruited for peripheral blood samples had ages ranging from 20 to 40 years, and their peripheral blood samples were obtained from the clinical laboratory department of The First Affiliated Hospital, Sun Yat-sen University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committees of the Zhongshan School of Medicine, Sun Yat-sen University (No. 20220120). Written informed consent was obtained from all patients/legal guardians and healthy donors.
Preparation of peripheral blood mononuclear cells (PBMCs), lamina propria cells, and lymphocytes
PBMCs were isolated by Ficoll-Hypaque (cat. LTS1077; Tianjin Hao Yang Biological Manufacture Co., Ltd., Tianjin, China); gradient centrifugation of sodium heparin-treated blood obtained from healthy donors or patients with CD. To isolate lamina propria T cells in this study, we adopted previously published methods (21). In general, biopsy specimens that were cut into 1–2 mm3 pieces were incubated for 2 hours at 37 ℃ in Hank’s balanced salt solution (HBSS) containing 1 mmol/L CaCl2, 1 mmol/L MgCl2, 1,000 U/mL collagenase IV, and 10 mg/mL DNase I (Sigma-Aldrich, St. Louis, MO, USA). The cells were resuspended in complete RPMI 1640 medium (cat. 11875093; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal calf serum (Hangzhou Si Ji Qing Biological Manufacture Co., Ltd., Hangzhou, China), 100 U/mL off penicillin (cat. 15071163), 100 mg/mL of streptomycin (cat. 15071163), 2 mM of L-glutamine (cat. 25030081), and 50 mM of 2-mercaptoethanol (cat. 21985023; Invitrogen). Lymphocytes were isolated by homogenizing the lymph node tissue, and erythrocytes were lysed using an ammonium chloride solution and resuspended in complete RPMI 1640 medium.
Flow cytometry analysis
Phenotypic characterization
T lymphocytes from the patients with CP were stained for flow cytometry. The following panel of mouse anti-human monoclonal antibodies (mAbs) used were all purchased from BD Biosciences (Franklin Lakes, NJ, USA) or eBioscience (San Diego, CA, USA): anti-human CD3-APC.Cy7 (cat. 557832, SK7; BD Biosciences), anti-human CD4-Percp.Cy5.5 (cat. 560650, RPA-T4; BD Biosciences), anti-human CD8-Alexa Fluor 700 (cat. 56-0088-42, RPA-T8; eBioscience), anti-human CCR7-PE.eFluor610 (cat. 61-1979-42, 3D12; eBioscience), anti-human CD62L-APC (cat. 17-0629-42, DREG56; eBioscience), anti-human CD45RO-FITC (11-0457-42, UCHL1; eBioscience), anti-human CD95-PE.Cy7 (cat. 561633, DX2; BD Biosciences), anti-human CD103-APC (cat. 17-1037-41, Ber-ACT8; eBioscience), and anti-human CD122-PE (cat. 554525; BD Biosciences). Cellular data were obtained from a BD LSRFortessa analytical flow cytometer. Unstained and single fluorochrome-stained cells served as controls for accurate compensation and data analysis. Cells in each sample were counted, and the data were analyzed with FlowJo version 10 (FlowJo, Ashland, OR, USA).
Intracellular staining
The T lymphocytes were incubated in 24-well plates at 2×106 cells per well in RP10 media [RPMI, 10% heat-inactivated fetal bovine serum (FBS)] alone or with phorbol 12-myristate13-acetate (PMA; 20 ng/mL final concentration) plus ionomycin (1 µg/mL) for 4 to 6 hours at 37 ℃ in the presence of brefeldin A (BFA; 10 µg/mL). The cells were harvested, washed with phosphate buffer saline (PBS), stained for the surface phenotypic markers, and fixed for 8 min at room temperature with 4% paraformaldehyde (PFA; Sigma-Aldrich). The cells were then permeabilized (0.01% saponin) overnight in the dark at 4 ℃, and the intracellular cytokines were stained using anti-human IFN-γ-APC (cat. 554702; BD Biosciences), anti-human IL-17A-V450 (cat. 560610, N49-653; BD Biosciences), and anti-human TNF-α-APC (cat. 17-7349-82, MAb11; eBioscience). All samples were analyzed using a BD LSRFortessa instrument. The data were analyzed using FlowJo software. PMA (cat. 16561-29-8; PMA), ionomycin (cat. 10634), BFA (cat. B7651), bovine serum albumin, and NaN3 were all purchased from Sigma-Aldrich.
