
Clinical outcomes of long-term transmural drainage with double pigtail stents in disconnected pancreatic duct syndrome
Highlight box
Key findings
• Long-term transmural drainage with double pigtail plastic stents is a safe and effective modality to prevent recurrent collections in patients with disconnected pancreatic duct syndrome (DPDS).
• Patients with DPDS are prone to develop sequela of chronic pancreatitis in the disconnected segment including diabetes and exocrine pancreatic insufficiency.
What is known and what is new?
• DPDS leads to recurrent pancreatic fluid collections and chronic pancreatitis.
• Long-term double pigtail stent placement prevents recurrence of these collections.
What is the implication, and what should change now?
• Follow-up interval imaging in patients DPDS is essential to tailor patients’ treatment plan.
• Randomized, prospective studies are needed to validate these findings.
Introduction
Pancreatic fluid collections (PFCs) are a common sequela of acute pancreatitis (AP) and are classified into four categories based on the age of the collection and the presence of necrotic tissue. Pseudocysts and walled off pancreatic necrosis (WOPN) are encapsulated collections with a well-defined inflammatory wall that typically forms around four weeks after AP (1). Drainage of PFCs is indicated in symptomatic patients presenting with abdominal pain, infection, gastric outlet obstruction, biliary obstruction, or vascular compression.
Open surgical necrosectomy was the traditional first line therapy for WOPN but is accompanied by substantial morbidity and mortality (2,3). In consequence, more recent practice involves implementing a step-up approach starting with either endoscopic transmural drainage or percutaneous drainage (3,4). When feasible, index endoscopic drainage is preferred due to fewer pancreatocutaneous fistulas and shorter hospital length of stay (5). Escalation of therapy to endoscopic necrosectomy or video-assisted retroperitoneal debridement (VARD) is reserved for patients who do not experience clinical improvement.
Disconnected pancreatic duct syndrome (DPDS) occurs when complete main pancreatic duct disruption isolates upstream viable pancreatic tissue, resulting in PFC formation (6,7). DPDS has been shown to increase PFC recurrence rates, hospital length of stay, and morbidity while decreasing the likelihood of successful percutaneous drainage (8,9). Unfortunately, transpapillary stenting is not effective for DPDS with success rates of only 20% (8). Instead, treatment for DPDS consists of long term endoscopic transmural drainage even after PFC resolution. While lumen apposing metal stents (LAMS) play a pivotal role in the initial management of PFCs, the risk of delayed adverse events, particularly bleeding, prohibit leaving the LAMS in indefinitely (10).
The current paradigm for managing DPDS involves early LAMS removal with plastic double pigtail stent (DPS) placement. Initial studies examining this strategy have demonstrated PFC recurrence rates of 1–5% with low adverse event rates (11,12). However, these studies were limited by relatively short-term follow-up and current long-term data is relatively sparse. As such, we aim to retrospectively evaluate the safety and efficacy of long-term indwelling transmural DPS in the management of DPDS. We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-94/rc).
Methods
Patients
Patients undergoing endoscopic drainage of symptomatic PFCs at a single, tertiary care center between November 2015 to May 2022 were identified and included in a prospectively maintained database. The database was secured in accordance with Institutional Review Board of Wake Forest School of Medicine (IRB No. 00035936, Retrospective Analysis of Acute Pancreatitis at an Academic Medical Center). A multidisciplinary team consisting of advanced endoscopists, abdominal radiologists, and a hepatopancreatobiliary surgeon directed the care for each patient. Patients with documented DPDS who underwent endoscopic drainage of a pancreatic pseudocyst or WOPN as defined by the revised Atlanta classification were included in the analysis (1). Patients with post-operative PFCs, pancreatic malignancies, partial pancreatic duct disruption, equivocal diagnosis of DPDS after magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP), or follow-up less than 6 months after the index procedure were excluded. Data on patient demographics, PFC characteristics, procedure details, adverse events, and relevant clinical outcomes were recorded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Diagnosis of DPDS
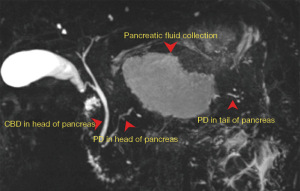
DPDS was defined as complete disruption of the main pancreatic duct with viable pancreas upstream from the injured portion of the pancreatic duct. MRCP was initially used to diagnose DPDS with ERCP reserved for cases where MRCP was non-diagnostic (Figure 1). On pancreatogram, contrast filling the pancreatic duct distal to the level of disruption was diagnostic for partial duct disruption. Patients with partial pancreatic duct disruption underwent transpapillary stenting. If the pancreatic duct distal to the disruption was not able to be opacified, this was diagnostic of DPDS. These patients were managed with endoscopic transmural drainage with LAMS followed by DPS placement as described in the procedure details section.
