The outcomes of COVID-19 and acute pancreatitis: a systematic review and meta-analysis
Highlight box
Key findings
• Coronavirus disease 2019 (COVID-19) may worsen the prognosis of patients with acute pancreatitis.
What is known and what is new?
• At present, studies have shown that severe acute respiratory syndrome corona virus 2 may cause pancreatic infection, and there are also some cases reporting that acute pancreatitis may be related to COVID-19. However, the interaction between the two remains unclear.
• We analyzed the clinical outcomes of patients with and without COVID-19, and used sensitivity analysis and Egger’s test to infer the association between COVID-19 infection and poor prognosis of acute pancreatitis.
What is the implication, and what should change now?
• Through this study, we found that COVID-19 infection may lead to poor prognosis in patients with acute pancreatitis, so as to better guide clinical work.
Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic caused by the severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) that emerged at the end of 2019 (1,2). SARS-CoV-2 utilizes angiotensin-converting enzyme 2 (ACE-2) receptors to enter human cells and TMPRSS2 for priming (3). The expression of these proteins is substantially elevated in the luminal cells of the gastrointestinal system as well as in pancreatic duct cells, acinar cells, and islet cells (4-6). Hence, we hypothesized that gland infection is possible, as the virus can disseminate from the duodenum to the pancreatic duct, subsequently affecting acinar and islet cells (4). This process is accompanied by cytolytic effects that facilitate the release of pancreatic amylase and/or lipase.
Patient samples of pancreatic pseudocysts were identified as SARS-CoV-2 in a case report of acute pancreatitis (AP) (7). AP has been linked to SARS-CoV-2 infection in several case reports. In a study conducted by Qurban et al. (8), a case of pancreatitis was documented based on findings from an fluorodeoxyglucose positron emission tomography (FDG-PET) scan. The researcher identified COVID-19-related pancreatitis as the most probable explanation for the observed diffuse moderate fluorodeoxyglucose (FDG) uptake in the pancreas after excluding other pertinent risk factors. The Szatmary et al. (9) study has provided a comprehensive description of thirty-five individuals diagnosed with AP. A total of ten patients with SARS-CoV-2 positive were found, out of which five cases lacked a definitive cause and were ascribed to infection with COVID-19. The current understanding of the association between COVID-19 and AP remains limited, and there is a notable absence of a thorough and systematic examination through a comprehensive review and meta-analysis. Given this context, our objective was to enhance our understanding of the involvement of SARS-CoV-2 in pancreatitis by conducting a systematic review and meta-analysis. This involved comparing the clinical outcomes of AP in individuals with COVID-19 and those without COVID-19. We present this article in accordance with the MOOSE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-58/rc).
Methods
The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, ID: CRD42023397011).
Search strategy
In this study, we searched the Web of Science, Cochrane Library, PubMed, Scopus, and Embase between January 2019 and December 2022. Our PubMed search term was: (“COVID-19”[MeSH Terms] OR “SARS-CoV-2”[MeSH Terms] OR “Coronavirus”[MeSH Terms] OR (“covid”[Title/Abstract] OR “COVID 19”[Title/Abstract] OR “2019-nCoV”[Title/Abstract] OR “severe acute respiratory syndrome coronavirus 2”[Title/Abstract])) AND (“Pancreatitis”[MeSH Terms] OR “pancreatitis, acute hemorrhagic”[MeSH Terms] OR “pancreatitis, acute necrotizing”[MeSH Terms] OR (“acute pancreatitis”[Title/Abstract] OR “pancreatic injur*”[Title/Abstract] OR “pancreas”[Title/Abstract] OR “pancreatic damage”[Title/Abstract] OR “interstitial pancreatitis”[Title/Abstract])). Supplementary material exhibits the search strategy for each database (Appendix 1).
Inclusion criteria
- With and without SARS-CoV-2 infection, patients who suffered from AP were included.