Statistical analysis
GraphPad Prism software version 5 (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis (22). The Mann-Whitney test (two-tailed) and unpaired Student t-test were used to identify significant differences. A value of P≤0.05 indicated statistical significance.
Results
A distinct compartmentalization of T cells was found in CD patients
We analyzed T lymphocyte distribution in post-treatment CD patients across peripheral blood, MLNs, and intestinal mucosa. PBMCs from healthy donors (HD-PBMCs) displayed balanced CD4+ and CD8+ T cells frequencies (Figure 1A). Conversely, post-treatment CD patients had increased CD8+ and decreased CD4+ T cells in PBMCs (P=0.0065 and P=0.0069, respectively; Figure 1A). In MLNs, CD4+ T cells were predominant, with differences between CD patients and healthy controls evident (Figure 1A). CD4+ T cells also dominated the intestinal mucosa (Figure S1). In essence, CD patients post-treatment showed a notable CD4+/CD8+ shift in PBMCs and MLNs.
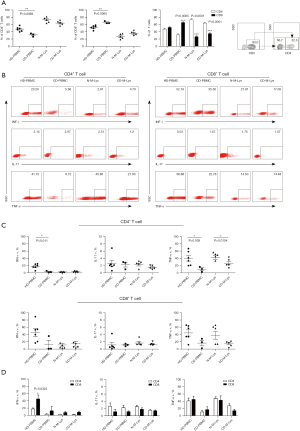
Post-treatment CD patient T cells, when stimulated with PMA and ionomycin, showed similar proportions of IL-17-producing CD4+ and CD8+ T cells in the blood and MLNs (Figure 1B,1C). CD4+ T cells in these patients had decreased IFN-γ and TNF-α production in blood compared to healthy donors, especially evident for TNF-α in lesions of the MLNs from patients with CD (CD-M-Lys) (Figure 1C). Both IL-17 and TNF-α production in CD4+ and CD8+ T cells were comparable in CD patient blood and MLNs, though CD8+ T cells showed higher IFN-γ expression (Figure 1D). Interestingly, in HD-PBMCs, IFN-γ-expressing CD8+ T cells were predominant over CD4+ counterparts (Figure 1D). Overall, post-treatment CD patients demonstrated reduced CD4+ T cell frequency and Th1 cytokine production relative to healthy individuals.
Tm cells show altered cytokine production in post-treatment CD patients
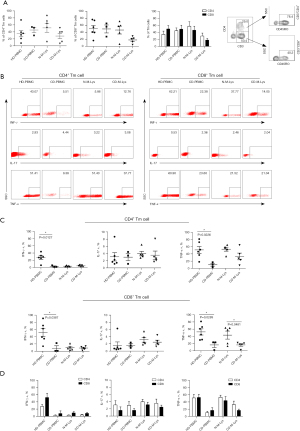
Tm cells, identified as CD45RA− and CD45RO+, can produce cytokines quickly upon recognizing a familiar antigen (23). Colitogenic memory CD4+ T cells, circulating throughout the body, are reactivated by antigen-presenting DCs in MLNs, contributing to chronic colitis. Immunosuppressants, like FTY720, have been shown to disrupt this process (18). Investigating the impact of immunosuppressive drugs on Tm cells in CD patients, we found reduced percentages of CD4+ Tm (27%) and CD8+ Tm (18%) cells in CD-M-Lys compared to healthy MLNs from patients with CD (N-M-Lys) (Figure 2A). Both healthy and lesioned small intestinal mucosa had similar CD4+ Tm proportions, but more CD8+ Tm cells (Figure S2). Notably, CD-PBMCs had fewer IFN-γ and TNF-α producing CD4+ and CD8+ Tm cells than HD-PBMCs (Figure 2B,2C). The IL-17 production from CD4+ T cells in the blood and MLNs of CD patients was consistent (Figure 2C). While IFN-γ-expressing CD8+ Tm cells predominated in the blood and MLNs, the blood contained significantly more IL-17-expressing CD4+ Tm cells than its CD8+ counterpart (Figure 2D). In summary, post-treatment CD patient blood exhibited diminished IFN-γ and TNF-α output from Tm cells.