Procedure details
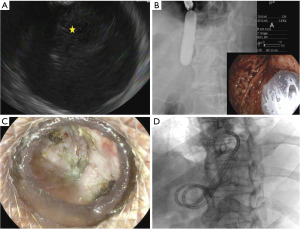
Endoscopic ultrasound (EUS) guided transmural drainage with a LAMS was performed in all patients with mature, symptomatic PFCs and a diagnosis of DPDS (Figure 2A). Informed consent was obtained from all patients prior to procedure. A curvilinear therapeutic echoendoscope (GF-UCT180; Olympus, Center Valley, PA, USA) was first used to identify an avascular path for stent placement. An electrocautery enhanced Axios stent (Boston Scientific, Marlborough, MA, USA) was then deployed into the PFC from either the stomach or the duodenum depending on the collection location. A 15 mm × 10 mm Axios stent was used for WOPN and a 10 mm × 10 mm Axios stent was used for pseudocysts. A CRE (Boston Scientific, Marlborough, MA, USA) wire-guided balloon was used to dilate the LAMS up to the stent diameter (Figure 2B). For WOPN, necrotic pancreatic tissue was debrided with a snare, basket, or rat tooth forceps. Following LAMS placement and necrosectomy, 100 mL of 3% hydrogen peroxide was infused into the cavity. No coaxial DPS were placed at the termination of the index procedure.
Follow-up protocol
Serial computed tomography (CT) scans were performed at scheduled intervals (at 1 week, 3–4 weeks, and 6 weeks after index procedure and 1 week after each necrosectomy). Decision for repeat necrosectomy was based on the patient’s symptoms and amount of residual necrosis on CT. LAMS removal was performed following complete clearance of the necrotic material from the cyst cavity with improvement in symptoms (Figure 2C). At the time of LAMS removal, one or two DPS (7 Fr × 4 cm or 10 Fr × 4 cm) were placed into the collection (Figure 2D). The tract was allowed to close if DPS were not able to be placed. A step-up approach was used for patients who failed endoscopic management.
After LAMS removal, patients had a repeat CT scan and clinic visit at 3 months. Follow-up was then conducted semiannually for 1 year and then annually. Patients were asked screening questions for exocrine pancreatic insufficiency (EPI) at each clinic visit. CT scans and MRCPs were also reviewed to detect changes of chronic pancreatitis (CP) in the disconnected segment. Glycemic control was monitored with regular glycosylated hemoglobin A1C (HbA1C) levels.
Definitions and clinical outcomes
Technical success was defined as successful placement of a LAMS into the PFC. Clinical success was defined as improvement in symptoms and resolution of the PFC. PFC resolution occurred when a collection measured less than 2 cm at 6 months follow-up on cross sectional abdominal imaging. PFCs that increased to greater than 2 cm following LAMS removal were categorized as recurrent collections.
The primary outcome was PFC recurrence following LAMS removal and DPS placement. Secondary outcomes included technical and clinical success, locoregional adverse events from indwelling DPS, new onset diabetes mellitus and EPI, and development of CP. Adverse events were defined and classified by the lexicon proposed by the American Society of Gastrointestinal Endoscopy (13). The diagnosis of CP was made using standard criteria based on imaging findings on CT and MRCP (14).
Statistical analysis
Descriptive statistics were performed for data analysis using statistical analysis system (version 9.4; Cary, NC, USA).