- Two of the following three characteristics must be presented according to the usual definition of AP (defined as clinically significant pancreatic damage) for inclusion in the research: (i) acute characteristic signs of pancreatitis are present on enhanced computed tomography and, less typically, on transabdominal ultrasound or magnetic resonance imaging; (ii) the presence of serum amylase or lipase activity for at least three times > upper limit of normal (ULN); and (iii) the presence of typical abdominal pain. Finally, the meta-analysis included both clinical trials and cohort investigations.
- The included studies reported at least one of the following outcomes using relevant data: (i) death; (ii) intensive care unit (ICU) admission; (iii) mechanical ventilation; (iv) Bedside Index of Severity in Acute Pancreatitis (BISAP) score (10-12); (v) idiopathic and unknown etiology; (vi) necrotizing pancreatitis; and (vii) length of hospital stay. A BISAP score ≥3 was considered severe pancreatitis.
- All studies were published in English.
- All studies had scores ≥6 on the Newcastle-Ottawa Scale (NOS) (13).
Exclusion criteria
Conferences, abstracts, meta-analyses, reviews, case reports, letters, comments, guidelines, or non-clinical studies were excluded. Exclusion was made for studies that lacked the necessary data.
Data extraction
Two researchers (C.Z. and H.W.) evaluated and retrieved all publications that fulfilled the requirements. Two people discussed any associated topics with a third author (X.Y.) and agreed. For each included study article, the first author, year published, gender and number of subjects, participants’ ages, exposures, quality evaluation, and outcome measures were noted.
Quality assessment
The cohort and case-control papers included in the study were assessed using the NOS scale, as reported earlier (13). Scores ranging from 1 to 4 indicated low quality, while scores ranging from 5 to 9 indicated excellent quality. The assessment of article quality was conducted independently by two authors.
Statistical analysis
The meta-analysis was conducted using Stata 12.0 software. The study employed statistical method to assess the relationship between AP and COVID-19 prognosis. Specifically, the 95% confidence interval (CI), odds ratio (OR), and weighted mean difference (WMD) were calculated for dichotomous and continuous variables, respectively. A significance level of P<0.05 was used to determine statistical significance. Statistics from Luo et al. (14) and Wan et al. (15) were used to transform continuous variables presented as median and range into sample means and standard deviations. The degree of heterogeneity was calculated using the I2 test, and we used the random effects model if it was larger than 50%. To conduct the sensitivity analysis, we initially omitted any research findings with severe characteristics. Based on using Egger’s test for publication bias, it may be concluded that a significant publication bias exists at a significance level of P<0.05.
Results
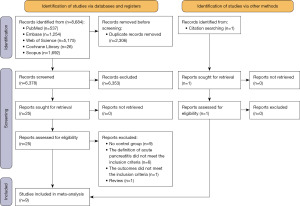
After searching for relevant articles in five databases, we screened 8,684, removed 2,306 duplicates, and reviewed the remaining 6,378 articles in full for 25 studies, plus one additional research found via a search of other literature (Figure 1). Eventually, this meta-analysis used data from nine research (16-24), all of which had NOS scores ≥6 (Table 1).