The expression of IFN-γ in Tscm cells differed in the blood and MLNs of patients with CD after treatment
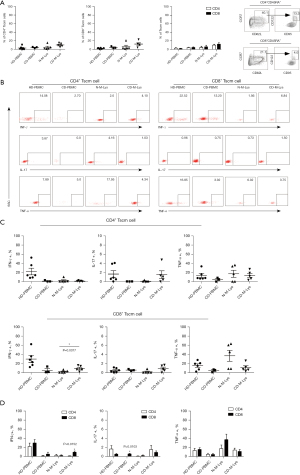
Tscm cells, present in the blood and lymph nodes of healthy individuals, carry markers of naïve T cells like CD45RA+ and also memory-associated markers CD95 and CD122 (11,24,25). These cells contribute to homeostatic proliferation and Tm cell survival, responding to IL-15 and IL-7. Notably, naïve-like CD4+/CD8+ T cells with CD95+CD122+ phenotype also exhibit the stem cell-like marker Bcl-2. Analyzing the Tscm cell proportions in CD patients’ peripheral blood and MLNs, we observed a decreased fraction (around 3%) post-treatment (Figure 3A). A higher percentage of both CD4+ and CD8+ Tscm cells was detected in CD-M-Lys compared to N-M-Lys (Figure 3A). After stimulation with PMA plus ionomycin, fewer CD4+/CD8+ Tscm cells in CD-PBMCs produced IFN-γ and TNF-α than in HD-PBMCs (Figure 3B,3C). Interestingly, no IL-17 production by CD4+ Tscm cells was seen in post-treatment CD patient blood. However, a significant elevation of IFN-γ-secreting CD8+ Tscm cells was noticed in CD-M-Lys (Figure 3B-3D). In summary, post-treatment CD patients showed reduced IFN-γ production in Tscm cells in the blood, with an increase in the lesions of MLNs.
The distinct frequencies of Trm-cell subsets in the MLNs and small intestinal mucosa of patients with CD after treatment
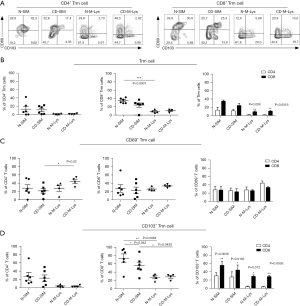
Trm cells, distinctly recognized by their absence in the blood but presence in non-lymphoid tissues like mucosal areas, play a pivotal role in local immune reactions. Their identification is largely facilitated by markers CD69 and CD103. While CD8+CD103+/-CD69+ Trm cells predominantly inhabit the epithelial layer, CD4+/CD8+CD103+/-CD69+ counterparts are mainly found in the lamina propria. It is noteworthy that not all intestinal Trm cells exhibit CD103 (26,27). In our investigation of Trm cell compositions in CD patient intestines, we focused on CD69 and CD103 expressions in small intestinal mucosa and MLNs (Figure 4A). Notable findings include: Reduced frequencies of CD4+CD69+CD103+ and CD8+CD69+CD103+ Trm cells in N-M-Lys compared to healthy small intestinal mucosa from patients with CD (N-SIM) (Figure 4B). In lesions from the small intestinal mucosa from patients with CD (CD-SIM), CD8+CD69+CD103+ Trm cells outnumbered their CD4+ counterparts, a trend also observed in both N-M-Lys and CD-M-Lys (Figure 4B). CD4+CD69+ Trm cell frequencies were diminished in CD-SIM relative to CD-M-Lys (Figure 4C). Frequencies of CD8+CD103+ Trm cells in N-M-Lys were less than in N-SIM, a pattern also evident in CD patients (Figure 4D). Post-treatment, CD patients exhibited a more significant count of CD8+CD103+ Trm cells than CD4+CD103+ Trm cells, evident in both MLNs and small intestinal mucosa (Figure 4D). Conclusively, post-treatment CD patients manifest unique Trm-cell subset frequencies in MLNs and the small intestinal mucosa.