Results
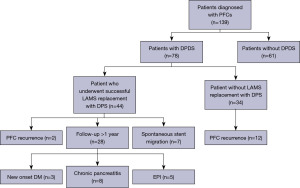
During the study period, 139 patients with AP underwent endoscopic management of symptomatic PFCs using LAMS. Of these, 78 (56%) patients were diagnosed with DPDS on MRCP and/or ERCP. Amongst patients with DPDS, 44 (56%) had successful placement of a DPS following LAMS removal after an average LAMS duration of 32 days. In the remaining 34 patients, DPS could not be placed due to collapse of the cyst cavity following LAMS removal (Figure 3).
In the study cohort consisting of DPDS patients treated with long term transmural DPS, 14 patients were female (32%), and the median age was 57 years. The etiology of AP was idiopathic in 13 (30%) patients, gallstone in 12 (27%) patients, alcohol in 11 (25%) patients, hypertriglyceridemia in 4 (9%) patients, and medication-induced in 4 (9%) patients. The median area of the PFC was 121.1 cm2 and 40 (91%) patients had necrotic debris within the cyst cavity. Infection of the PFC was present in 22 (50%) patients. PFCs were primarily drained via a transgastric approach (95%). One or two DPS were placed after LAMS removal with 10 Fr diameter stents most commonly used (61%) (Tables 1,2).
Table 1
| Patient characteristics | Data |
|---|---|
| Age, years, median [IQR] | 57 [48, 64] |
| Female, n [%] | 14 [32] |
| Etiology of pancreatitis, n [%] | |
| Alcohol | 11 [25] |
| Gallstone | 12 [27] |
| Idiopathic | 13 [30] |
| Other | 8 [18] |
| Presence of infection, n [%] | 22 [50] |
| Type of collection, n [%] | |
| Pseudocyst | 4 [9] |
| Walled-off necrosis | 40 [91] |
| Area of collection, cm2, median [IQR] | 121.1 [75.6, 168.7] |
PFC, pancreatic fluid collection; DPDS, disconnected pancreatic duct syndrome; IQR, interquartile range.
Table 2
| Variables | Outcomes |
|---|---|
| Endoscopic approach, n [%] | |
| Transgastric | 42 [95] |
| Transduodenal | 2 [5] |
| Size of DPS, n [%] | |
| 7 Fr | 17 [39] |
| 10 Fr | 27 [61] |
| Duration of DPS, days, median [IQR] | 394 [245, 853] |
| Length of follow-up, days, median [IQR] | 517 [249, 1,025] |
| Recurrence, n [%] | 2 [5] |
| Spontaneous migration, n [%] | 7 [16] |
| New onset diabetes mellitus, n [%] | 3 [7] |
| New onset chronic pancreatitis, n [%] | 10 [23] |
| New onset exocrine pancreatic insufficiency, n [%] | 8 [18] |
| Adverse events related to indwelling stents, n [%] | 0 |
| Overall mortality, n [%] | |
| Related to DPS placement | 0 |
| Unrelated causes | 3 [7] |
DPDS, disconnected pancreatic duct syndrome; DPS, double pigtail stents; IQR, interquartile range.
The median duration of indwelling DPS was 394 days with a median follow-up duration of 517 days. PFC recurrence occurred in 2 (5%) patients, with spontaneous DPS migration seen in one of them. Six additional patients experienced spontaneous migration of DPS without developing recurrent PFCs. Of the two recurrences (1 with and 1 without DPS), neither were clinically significant thus repeat endoscopic intervention was not necessary. No patients experienced loco-regional adverse events related to the indwelling DPS. Throughout the follow-up period, 3 (7%) patients developed new onset diabetes with HbA1C increasing from an average of 6% to 11%, requiring medical treatment. Serial cross-sectional imaging with either CT or magnetic resonance imaging (MRI) identified changes consistent with CP in 10 (23%) patients. A total of eight (18%) patients required pancreatic enzyme supplementation for EPI. The overall mortality in the study cohort was 7% (3 patients), none of which was related to initial LAMS placement or long-term placement of DPS (Table 2).
Twenty-eight (63%) patients had indwelling DPS for greater than one year with a median DPS duration of 665 days. The clinical outcomes for patients in this cohort are illustrated in Table 3. Three (11%) patients required treatment for new onset diabetes mellitus. Lastly, eight (29%) patients had imaging evidence of CP with five (18%) requiring pancreatic enzyme replacement therapy for EPI.
