
Efficacy and safety of vibrating capsule in treatment of chronic idiopathic constipation: a systematic review and meta-analysis of randomized controlled trials
Highlight box
Key findings
• This study found that vibrating capsule (VC) increased spontaneous bowel movements (SBMs) defined as bowel movements without the use of laxatives. However, there was no improvement in complete SBM defined as SBM with sense of complete evacuation. VC didn’t show an increase in pooled adverse events.
What is known and what is new?
• Current randomized controlled trials (RCT) demonstrating safety however inconsistently showing efficacy of this novel device in chronic idiopathic constipation (CIC).
• This meta-analysis reaffirms the safety of VC however efficacy of VC remains unknown. This is after pooling the data of available RCTs with heterogenous VC protocol.
What is the implication, and what should change now?
• There is need for large RCTs with homogenous VC protocol to evaluate the efficacy of VC in CIC.
Introduction
Chronic idiopathic constipation (CIC) is a common functional bowel disorder that affects 14% [95% confidence interval (CI): 12–17%] of the global population (1). This disease has increased prevalence in racial minorities, women, elderly, low socioeconomic status groups, and rates are projected to increase in the upcoming years (2,3). This chronic disease is perceived by 28% as a severe illness and is associated with significant financial burden, impaired quality of life, and work productivity (4-6). Treatment for constipation is mainly dietary therapy, lifestyle interventions, pharmacological therapy, trans-anal irrigation, and rarely surgery (7). Population surveys have shown that 40–50% of patients are not satisfied with their pharmacotherapy and are willing to try new modalities (8). In addition to low satisfaction, cost and side effects limit use of pharmacotherapy in constipation and make the field open to new treatment modalities (7).
Vibrating capsule (VC) is an orally ingested programmable capsule device that was first introduced by Ron et al. in a series of safety studies for treatment of constipation (9). The mechanism is thought to be via mechanical stimulation of the colon wall and augmenting the circadian rhythm of colonic peristaltic activity resulting in an increase of bowel movements (10). Recent studies evaluated the efficacy and safety of VC in patients with CIC with variable results. We aimed to perform a systematic review and meta-analysis of available studies to assess the impact of VC on patients with CIC. We present this article in accordance with the PRISMA reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-64/rc) (11).
Methods
Search strategy
A systematic search of the literature was performed through June 14th, 2023, in databases including Embase (Embase.com, Elsevier), PubMed/MEDLINE (Ovid), Cochrane Central (CochraneLibrary.com, Wiley), Web of Science (Clarivate), Global Index Medicus (World Health Organization), and Google Scholar via the Publish or Perish software. The author M.A. formulated the initial search that was refined by librarian (W.L.S.). The core concepts of “vibrating capsule”, “constipation”, and “spontaneous bowel movement” were used to develop a subject term and truncated keyword search strategy for Embase that was translated for the other databases. The specific strategies used are illustrated in Tables S1-S6. All studies were exported to EndNote 20 (Clarivate, Philadelphia, PA, USA) and, subsequently, all duplicates were removed by successive iterations of EndNote’s duplicate detection algorithms and manual inspection.
Definitions
Spontaneous bowel movement (SBM) is defined as a bowel movement without the use of rescue medicines within the last 48 hours or use of digital maneuvers. Complete SBM (CSBM) is defined as a SBM with sense of complete evacuation. CSBM 1 and CSBM 3 are defined as the proportion of the patients with an increase of at least 1 and 3 CSBMs per week from the baseline, respectively.
Inclusion and exclusion criteria
We included only full texts of randomized controlled trials (RCTs) assessing efficacy and safety of VC in subjects with constipation. All studies addressing the following were selected: (I) patients: patients with constipation. (II) Outcomes: SBM, CSBM. Rescue medications used and adverse events were also recorded (III) comparison/intervention: placebo/VC. We excluded case reports, case series (<10 patients), review articles, scientific letters, guidelines, observational studies, cohort studies, and abstracts. Abstracts were excluded as lack of detailed methodology does not allow assessment of potential biases. No restrictions of language were applied.
Screening and data extraction
Two independent reviewers (H.H. and N.Z.) performed the manual screening and data extraction using the title and abstracts. All conflicts were resolved using a third reviewer (M.A.). Microsoft Excel (Microsoft, Redmond, WA, USA) was the software used by reviewers to record study characteristics, patient demographics, primary, and secondary outcomes.
Statistical analysis
OpenMeta[Analyst] (CEBM, Brown University, Providence, RI, USA) software was used to perform statistical analysis of pooled data. Mean difference (MD) and odds ratio (OR) for continuous and proportional outcomes respectively, along with 95% CI with a P value of <0.05 for statistical significance were used. DerSimonian-Laird method and random effects model for pooling data were utilized. Heterogeneity was calculated with I2 statistic with values of 0%, 25%, 50%, and 75% as absent, low, moderate, and high, respectively (12).