Table 1
| Study | Groups | Patients | Age (year) | Males % | Number of deaths | ICU occupancy | Mechanical ventilation | Etiology (idiopathic & unknown) | BISAP ≥3 | Necrotizing | Length of hospital stay (days) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inamdar (22), [2020] | Positive | 32 | 53.44±16.60 | 43.8 | 4 | NA | 9 | 22 | 12 | 4 | 21.22±26.91 | 8 |
| Negative | 157 | 52.14±19.80 | 38.9 | 8 | NA | 10 | 33 | 71 | 7 | 6.36±5.83 | ||
| Dirweesh (18), [2020] | Positive | 14 | 55.2±14.8 | 50.0 | 3 | NA | 6 | 9 | 6 | 2 | 10.8±7.2 | 6 |
| Negative | 61 | 48.4±14.1 | 44.0 | 1 | NA | 3 | 3 | 3 | 6 | 6.5±6.7 | ||
| Karaali (20), [2021] | Positive | 83 | 57.43±18.85 | 50.6 | 13 | 6 | NA | 34 | 27 | 14 | 7.7 | 7 |
| Negative | 106 | 52.94±17.12 | 45.3 | 3 | 1 | NA | 26 | 15 | 12 | 5.5 | ||
| Pandanaboyana (23), [2021] | Positive | 110 | 59.9±17.2 | 62.8 | 15 | 27 | NA | NA | NA | 24 | 9 [5–17] | 8 |
| Negative | 1,373 | 54.5±18.1 | 51.5 | 34 | 100 | NA | NA | NA | 177 | 4 [3–8] | ||
| Miró (17), [2021] | Positive | 54 | 68 [53–79] | 72.2 | 9 | 5 | NA | 15 | 19 | NA | NA | 7 |
| Negative | 162 | 61 [49–77] | 56.2 | 6 | 7 | NA | 36 | 24 | NA | NA | ||
| Akarsu (16), [2022] | Positive | 40 | 55 [26–84] | 65.0 | 13 | 25 | 23 | NA | NA | NA | 14.7±9.5 | 7 |
| Negative | 276 | 54 [26–87] | 58.3 | 22 | 52 | 50 | NA | NA | NA | 11.2±6.4 | ||
| EBiK (19), [2022] | Positive | 7 | 51.4±12.5 | 57.1 | 0 | NA | NA | 4 | NA | NA | NA | 6 |
| Negative | 315 | 57.17±19.1 | 32.7 | 4 | NA | NA | 53 | NA | NA | NA | ||
| Samanta (24), [2022] | Positive | 85 | 41.1±13.0 | 68.2 | 28 | 48 | 5 | NA | NA | NA | NA | 7 |
| Negative | 230 | 40.07±11.9 | 68.3 | 44 | 85 | 10 | NA | NA | NA | NA | ||
| Haydar (21), [2022] | Positive | 21 | 63.7±16.8 | 66.7 | 6 | NA | NA | NA | NA | NA | NA | 8 |
| Negative | 34 | 58.2±18.5 | 50.0 | 1 | NA | NA | NA | NA | NA | NA |
Data presentation of age (year) and length of hospital stay (days) are mean ± SD, median [p75–p25] or average. ICU, intensive care unit; BISAP, Bedside Index of Severity in Acute Pancreatitis; NOS, Newcastle-Ottawa Scale; NA, not applicable.
Mortality
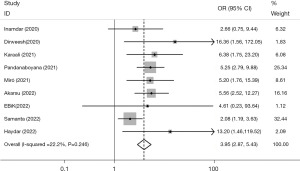
The analysis of nine studies reporting mortalities revealed a significant difference in death rates between individuals with AP with COVID-19 and those without COVID-19 (OR =3.95, 95% CI: 2.87, 5.43, P<0.001), indicating a higher mortality rate among individuals with both conditions. This conclusion is supported by a low level of heterogeneity (I2=22.2%, P=0.246), as shown in Figure 2. The application of regression analysis, specifically employing the Egger test, yielded results indicating the absence of any discernible dispersion bias (β=1.25, P=0.129).
ICU admission
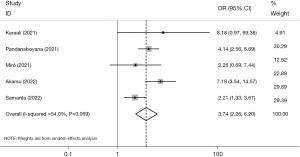
The analysis revealed a considerable level of heterogeneity (I2=54.0%, P=0.069) among the five studies that included data on ICU admissions (Figure 3). However, our findings indicate that persons diagnosed with AP and COVID-19 had a significantly increased likelihood of being admitted to the ICU compared to those without COVID-19 (OR =3.74, 95% CI: 2.26, 6.20, P<0.001). Low between-study heterogeneity (I2=10.4%, P=0.341) and greater significance (OR =4.66, 95% CI: 3.07, 7.08, P<0.001) after excluding Samanta et al. (24) were observed. There was also no statistically significant dispersion bias, as measured by the Egger test regression (β=0.75, P=0.699).