Discussion
The systemic distribution of CD4+ T cells and CD8+ T cells depends on bacterial signals (2). The bacterial gradients in distinct sites from patients with CD differ, with a higher bacterial concentration in the small intestine than the blood. Antigen concentration significantly influences the strength of the immune response, critically affecting Tm cells’ formation. It’s worth noting that the beta7 integrin expression has historically played a role in the differentiation of circulating Tm cells, especially those primed in the intestine. Hart et al. (9) emphasized that the beta7+ population was enriched for cytokine-producing effector cells, which aligns with our observations on the cytokine environment in the intestines of patients with CD. Antibiotic administration alters the intestinal microbial population, influencing immune system function (28,29). Whether anti-inflammatory drugs affect the characteristics of Tm-cell subsets in diseased tissues remains to be clarified. In our study, the compartmentalization and function of Tm cells in inflammatory bowel disease post-treatment were examined. The findings showed considerable fractions of CD4+ T cells in the intestinal mucosa and MLNs in treated CD patients. Interestingly, this aligns with Hart et al. who pointed out that beta7+ cells, primed in the intestine, could home back to the gut, reflecting the specialized mucosal compartment in which they were primed (9).
Patients with CD exhibit an aberrant immune response, characterized by abnormal T-cell activation and cytokine production responses to intestinal bacteria (26,27). Notably, while Hart et al. identified increased cytokine production in the beta7hi population compared to the beta7int population, suggesting a gradient of cytokine production based on beta7 integrin expression, our research adds depth to this understanding (9). Our study revealed that anti-inflammatory or immunosuppressive drugs did not influence the Tm cell frequency but suppressed certain cytokine productions in CD patients’ blood.
It’s crucial to understand the immune subsets of CD4+ T cells, like Th1, Th2, Th17, and regulatory T cells (Tregs), which have a significant role in modulating inflammatory bowel disease (30,31). The immune subsets of CD4+ T cells include several cell types, such as Th1, Th2, Th17, and Tregs. Tm cell exerts important effect in regulating anti-tumor responses in solid cancers (32). Mucosal macrophages prevent the interconversion of Th1 and Th17 cells, thus promoting Tregs differentiation (33). Th2 cells could secret IL-5, IL-6, and IL-13 to form inflammatory intestine environment (34). Tregs down-regulate Th1 and Th17 responses to inhibit colonic inflammation (35). Phosphoglycerate-kinase-1 and/or aldolase-A blocking could treat CD via regulating the functions of Th17-cells (36). T cell responses are also regulated by selenium (37). Atorvastatin could shift the immune response from Th17 towards Th2 to improve the inflammatory reaction and clinic symptoms (38). The level of long non-coding RNA (lncRNA) ANRIL is negatively correlated with CD activity index, suggesting the potential therapeutic effect of lncRNA ANRIL (39).