Bias assessment
The method outlined in the Cochrane Handbook for Systematic Reviews of Interventions was used for risk of bias assessment for the studies (13). Publication bias was assessed qualitatively and quantitatively using funnel plots and Egger’s regression respectively, where applicable.
Results
The search strategy yielded a total of 117 articles. After removing duplicates, 67 studies remained. Three articles with four studies consisting of 705 total patients were finalized after implementing inclusion and exclusion criteria as illustrated in the PRISMA diagram (Figure 1) (10,14,15). The studies were published between 2019 and 2023 and totaled 386 VC and 319 placebo (Table 1). The mean age ranged from 41.3 to 46.8 years old (Table 1). One publication by Rao et al., comprised two separate RCTs (10). Two different VCs were used: (I) VibrantTM (Vibrant Ltd., Hakochav Yokneam, Israel) (10,14), and (II) VibravotTM (Ankon Medical Technology Co., Ltd., Wuhan, China) (15).
Table 1
| Study | Year | Total, n | VC, n | Placebo, n | Age (years), mean | Sex, n | |||
|---|---|---|---|---|---|---|---|---|---|
| VC | Placebo | VC | Placebo | ||||||
| Rao1 | 2019 | 182 | 89 | 93 | 45.3 | 42.7 | F: 71, M: 18 | F: 71, M: 22 | |
| Rao2 | 2019 | 68 | 44 | 24 | 42.9 | 41.3 | F: 37, M: 7 | F: 21, M: 3 | |
| Rao | 2023 | 349 | 200 | 149 | 46.8 | 45.9 | F: 172, M: 28 | F: 126, M: 23 | |
| Zhu | 2022 | 106 | 53 | 53 | 42.8 | 43.2 | F: 48, M: 5 | F: 48, M: 5 | |
VC, vibrating capsule; F, female; M, male.
Efficacy
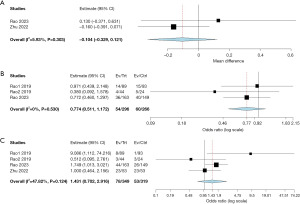
All studies reported mean change in CSBM and CSBM 1. Compared to placebo/sham treatment, the mean CSBM (MD =0.153; 95% CI: −0.218 to 0.523; P=0.422; I2=61.99%) and CSBM 1 (OR =1.628; 95% CI: 0.843 to 3.145; P=0.147; I2=71.76%), did not improve significantly with VC (Table 2, Figure 2A,2B). Mean SBM improvement and CSBM 3 were reported in two studies (14,15). The mean SBM and CSBM 3 showed statistical improvement with VC (MD =0.159; 95% CI: 0.095 to 0.223; P<0.001; I2=0%) and (OR =2.086; 95% CI: 1.155 to 3.768; P=0.015; I2=0%), respectively (Table 2, Figure 2C,2D). Neither the mean number of rescue medications used (MD =−0.104; 95% CI: −0.329 to 0.121; P=0.366; I2=5.93%), nor the patients requiring rescue medications (OR =0.774; 95% CI: 0.511 to 1.172; P=0.226; I2=0%), were statistically different in VC group compared to placebo (Table 3, Figure 3A,3B).
Table 2
| Study | Total, n | VC, n | Placebo, n | Change in CSBM score, mean (SD) | CSBM 1, n | CSBM 3, n | Change in SBM score, mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VC | Placebo | VC | Placebo | VC | Placebo | VC | Placebo | |||||||
| Rao1, 2019 | 182 | 89 | 93 | 1.14 (1.73) | 1.45 (1.91) | 25 | 33 | NR | NR | NR | NR | |||
| Rao2, 2019 | 68 | 44 | 24 | 1.22 (1.57) | 1.41 (1.71) | 19 | 8 | NR | NR | NR | NR | |||
| Rao, 2023 | 349 | 200 | 149 | 1.07 (0.26) | 0.71 (0.27) | 75 | 33 | 21 | 8 | 1.40 (0.28) | 1.24 (0.30) | |||
| Zhu, 2022 | 106 | 53 | 53 | 1.53 (1.601) | 1.06 (1.54) | 34 | 19 | 20 | 14 | 1.59 (1.76) | 1.52 (1.64) | |||
VC, vibrating capsule; CSBM, complete spontaneous bowel movement; SD, standard deviation; CSBM 1, increase in CSBMs of at least 1 per week; CSBM 3, increase in CSBMs of at least 3 per week; SBM, spontaneous bowel movement; NR, not reported.