Mechanical ventilation
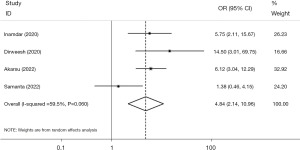
The four studies that reported mechanical ventilation had significant heterogeneity (I2=59.5%, P=0.060). However, our analysis revealed that patients with AP and COVID-19 had a significantly increased likelihood of requiring mechanical ventilation compared to those without COVID-19 (OR =4.84, 95% CI: 2.14, 10.96, P<0.001) (Figure 4). Moreover, low between-study heterogeneity (I2=0.0%, P=0.582) and greater significance (OR =6.65, 95% CI: 3.88, 11.39, P<0.001) after excluding Samanta et al. (24) were observed. There was also no statistically significant dispersion bias, as measured by the Egger test regression (β=0.04, P=0.992).
Severe pancreatitis
The present study analyzed data from four studies to determine the prevalence of severe pancreatitis (defined as BISAP scores ≥3) among patients with AP, comparing those with and without COVID-19. The analysis revealed a significantly higher proportion of severe pancreatitis cases among individuals with COVID-19 compared to those without (OR =2.71, 95% CI: 1.04, 7.04, P=0.042). However, it is important to note that there was a substantial degree of heterogeneity in the results (I2=79.7%, P=0.002) (Figure 5). The findings are more significant (OR =3.83, 95% CI: 1.94, 7.57, P<0.001) when excluding Inamdar et al. (22), with low between-study heterogeneity (I2=42.7%, P=0.175). In addition, the Egger test regression found no evidence of a dispersion bias (β=3.68, P=0.5).
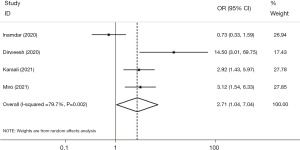
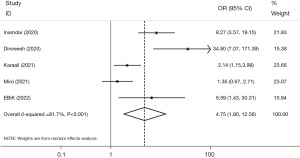
Etiology of idiopathic and unknown
Five studies reported the patients with various etiologies of pancreatitis, and the analysis found a higher proportion of patients with idiopathic and unknown etiologies of AP with COVID-19 than those without it (OR =4.75, 95% CI: 1.80, 12.56, P=0.002). The findings also revealed significant heterogeneity (I2=81.7%, P<0.001) (Figure 6). In addition, applying sensitivity analysis, wherein each of the five studies was systematically excluded 1 at a time, further demonstrated the presence of substantial heterogeneity. The application of regression analysis, specifically employing the Egger test, yielded results that did not provide any substantiated indication of a dispersion bias (β=4.70, P=0.132).
Proportion of necrotizing pancreatitis
Four studies reported the number of patients with necrotizing pancreatitis. Patients with combined COVID-19 had a higher risk of developing necrotizing pancreatitis than patients with AP alone (OR =1.88, 95% CI: 1.28, 2.76, P=0.001), and this difference was not heterogeneous (I2=0.0%, P=0.859) (Figure 7). Regression using the Egger test found no evidence of a dispersion bias (β=0.16, P=0.833).

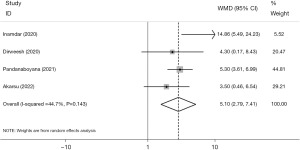
Length of hospital stay
Five studies reported the length of hospital stay. The research by Karaali et al. (20), which only provided the mean length of stay, was omitted from the meta-analysis, and the other four investigations were included. Although heterogeneity was modest (I2=44.7%, P=0.143) (Figure 8), AP cases with COVID-19 had a significantly longer duration of stay than those without it (WMD =5.10, 95% CI: 2.79, 7.41, P<0.001). Upon doing sensitivity analysis and removing Inamdar et al. (22) from the meta-analysis, it was observed that there was no heterogeneity among the trials (I2=0.0%, P=0.579). Consequently, the results exhibited statistical significance (WMD =4.81, 95% CI: 3.42, 6.20, P<0.001). Additionally, the presence of dispersion bias was not statistically significant, as assessed by applying the Egger test regression (β=1.11, P=0.538).
Discussion
This meta-analysis analyzed a total of nine studies encompassing a sample size of 3,160 patients. Our study revealed notable elevations in mortality rates associated with AP, as well as increased rates of ICU admission, mechanical ventilation usage, prevalence of severe disease, cases with idiopathic and unknown causes, instances of necrotizing pancreatitis, and lengthier hospital stays among patients diagnosed with COVID-19 compared to those without COVID-19.