The cause of pathology of CD is the persistence of local inflammatory molecules, which are mainly produced by Th1 and Th17 cells. More than 95% of the effector T cells will undergo apoptosis by activation-induced cell death (AICD) after clearing the antigen, and about 5% of effector T cells will develop into Tm cells. CD4+ effector T cells are the main source of cytokines (IFN-γ, TNF-α, IL-17) during persistent infection in colon. Continued differentiation of Tm cells into effector T cells creates a locally persistent inflammatory state. A newly defined subpopulation of Tm cells, Tscm cells can have the capacity to self-renew and differentiate into Tcm cells, Tem cells, and effector T cells; Trm cells which colonize nonlymphocytic tissue mediate local immune response (40). The distributions of Tscm cells in human blood and lung lymph nodes (24) and tumor-infiltrating lymphocytes in lung cancer have been determined (41). Tm cells, particularly the subpopulations like Tscm cells, play a pivotal role in chronic inflammation seen in patients with CD. Hart et al. (9) alluded to the role of beta7 integrin in influencing cytokine production; our findings further suggest that an increase in Tscm cells in MLNs lesions might contribute to chronic inflammation in CD patients. Our results indicated that in the mucosal immune system, the proportion of Tscm cells is higher in the lesions of MLNs than in healthy MLNs. By functional analysis, we found that CD8+ Tscm cells in the lesions of MLNs produced more IFN-γ than did those in healthy MLNs, while in the blood, the number of IFN-γ-producing CD4+ Tscm and CD8+ Tscm cells from patients with CD with treatment was lower compared to that in healthy donors. More Tscm cells in the lesions of MLNs may differentiate into Tm cells and effector T cells, which may cause chronic persistent inflammation in patients with CD. The mechanism by which anti-inflammatory agents and/or immunosuppressive drugs affect Tscm cell proliferation and IFN-γ secretion at different sites remains to be further studied.
CD8+ Trm cells are identified as Trm cells; meanwhile, CD4+ Trm cells remain the predominant subtype in various tissues, like lungs (42,43), genital mucosa (44), and skin (45), and they permanently reside in Peyer’s patches and lymph nodes (46). Our study showed fewer Trm cells in MLNs than in the small intestinal mucosa, which also agrees with the earlier findings of Hart et al. that beta7+ cells were enriched for cells primed in the intestine, emphasizing the unique environment of the intestine and the significance of T cells’ role therein (9). Moreover, CD8+ Trm cells outnumbered CD4+ Trm cells in both MLNs and the small intestinal mucosa, which was also true for the CD103+ Trm-cell subset. Meanwhile, the frequency of CD4+CD69+ Trm cells and CD8+CD69+ Trm cells was comparable between MLNs and the small intestinal mucosa. Anti-inflammatory and/or immunosuppressive agents inhibit the proliferation of CD69+ Trm cells in the small intestinal mucosa; however, because the acquisition of T cells from the small intestinal mucosa was limited, we did not analyze the function of Trm cells or the expression of surface markers related to recirculation. It is important to determine the properties of Trm-cell subsets in the Trm cell-mediated immune response in the mucosal system.
This study has several limitations, notably in the sample size and demographic characteristics. We recruited only six male patients aged 14 to 33 years diagnosed with CD and treated with anti-inflammatory agents and/or immunosuppressive drugs. Acknowledging the constraints in our sample size and lack of gender diversity, we emphasize that these participants were the only available and willing subjects during the research period. This limitation in sample size and demographic range, while introducing potential biases especially in sex and age, was addressed through rigorous statistical measures to bolster the validity and robustness of our findings. Moreover, due to the limited number of Trm cells, we were unable to analyze cytokine production from these cells, which could impact the comprehensive understanding of Trm cell functions. Despite these constraints, our research serves as a preliminary study offering valuable insights into Tm-cell subset responses to treatment in CD patients. In recognizing the need for more inclusive research, our study underscores the importance of larger, more diverse studies in the future, incorporating a broader age range and including female patients, to enhance the understanding of observed effects. We have discussed these aspects and the necessity for expanded research in the manuscript, aiming for a more holistic understanding of the treatment outcomes in CD.
Conclusions
Our findings describe the frequencies and functions of Tm-cell subsets in the mucosal immune system of patients with CD after treatment. A better understanding of the effect of anti-inflammatory agents and/or immunosuppressive drugs on Tm cells will offer new strategies for the treatment of human CD.