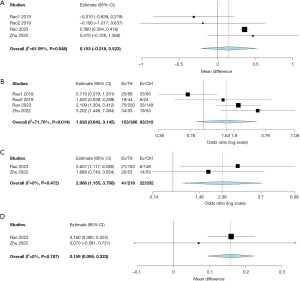
Table 3
| Study | Total, n | VC, n | Placebo, n | Patients requiring rescue medications, n | Rescue medications used, mean (SD) | Total adverse events, n | Vibration sensation, n | Diarrhea, n | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VC | Placebo | VC | Placebo | VC | Placebo | VC | Placebo | VC | Placebo | ||||||||
| Rao1, 2019 | 182 | 89 | 93 | 14 | 15 | NR | NR | 8 | 1 | 8 | 1 | 0 | 1 | ||||
| Rao2, 2019 | 68 | 44 | 24 | 4 | 5 | NR | NR | 3 | 3 | 3 | 3 | 1 | 1 | ||||
| Rao, 2023 | 349 | 200 | 149 | 36 | 40 | 0.85 (2.7) | 0.72 (1.75) | 44 | 26 | 18 | 0 | 2 | 0 | ||||
| Zhu, 2022 | 106 | 53 | 53 | NR | NR | 0.22 (0.4) | 0.38 (0.75) | 23 | 23 | NR | NR | 0 | 3 | ||||
VC, vibrating capsule; SD, standard deviation; NR, not reported.
Adverse events
All studies reported adverse events that included vibration sensation, abdominal pain/discomfort, musculoskeletal, diarrhea, pharyngitis, etc. Vibration sensation was the most common adverse event reported in most studies (10,14). No mortality was reported in the studies. The pooled total adverse events showed no statistical difference between VC and placebo (OR =1.431; 95% CI: 0.702 to 2.916; P=0.324; I2=47.82%) (Table 3, Figure 3C).
Risk of bias
All studies were high-quality multicenter double-blinded RCTs, and the risk of bias was calculated to be low based on using Cochrane risk of bias tool (Table S7). Due to limited number of studies, publication bias was not assessed.
Discussion
This systematic review and meta-analysis showed an improvement in CSBM 3 and mean SBM in adult CIC patients receiving VC compared with placebo. Nevertheless, VC did not show an improvement in mean CSBM, CSBM 1, or mean required rescue medications compared with placebo. On closer inspection, two studies with sample sizes of 349 and 106 were noted to show positive results that resulted in statistically significant results when mean SBM and CSBM 3 were compared (14,15). There are possible explanations for this finding; first, there was heterogeneity regarding capsule dosing regimen. In the studies with negative results, the investigators reduced the VC exposure from 5 to 2 days per week regimen in the second half of the study or the VC exposure was only for a one 2-hour session from the beginning (10). Zhu et al. hypothesized that this may be due to the different size and vibration frequency used in these studies (15). Since the most recent study used the same VC type, it is possible that the key to clinical efficacy was the higher exposure protocol of two vibration sessions, an intensity not achieved by prior studies (10,14). Interestingly, although no statistically discernible improvement was seen with VC, post-hoc analysis demonstrated a temporal correlation of achieving CSBM within 3 hours of feeling of vibration (10). This may support the theory that there is a VC exposure threshold under which one may not achieve clinical improvement. Of note, the total duration of treatment is likely not playing a role in different efficacies as results were seen as early as the second week of treatment (14). Requirements for rescue medications in the VC group were not different from that of placebo. This may be due to the subjective nature of opting for rescue medications, for instance vibration sensation being a trigger to do so. Validity of this claim needs to be further studied. The implications of this finding are in subjective aspect and patient satisfaction with VC.
Regarding adverse events, there was no statistically significant difference between VC and the placebo (that consisted of a dissolvable capsule in the small intestine). This included both types of capsule sizes with different stimulation paradigms (10,14,15). The reported adverse events were mostly mild with the most notable adverse event being a sense of vibration mainly in the VC group (10,14). This adverse event although not severe, needs to be further investigated in patients with irritable bowel syndrome in whom the subjective and perceptive aspects of treatment will likely play a bigger role. Another important caveat is that to avoid complications, studies had an exhaustive range of exclusion criteria consisting of patients with pregnancy, lactation, cardiac pacemakers, history of dysphagia, Barrett’s esophagus, achalasia, esophageal stricture, eating disorder, bariatric surgery, Zenker’s diverticulum, inflammatory bowel disease, gastroparesis, gastrointestinal malignancy, defecatory disorder with digital maneuvers to evacuate or feeling of anal blockage, rectocele, megarectum, complicated diverticular disease, intestinal obstruction, irritable bowel syndrome, diabetes mellitus, weight loss, or rectal bleed (10,14,15). In addition, patients taking opioids, calcium channel blockers, prokinetics, anti-Parkinsonian medications, or chronic use of non-steroidal anti-inflammatory drugs were also excluded. Last, a recent colonoscopy was required for inclusion unless in patients younger than 50 and not having alarming features (14). These criteria will likely be a major obstacle to acceptance of this modality amongst patients and providers. Possibly a patency capsule with subsequent motility capsule evaluation to rule out major anatomic and functional pathology prior to initiation of VC can substitute some of the exhaustive exclusion criteria (16,17).