This phenomenon can perhaps be elucidated by the observation that individuals afflicted with COVID-19 tend to have a higher degree of disease severity. In the context of SARS-CoV-2 viral infection, the condition of patients deteriorates, resulting in a heightened occurrence of necrotizing pancreatitis and a greater proportion of severe cases. Consequently, this leads to an elevated admission rate to ICUs and the need for mechanical ventilation. Patients experience prolonged hospital stays, resulting in a substantial rise in mortality rates. Furthermore, our study also revealed a notable prevalence of patients with idiopathic pancreatitis and unclear origin among individuals diagnosed with COVID-19. In the five studies incorporated in our analysis, the investigators did not provide a specific definition for idiopathic pancreatitis. However, it is noteworthy that the studies consistently excluded major etiological factors such as alcohol consumption, gallstones, and hyperlipidemia. Given these exclusions, it is plausible to consider a potential association between COVID-19 and cases of pancreatitis that are idiopathic or lack a clear explanation.
Some studies have pointed out that the extracellular structural domain of ACE2 interacts with stinging proteins to induce cytokine storms (25,26). ACE2 is the primary receptor for SARS-CoV-2 entrance into target cells and is found in cells of numerous organ tissues of the human body. In addition to the respiratory system, ACE2 is also expressed at high levels in pancreatic exocrine glands and islets (27). Some individuals with COVID-19 have had higher blood amylase and lipase values (28), but no abdominal symptoms or imaging abnormalities were seen, thereby characterizing the condition as “pancreatic injury” and, presumably, the link between COVID-19 and pancreatic injury. Mildly raised levels of pancreatic enzymes in the blood of COVID-19 individuals may have several causes. The concentration of amylase and lipase in the bloodstream is regulated by the balance between their synthesis and elimination processes (29). Due to the inadequacy of assessing a solitary pancreatic enzymology indicator in patients, it is also impossible to establish a causal relationship between COVID-19 and idiopathic or unexplained pancreatitis. Further comprehensive research is necessary to establish a conclusive correlation between SARS-CoV-2 and pancreatic damage (30).
There are still several limitations inherent in our study. First, three of the nine studies used in our analysis were conducted prospectively, while the remaining six were conducted retrospectively. For the statistics of mortality in the included studies, it was clearly stated in Miró et al. (17) and Samanta et al. (24) that it was in-hospital mortality, and in Pandanaboyana et al. (23) that it was 30-day mortality, while the other studies did not clearly state it, only “mortality” was stated. Consequently, our study focused on reporting death rates without distinct comparisons. The factor mentioned above may have exerted an influence on the outcome.
Furthermore, our study revealed that individuals diagnosed with AP and COVID-19 exhibited a higher degree of severe illness. This observation suggests a potential exacerbation of AP by COVID-19; nevertheless, it is important to note that the possibility of COVID-19 independently influencing the severity of the disease cannot be entirely excluded. This study primarily compared two distinct cohorts of patients with AP, one affected by COVID-19 and AP, and the other only affected by AP. Consequently, it is unable to elucidate the individual impact of COVID-19. We anticipate more clinical investigations to compare the management of patients with COVID-19 AP to those without this condition. Therefore, it is a better option to present the independent effect of COVID-19 on the unfavorable prognosis of individuals suffering from AP.
There may be a variety of confounding factors in this study. The included studies did not document the effect of various treatments, such as paxlovid, on patient outcomes, and some treatments may have exacerbated pancreatic injury. The World Health Organization (WHO) reported more than 525 drugs as suspected causative agents (31,32) of these drugs have been used in patients with COVID-19 and have caused direct or indirect pancreatic damage (31-34). Paramythiotis et al. (35) summarized and analyzed the medications used to treat COVID-19 infection and possibly associated with drug-induced AP in hospitalized patients with COVID-19. These drugs mainly include antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, glucocorticoids, monoclonal antibodies, antiviral agents, estrogens and anesthetic agents. To address this issue, it is imperative to conduct additional rigorous clinical investigations that compare the effectiveness of different treatment approaches and medications on the pancreas. This will help eliminate potential confounding factors, establish more robust evidence connecting SARS-CoV-2 infection and AP, and guide the appropriate clinical utilization of drugs to minimize the risk of adverse outcomes.