Acknowledgments
We thank the fellow members of the laboratory for their assistance.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE and AME Case Series reporting checklists. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-21/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-21/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-21/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-21/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committees of the Zhongshan School of Medicine, Sun Yat-sen University (No. 20220120). Written informed consent was obtained from all patients/legal guardians and healthy donors.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010;28:573-621. [Crossref] [PubMed]
- Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol 2010;28:623-67. [Crossref] [PubMed]
- Duchmann R, Kaiser I, Hermann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995;102:448-55. [Crossref] [PubMed]
- Macpherson A, Khoo UY, Forgacs I, et al. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 1996;38:365-75. [Crossref] [PubMed]
- Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 2009;31:677-89. [Crossref] [PubMed]
- Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485-98. [Crossref] [PubMed]
- Zaph C, Du Y, Saenz SA, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med 2008;205:2191-8. [Crossref] [PubMed]
- Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 2008;455:808-12. [Crossref] [PubMed]
- Hart AL, Kamm MA, Knight SC, et al. Quantitative and functional characteristics of intestinal-homing memory T cells: analysis of Crohn's disease patients and healthy controls. Clin Exp Immunol 2004;135:137-45. [Crossref] [PubMed]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004;22:745-63. [Crossref] [PubMed]
- Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer 2012;12:671-84. [Crossref] [PubMed]
- Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity 2014;41:886-97. [Crossref] [PubMed]
- Fan X, Rudensky AY. Hallmarks of Tissue-Resident Lymphocytes. Cell 2016;164:1198-211. [Crossref] [PubMed]
- Kanai T, Nemoto Y, Tomita T, et al. Persistent retention of colitogenic CD4+ memory T cells causes inflammatory bowel diseases to become intractable. Inflamm Bowel Dis 2009;15:926-34. [Crossref] [PubMed]
- Nemoto Y, Kanai T, Kameyama K, et al. Long-lived colitogenic CD4+ memory T cells residing outside the intestine participate in the perpetuation of chronic colitis. J Immunol 2009;183:5059-68. [Crossref] [PubMed]
- Tomita T, Kanai T, Nemoto Y, et al. Colitogenic CD4+ effector-memory T cells actively recirculate in chronic colitic mice. Inflamm Bowel Dis 2008;14:1630-40. [Crossref] [PubMed]
- Takahara M, Nemoto Y, Oshima S, et al. IL-7 promotes long-term in vitro survival of unique long-lived memory subset generated from mucosal effector memory CD4+ T cells in chronic colitis mice. Immunol Lett 2013;156:82-93. [Crossref] [PubMed]
- Kanai T, Watanabe M, Hibi T. Systemically circulating colitogenic memory CD4+T cells may be an ideal target for the treatment of inflammatory bowel diseases. Keio J Med 2009;58:203-9. [Crossref] [PubMed]
- Lutter L, Roosenboom B, Brand EC, et al. Homeostatic Function and Inflammatory Activation of Ileal CD8(+) Tissue-Resident T Cells Is Dependent on Mucosal Location. Cell Mol Gastroenterol Hepatol 2021;12:1567-81. [Crossref] [PubMed]
- Zhao X, Yang W, Yu T, et al. Th17 Cell-Derived Amphiregulin Promotes Colitis-Associated Intestinal Fibrosis Through Activation of mTOR and MEK in Intestinal Myofibroblasts. Gastroenterology 2023;164:89-102. [Crossref] [PubMed]
- Sylvester FA, Draghi A, Menoret A, et al. Distinctive colonic mucosal cytokine signature in new-onset, untreated pediatric Crohn disease. J Pediatr Gastroenterol Nutr 2014;59:553-61. [Crossref] [PubMed]