Table 3
| Variables | Outcomes |
|---|---|
| Duration of DPS, days, median [IQR] | 665 [453, 1,163] |
| Length of follow-up, days, median [IQR] | 629 [304, 1,087] |
| Recurrence, n [%] | 2 [7] |
| Spontaneous migration, n [%] | 4 [14] |
| New onset diabetes mellitus, n [%] | 3 [11] |
| New onset chronic pancreatitis, n [%] | 8 [29] |
| New onset exocrine pancreatic insufficiency, n [%] | 5 [18] |
DPDS, disconnected pancreatic duct syndrome; DPS, double pigtail stents; IQR, interquartile range.
Discussion
DPDS is a major complication observed in patients with acute necrotizing pancreatitis and greatly increases the risk of PFC recurrence following LAMS removal (8,11). Placement of a transmural DPS at the time of LAMS removal has proven to be an effective method of reducing PFC recurrence rates with a good short term safety profile (11,12). The impact of indefinite indwelling DPS on PFC recurrence and adverse events, however, is less well known. The results of our retrospective study suggest that in patients with DPDS, long-term transmural DPS produces low rates of PFC recurrence with an acceptable risk of adverse events.
In our study, PFC recurrence was observed in 2 (5%) patients following placement of DPS. Similarly, Rana et al. reported one recurrence (3%) in a cohort of 30 patients with DPDS treated with long-term DPS placement with a median follow-up of 20.4 months (15). In that patient, DPS migration was observed. Furthermore, in a cohort of 56 patients treated with indwelling transmural DPS for at least 3 years, PFC recurrence only occurred in 1 patient where the stent remained in situ (6). These results highlight the efficacy of permanent indwelling transmural DPS in DPDS.
Despite its efficacy, concerns have been raised regarding the safety of chronic indwelling DPS, particularly with regards to bleeding, infection, perforation, and stent migration. In our study, there were no loco-regional adverse events related to indwelling DPS. While several other studies have reported instances of colonic perforation from indwelling DPS, we did not encounter any such cases (6,16). Stent migration was observed in our cohort in 7 (16%) patients and was only clinically relevant in 1 such patient who developed PFC recurrence. Rana et al. reported similar stent migration rates the only clinical consequence being PFC recurrence (15). While not observed in our cohort, stent migration leading to small bowel obstruction has been reported (17).
Changes suggestive of CP in the disconnected segment are a common sequela of DPDS and can lead to both endocrine and exocrine pancreatic dysfunction (18). The impact of indwelling DPS on this phenomenon is poorly understood. Rana et al. noted 15/48 (31%) patients met EUS criteria for CP in the disconnected pancreas but not in the downstream portion of the pancreas (6). In our study, changes of CP in the disconnected segment were identified in 10 (23%) patients based on CT or MRI findings. EPI and diabetes mellitus was observed in 8 (18%) and 3 (7%) patients, respectively. In contrast, in a retrospective study of 21 patients managed with two indwelling DPS, 28% developed EPI and 52% developed new onset diabetes (19). The longer follow-up duration of this study compared to ours may explain these results.
Our study has several important strengths. Notably, we utilized a multidisciplinary approach in the clinical decision making for these complex patients. Second, we implemented a regimented follow-up protocol across a single tertiary care center which ensured uniformity in the care that these patients received. There are a few limitations that must be considered, including potential selection bias given that this was a retrospective review of a prospectively maintained database. Additionally, as this was a single center study, the small sample size is relatively small, limiting statistical power. Finally, all procedures were completed by experienced endoscopists at a high-volume center and as such, results might not be fully generalizable.
Conclusions
In conclusion, long-term placement of DPS for DPDS is a safe and effective method for preventing PFC recurrence, though it is not without potential complications. Future studies are needed to identify protective factors for PFC recurrence so candidates for earlier DPS removal can be selected.