In addition, gender, age, comorbidities, and other factors will also affect the results to a certain extent. Based on Balani et al. (36), elderly patients receiving multiple medications are at high risk for AP. In this context, combining medications for chronic diseases with COVID-19 treatment during hospitalization may result in severe pancreatic damage due to the patient’s decreased metabolism due to old age (33,36-38). Several risk factors for COVID-19 have been discovered, including male gender, age, hypertension, diabetes, obesity, chronic lung illness, cardiac disease, liver disease, renal disease, and neoplasms (39-42). COVID-19 patients combined with risk factors may have more adverse outcomes (43-45).
Some limitations remain to be addressed in the future. First, the heterogeneity across research on idiopathic and unclear etiology remained significant when we did a sensitivity analysis to eliminate the relevant studies. It was speculated that this may be due to the limited number of studies that were analyzed. More multicenter prospective clinical studies are needed to prove the causal relationship between COVID-19 and AP.
Second, it is impossible to prove that SARS-CoV-2 is the sole factor causing AP since the influence of various treatment measures on patient outcomes was not recorded in the included investigations and some of the therapeutic approaches may have aggravated the pancreatic damage.
To alleviate this problem, more high-quality clinical studies comparing the efficacy of various treatment modalities and drugs on the pancreas are needed to eliminate this confounding variable, provide stronger evidence linking SARS-CoV-2 infection and AP, and direct the clinical application of drugs in order to lessen the likelihood of adverse effects.
Third, several risk factors for COVID-19 have been discovered, including male gender, age, hypertension, diabetes, obesity, chronic lung illness, cardiac disease, liver disease, renal disease, and neoplasms (39-42). COVID-19 patients combined with risk factors may have more adverse outcomes (43-45). Therefore, as previously indicated, the research is influenced by the comorbidities of COVID-19 patients. The investigation conducted in the present study did not completely examine comorbidities due to the limited number of studies that specifically tested for them. Additional research is required to investigate the influence of comorbidities on the prognosis of individuals with COVID-19.
The statistical analysis revealed that there was still a high level of heterogeneity in the idiopathic and uncertain etiology research, even after conducting a sensitivity analysis to exclude relevant papers. The observed phenomenon could be attributed to the restricted quantity of research subjected to analysis. Due to insufficient evidence, further multicenter prospective clinical investigations are required to establish the causal association between COVID-19 and AP.
There are some guidelines on AP (46), as well as COVID-19, but there is still a lack of relevant guidelines and systematic treatment measures for COVID-19 complicated with AP. We anticipate the input of authoritative professionals to develop pertinent guidelines that might effectively guide clinical treatment.
In summary, it is important to note that the scope of this study was limited to individuals diagnosed with AP. Therefore, individuals with chronic pancreatitis with a prior history of AP should prioritize self-preventive measures to mitigate the risk of viral infections and adverse outcomes.
Conclusions
In conclusion, those diagnosed with COVID-19 confront a higher susceptibility to the manifestation of severe illness than those who do not contract the virus. Additional research is required to validate or challenge the hypothesis that the concurrent presence of SARS-CoV-2 infection may contribute to an increased probability of adverse outcomes in cases with AP.