Our study had some limitations. First, only four RCTs were available that assessed the efficacy and safety of VC in CIC patients. This limitation was likely due to novelty of VC, limited availability, and our search criteria limiting the results to high-quality multicenter double-blinded RCTs with strong methodologies. Second, there was heterogeneity regarding patient demographics, capsule device type, and dosing regimen. Nevertheless, restrictive inclusion criteria and narrow age range make the study population more homogenous. Third, the authors did not investigate VC efficacy in different severities of constipation or in patients with irritable bowel syndrome due to unavailability of data. Fourth, the age range of studied population is narrow which limits generalizability of the data to patients far from their forties. Last, due to limited follow-up in available studies, long-term efficacy and safety data is lacking. This area requires future studies with long follow-up.
Conclusions
In conclusion, this systematic review and meta-analysis suggested safety of VC. However, larger RCTs are needed to better determine the efficacy and cost-effectiveness of this novel modality.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-64/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-64/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-64/coif). M.A. serves as an unpaid editorial board member of Translational Gastroenterology and Hepatology from September 2022 to August 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol 2011;106:1582-91; quiz 1581; 1592. [Crossref] [PubMed]
- Gallegos-Orozco JF, Foxx-Orenstein AE, Sterler SM, et al. Chronic constipation in the elderly. Am J Gastroenterol 2012;107:18-25; quiz 26. [Crossref] [PubMed]
- Schiller LR. Chronic constipation: new insights, better outcomes? Lancet Gastroenterol Hepatol 2019;4:873-82. [Crossref] [PubMed]
- Nag A, Martin SA, Mladsi D, et al. The Humanistic and Economic Burden of Chronic Idiopathic Constipation in the USA: A Systematic Literature Review. Clin Exp Gastroenterol 2020;13:255-65. [Crossref] [PubMed]
- Black CJ, Drossman DA, Talley NJ, et al. Functional gastrointestinal disorders: advances in understanding and management. Lancet 2020;396:1664-74. [Crossref] [PubMed]
- Quigley EM, Neshatian L. Advancing treatment options for chronic idiopathic constipation. Expert Opin Pharmacother 2016;17:501-11. [Crossref] [PubMed]
- Bassotti G, Usai Satta P, Bellini M. Chronic Idiopathic Constipation in Adults: A Review on Current Guidelines and Emerging Treatment Options. Clin Exp Gastroenterol 2021;14:413-28. [Crossref] [PubMed]
- Oh SJ, Fuller G, Patel D, et al. Chronic Constipation in the United States: Results From a Population-Based Survey Assessing Healthcare Seeking and Use of Pharmacotherapy. Am J Gastroenterol 2020;115:895-905. [Crossref] [PubMed]
- Ron Y, Halpern Z, Safadi R, et al. Safety and efficacy of the vibrating capsule, an innovative non-pharmacological treatment modality for chronic constipation. Neurogastroenterol Motil 2015;27:99-104. [Crossref] [PubMed]
- Rao SSC, Lembo A, Chey WD, et al. Effects of the vibrating capsule on colonic circadian rhythm and bowel symptoms in chronic idiopathic constipation. Neurogastroenterol Motil 2020;32:e13890. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Rao SSC, Quigley EMM, Chey WD, et al. Randomized Placebo-Controlled Phase 3 Trial of Vibrating Capsule for Chronic Constipation. Gastroenterology 2023;164:1202-1210.e6. [Crossref] [PubMed]
- Zhu JH, Qian YY, Pan J, et al. Efficacy and safety of vibrating capsule for functional constipation (VICONS): A randomised, double-blind, placebo-controlled, multicenter trial. EClinicalMedicine 2022;47:101407. [Crossref] [PubMed]
- Fairbrass K, Hoshen D, Bailey H, et al. Cost-effectiveness of patency capsule test prior to wireless capsule endoscopy. Clin Med (Lond) 2020;20:s30-1. [Crossref] [PubMed]
- Irshad Q, Abu-Sbeih H, Stroehlein J, et al. 491 Wireless Motility Capsule Testing Results in New Diagnostic Information in 38% of Patients With Suspected GI Dysmotility: A 6-Year Review. Am J Gastroenterol 2019;114:S285. [Crossref]
Cite this article as: Haghbin H, Zakirkhodjaev N, Gangwani MK, Beran A, Lee-Smith W, Piper MH, Aziz M. Efficacy and safety of vibrating capsule in treatment of chronic idiopathic constipation: a systematic review and meta-analysis of randomized controlled trials. Transl Gastroenterol Hepatol 2024;9:8.