Acknowledgments
Funding: None
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-94/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-94/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-94/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-94/coif). R.P. serves as an unpaid editorial board member of Translational Gastroenterology and Hepatology from July 2023 to June 2025. R.P. is a consultant for Boston Scientific and Cook Medical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The database was secured in accordance with Institutional Review Board of Wake Forest School of Medicine (IRB No. 00035936, Retrospective Analysis of Acute Pancreatitis at an Academic Medical Center). Informed consent was obtained from all patients prior to procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491-502. [Crossref] [PubMed]
- Baron TH, DiMaio CJ, Wang AY, et al. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020;158:67-75.e1. [Crossref] [PubMed]
- IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1-15. [Crossref] [PubMed]
- van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet 2018;391:51-8. [Crossref] [PubMed]
- Rana SS, Shah J, Sharma RK, et al. Clinical and morphological consequences of permanent indwelling transmural plastic stents in disconnected pancreatic duct syndrome. Endosc Ultrasound 2020;9:130-7. [Crossref] [PubMed]
- Vanek P, Urban O, Trikudanathan G, et al. Disconnected pancreatic duct syndrome in patients with necrotizing pancreatitis. Surg Open Sci 2023;11:19-25. [Crossref] [PubMed]
- Jang JW, Kim MH, Oh D, et al. Factors and outcomes associated with pancreatic duct disruption in patients with acute necrotizing pancreatitis. Pancreatology 2016;16:958-65. [Crossref] [PubMed]
- Maatman TK, Mahajan S, Roch AM, et al. Disconnected pancreatic duct syndrome predicts failure of percutaneous therapy in necrotizing pancreatitis. Pancreatology 2020;20:362-8. [Crossref] [PubMed]
- Bang JY, Hawes RH, Varadarajulu S. Lumen-apposing metal stent placement for drainage of pancreatic fluid collections: predictors of adverse events. Gut 2020;69:1379-81. [Crossref] [PubMed]
- Bang JY, Mel Wilcox C, Arnoletti JP, et al. Importance of Disconnected Pancreatic Duct Syndrome in Recurrence of Pancreatic Fluid Collections Initially Drained Using Lumen-Apposing Metal Stents. Clin Gastroenterol Hepatol 2021;19:1275-1281.e2. [Crossref] [PubMed]
- Pawa R, Dorrell R, Russell G, et al. Long-term transmural drainage of pancreatic fluid collections with double pigtail stents following lumen-apposing metal stent placement improves recurrence-free survival in disconnected pancreatic duct syndrome. Dig Endosc 2022;34:1234-41. [Crossref] [PubMed]
- Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71:446-54. [Crossref] [PubMed]
- Kamat R, Gupta P, Rana S. Imaging in chronic pancreatitis: State of the art review. Indian J Radiol Imaging 2019;29:201-10. [Crossref] [PubMed]
- Rana SS, Bhasin DK, Rao C, et al. Consequences of long term indwelling transmural stents in patients with walled off pancreatic necrosis & disconnected pancreatic duct syndrome. Pancreatology 2013;13:486-90. [Crossref] [PubMed]
- Yamauchi H, Iwai T, Kida M, et al. Complications of Long-Term Indwelling Transmural Double Pigtail Stent Placement for Symptomatic Peripancreatic Fluid Collections. Dig Dis Sci 2019;64:1976-84. [Crossref] [PubMed]
- Varadarajulu S, Wilcox CM. Endoscopic placement of permanent indwelling transmural stents in disconnected pancreatic duct syndrome: does benefit outweigh the risks? Gastrointest Endosc 2011;74:1408-12. [Crossref] [PubMed]
- Verma S, Rana SS. Disconnected pancreatic duct syndrome: Updated review on clinical implications and management. Pancreatology 2020;20:1035-44. [Crossref] [PubMed]
- Téllez-Aviña FI, Casasola-Sánchez LE, Ramírez-Luna MÁ, et al. Permanent Indwelling Transmural Stents for Endoscopic Treatment of Patients With Disconnected Pancreatic Duct Syndrome: Long-term Results. J Clin Gastroenterol 2018;52:85-90. [Crossref] [PubMed]
Cite this article as: Koutlas N, Bentley B, Dorrell R, Ferris T, Pawa R. Clinical outcomes of long-term transmural drainage with double pigtail stents in disconnected pancreatic duct syndrome. Transl Gastroenterol Hepatol 2024;9:4.