Acknowledgments
Funding: This work was supported by Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-58/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-58/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-58/coif). All authors report that this study was supported by Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hui DS, I, Azhar E, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020;91:264-6. [Crossref] [PubMed]
- Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol 2020;92:401-2. [Crossref] [PubMed]
- Samanta J, Gupta R, Singh MP, et al. Coronavirus disease 2019 and the pancreas. Pancreatology 2020;20:1567-75. [Crossref] [PubMed]
- de-Madaria E, Capurso G. COVID-19 and acute pancreatitis: examining the causality. Nat Rev Gastroenterol Hepatol 2021;18:3-4. [Crossref] [PubMed]
- Liu F, Long X, Zhang B, et al. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol 2020;18:2128-2130.e2. [Crossref] [PubMed]
- Xiao F, Tang M, Zheng X, et al. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020;158:1831-1833.e3. [Crossref] [PubMed]
- Schepis T, Larghi A, Papa A, et al. SARS-CoV2 RNA detection in a pancreatic pseudocyst sample. Pancreatology 2020;20:1011-2. [Crossref] [PubMed]
- Qurban Z, Mullan D. COVID-19-Related Incidental Pancreatitis Detected on FDG-PET Scan. Cureus 2022;14:e31730. [Crossref] [PubMed]
- Szatmary P, Arora A, Thomas Raraty MG, et al. Emerging Phenotype of Severe Acute Respiratory Syndrome-Coronavirus 2-associated Pancreatitis. Gastroenterology 2020;159:1551-4. [Crossref] [PubMed]
- Gompertz M, Fernández L, Lara I, et al. Bedside index for severity in acute pancreatitis (BISAP) score as predictor of clinical outcome in acute pancreatitis: retrospective review of 128 patients. Rev Med Chil 2012;140:977-83. [Crossref] [PubMed]
- Chan KS, Shelat VG. Diagnosis, severity stratification and management of adult acute pancreatitis-current evidence and controversies. World J Gastrointest Surg 2022;14:1179-97. [Crossref] [PubMed]
- Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008;57:1698-703. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785-805. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Akarsu C, Karabulut M, Aydin H, et al. Association between Acute Pancreatitis and COVID-19: Could Pancreatitis Be the Missing Piece of the Puzzle about Increased Mortality Rates? J Invest Surg 2022;35:119-25. [Crossref] [PubMed]
- Miró Ò, Llorens P, Jiménez S, et al. A case-control emergency department-based analysis of acute pancreatitis in Covid-19: Results of the UMC-19-S(6). J Hepatobiliary Pancreat Sci 2021;28:953-66. [Crossref] [PubMed]
- Dirweesh A, Li Y, Trikudanathan G, et al. Clinical Outcomes of Acute Pancreatitis in Patients With Coronavirus Disease 2019. Gastroenterology 2020;159:1972-4. [Crossref] [PubMed]
- EBiK B. Bacaksiz F, EKiN N. Does COVID-19 cause pancreatitis? Arq Gastroenterol 2022;59:71-4. [Crossref] [PubMed]
- Karaali R, Topal F. Evaluating the effect of SARS-Cov-2 infection on prognosis and mortality in patients with acute pancreatitis. Am J Emerg Med 2021;49:378-84. [Crossref] [PubMed]
- Haydar FG, Otal Y, Avcioglu G. Evaluation of patients with acute pancreatitis associated with SARS-CoV-2 (COVID-19); The importance of lipase/lymphocyte ratio in predicting mortality. Bratisl Lek Listy 2022;123:428-34. [Crossref] [PubMed]
- Inamdar S, Benias PC, Liu Y, et al. Prevalence, Risk Factors, and Outcomes of Hospitalized Patients With Coronavirus Disease 2019 Presenting as Acute Pancreatitis. Gastroenterology 2020;159:2226-2228.e2. [Crossref] [PubMed]
- Pandanaboyana S, Moir J, Leeds JS, et al. SARS-CoV-2 infection in acute pancreatitis increases disease severity and 30-day mortality: COVID PAN collaborative study. Gut 2021;70:1061-9. [Crossref] [PubMed]