- Li B, Wang Z, Yu M, et al. miR-22-3p enhances the intrinsic regenerative abilities of primary sensory neurons via the CBL/p-EGFR/p-STAT3/GAP43/p-GAP43 axis. J Cell Physiol 2020;235:4605-17. [Crossref] [PubMed]
- Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol 1998;16:201-23. [Crossref] [PubMed]
- Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 2012;12:749-61. [Crossref] [PubMed]
- Hong H, Gu Y, Sheng SY, et al. The Distribution of Human Stem Cell-like Memory T Cell in Lung Cancer. J Immunother 2016;39:233-40. [Crossref] [PubMed]
- Cong Y, Brandwein SL, McCabe RP, et al. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med 1998;187:855-64. [Crossref] [PubMed]
- Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science 2006;312:1355-9. [Crossref] [PubMed]
- Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol 2012;30:759-95. [Crossref] [PubMed]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014;157:121-41. [Crossref] [PubMed]
- Imam T, Park S, Kaplan MH, et al. Effector T Helper Cell Subsets in Inflammatory Bowel Diseases. Front Immunol 2018;9:1212. [Crossref] [PubMed]
- Tindemans I, Joosse ME, Samsom JN. Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD. Cells 2020;9:110. [Crossref] [PubMed]
- Marceaux C, Weeden CE, Gordon CL, et al. Holding our breath: the promise of tissue-resident memory T cells in lung cancer. Transl Lung Cancer Res 2021;10:2819-29. [Crossref] [PubMed]
- Denning TL, Wang YC, Patel SR, et al. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 2007;8:1086-94. [Crossref] [PubMed]
- Negi S, Saini S, Tandel N, et al. Translating Treg Therapy for Inflammatory Bowel Disease in Humanized Mice. Cells 2021;10:1847. [Crossref] [PubMed]
- Ogino H, Nakamura K, Ihara E, et al. CD4+CD25+ regulatory T cells suppress Th17-responses in an experimental colitis model. Dig Dis Sci 2011;56:376-86. [Crossref] [PubMed]
- Vuerich M, Wang N, Graham JJ, et al. Blockade of PGK1 and ALDOA enhances bilirubin control of Th17 cells in Crohn's disease. Commun Biol 2022;5:994. [Crossref] [PubMed]
- Huang LJ, Mao XT, Li YY, et al. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn's disease. Immunity 2021;54:1728-1744.e7. [Crossref] [PubMed]
- Wada K, Nakajima A, Katayama K, et al. Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem 2006;281:12673-81. [Crossref] [PubMed]
- Rui Q, Lu C, Song S, et al. Identification and validation of key long non-coding RNAs using co-expression network analysis in Crohn's disease. Ann Palliat Med 2021;10:9627-39. [Crossref] [PubMed]
- Hornung F, Zheng L, Lenardo MJ. Maintenance of clonotype specificity in CD95/Apo-1/Fas-mediated apoptosis of mature T lymphocytes. J Immunol 1997;159:3816-22. [Crossref] [PubMed]
- Sheng SY, Gu Y, Lu CG, et al. The Characteristics of Naive-like T Cells in Tumor-infiltrating Lymphocytes From Human Lung Cancer. J Immunother 2017;40:1-10. [Crossref] [PubMed]
- Turner DL, Bickham KL, Thome JJ, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 2014;7:501-10. [Crossref] [PubMed]
- Teijaro JR, Turner D, Pham Q, et al. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 2011;187:5510-4. [Crossref] [PubMed]
- Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 2014;346:93-8. [Crossref] [PubMed]
- Glennie ND, Yeramilli VA, Beiting DP, et al. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med 2015;212:1405-14. [Crossref] [PubMed]
- Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 2016;16:79-89. [Crossref] [PubMed]
(English Language Editor: J. Gray)
Cite this article as: Chen ZH, Tang YY, Sheng SY, Lu CG, Xu KW, Chen GJ, Wang YF, Gu Y, Song XM, Hong H. Crohn’s disease treatment and memory T-cell subset changes: insights from a case series. Transl Gastroenterol Hepatol 2024;9:18.