- Samanta J, Mahapatra SJ, Kumar N, et al. Virus related acute pancreatitis and virus superinfection in the 'Dual disease' model of acute pancreatitis and SARS-Co-V2 infection: A multicentre prospective study. Pancreatology 2022;22:339-47. [Crossref] [PubMed]
- Ramasamy S, Subbian S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin Microbiol Rev 2021;34:e00299-20. [Crossref] [PubMed]
- Walls AC, Park YJ, Tortorici MA, et al. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020;181:281-292.e6. [Crossref] [PubMed]
- Wang F, Wang H, Fan J, et al. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology 2020;159:367-70. [Crossref] [PubMed]
- Mukherjee R, Smith A, Sutton R. Covid-19-related pancreatic injury. Br J Surg 2020;107:e190. [Crossref] [PubMed]
- de-Madaria E, Siau K, Cárdenas-Jaén K. Increased Amylase and Lipase in Patients With COVID-19 Pneumonia: Don't Blame the Pancreas Just Yet! Gastroenterology 2021;160:1871. [Crossref] [PubMed]
- Yuan Y, Jiao B, Qu L, et al. The development of COVID-19 treatment. Front Immunol 2023;14:1125246. [Crossref] [PubMed]
- Brikman S, Denysova V, Menzal H, et al. Acute pancreatitis in a 61-year-old man with COVID-19. CMAJ 2020;192:E858-9. [Crossref] [PubMed]
- Muzahim YE, Parish DC, Goyal H. Insights into Acute Pancreatitis Associated COVID-19: Literature Review. J Clin Med 2021;10:5902. [Crossref] [PubMed]
- Simons-Linares CR, Elkhouly MA, Salazar MJ. Drug-Induced Acute Pancreatitis in Adults: An Update. Pancreas 2019;48:1263-73. [Crossref] [PubMed]
- Juhász MF, Ocskay K, Kiss S, et al. Insufficient etiological workup of COVID-19-associated acute pancreatitis: A systematic review. World J Gastroenterol 2020;26:6270-8. [Crossref] [PubMed]
- Paramythiotis D, Karlafti E, Veroplidou K, et al. Drug-Induced Acute Pancreatitis in Hospitalized COVID-19 Patients. Diagnostics (Basel) 2023;13:1398. [Crossref] [PubMed]
- Balani AR, Grendell JH. Drug-induced pancreatitis: incidence, management and prevention. Drug Saf 2008;31:823-37. [Crossref] [PubMed]
- Hung WY, Abreu Lanfranco O. Contemporary review of drug-induced pancreatitis: A different perspective. World J Gastrointest Pathophysiol 2014;5:405-15. [Crossref] [PubMed]
- Wolfe D, Kanji S, Yazdi F, et al. Drug induced pancreatitis: A systematic review of case reports to determine potential drug associations. PLoS One 2020;15:e0231883. [Crossref] [PubMed]
- Mutneja HR, Bhurwal A, Arora S, et al. Acute pancreatitis in patients with COVID-19 is more severe and lethal: a systematic review and meta-analysis. Scand J Gastroenterol 2021;56:1467-72. [Crossref] [PubMed]
- Du H, Dong X, Zhang JJ, et al. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy 2021;76:510-32. [Crossref] [PubMed]
- Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547. [Crossref] [PubMed]
- Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV 2020;7:e554-64. [Crossref] [PubMed]
- Chan WF, Toh YN. Acute Necrotizing Pancreatitis Complicated by Multiple Splanchnic Venous Thromboses and Bilateral Renal Infarctions in a Patient With Recent COVID-19 Infection: A Case Report. Cureus 2022;14:e28049. [Crossref] [PubMed]
- Lam ICH, Wong CKH, Zhang R, et al. Long-term post-acute sequelae of COVID-19 infection: a retrospective, multi-database cohort study in Hong Kong and the UK. EClinicalMedicine 2023;60:102000. [Crossref] [PubMed]
- Devi SM, Pamreddy A, Narendra VR. Risks associated with acute pancreatitis (AP) with diabetic ketoacidosis (DKA) in COVID-19 patients: a literature review. J Diabetes Metab Disord 2023;22:135-46. [Crossref] [PubMed]
- Oland GL, Hines OJ. New guidelines for the treatment of severe acute pancreatitis. Hepatobiliary Surg Nutr 2022;11:913-6. [Crossref] [PubMed]
Cite this article as: Zhu C, Wu H, Yang X, Gao J. The outcomes of COVID-19 and acute pancreatitis: a systematic review and meta-analysis. Transl Gastroenterol Hepatol 2024;9:6.