
The current state of gastrointestinal motility evaluation in cystic fibrosis: a comprehensive literature review
Introduction
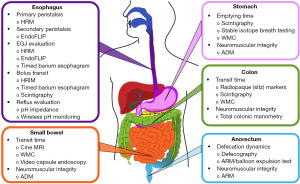
The gastrointestinal (GI) tract plays a fundamental role in overall health and well-being through the ability to digest food with subsequent nutrient absorption and excretion of waste products. To adequately perform its function and preserve the microbiome, the GI tract requires coordinated movement of luminal contents from the esophagus through the anorectum. The impact of GI dysmotility is far-reaching with a range of severity, and may have significant implications for patients with other chronic diseases, such as cystic fibrosis (CF), in which nutritional status is of utmost importance. CF, which is a multiorgan disorder secondary to mutation of the CF transmembrane conductance regulator (CFTR) gene, may present with a wide array of GI comorbidities spanning from gastroesophageal reflux disease (GERD) and chronic abdominal pain to small intestinal bacterial overgrowth (SIBO), distal intestinal obstruction syndrome (DIOS), and chronic constipation (1-3). Despite knowledge of these potential GI-related complications, the unifying theme of these conditions is an underlying predisposition to intestinal dysmotility, which remains largely understudied in patients with CF (pwCF). In the current article, we aim to provide a comprehensive review of diagnostic modalities at the disposal of the clinician in the evaluation of pwCF presenting with GI complaints (Figure 1). Furthermore, we aim to highlight the available literature regarding utilization of these modalities in CF, in addition to their shortcomings, and emphasize areas within the motility literature where further research is essential. We present this article in accordance with the Narrative Review reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-59/rc).
Methods
A comprehensive review of all available literature in the English language through December 1, 2022 utilizing PubMed was conducted. Our search was limited to GI motility/transit and dysmotility in pwCF. Two researchers independently screened references for applicable articles and extracted pertinent data. Table 1 includes further methodology details of how our search was conducted.
Table 1
| Items | Specification |
|---|---|
| Date of search | December 1, 2022 |
| Database searched | PubMed |
| Search terms | All terms were placed in the context of CF, including GI motility, GI dysmotility, GI tract, GI transit, GI comorbidities |
| Timeframe | All published articles up to December 1, 2022 |
| Inclusion criteria | Inclusion: articles that included pwCF and GI motility/dysmotility/transit testing or comorbidities |
| Selection process | Two researchers independently screened references for applicable articles |
CF, cystic fibrosis; GI, gastrointestinal; pwCF, patients with CF.
The significance of CF-related GI dysfunction
In the 1970s, the average pwCF in the United States had a median survival age of 11 years which is dramatically different from the current life expectancy of 48 years (4). This improvement in longevity is directly influenced by the rise of highly effective modulators, such as elexacaftor/tezacaftor/ivacaftor (ETI), which was demonstrated to provide significant improvements in lung pathology through the PROMISE Study (5,6). Extra-pulmonary comorbidities, including intestinal inflammation and bacterial overgrowth, have become more apparent as lifespan has increased, likely related to the systemic impact of CFTR mutation (7). The outcome of these undesirable sequelae is that the physiologic function of the digestive system including neuromuscular integrity and associated motility is vastly impacted, causing a range of disease processes that influence all sections of the GI tract from the esophagus through the anal canal. Prior work has indicated that the presence of certain GI symptoms may lead to worsening respiratory function and vice versa in the CF population (8). Interestingly, initiation of ETI has been shown to positively influence GI symptoms and reduce markers of intestinal inflammation; however, whether these improvements are secondary to improved regulation of GI motility remains to be studied (5,6,9).
Indeed, GI disease in CF has been identified as a key area of focus in the 2020–2024 strategic vision from the CF Foundation, and set as a top-ten priority by the James Lind Alliance Priority Setting Partnership. Furthermore, recent survey-based studies have demonstrated that a majority of pwCF suffer from some form of GI disease (3,10). With a shift in focus toward GI comorbidities in pwCF, the GALAXY Study Group was developed with a vision of developing standardized outcome measures to detect and address GI symptomatology (7). This prospective, longitudinal endeavor has employed several validated GI patient-reported outcome measures (PROMs) in clinical assessment of GI symptoms in pwCF (7). Similarly, alternative questionnaires such as the CF Abdomen Score, which is validated in pwCF, have been developed more recently to identify signs and symptoms impacting quality of life and clinical outcomes (11). Conversely, despite foundational encouragement and knowledge of concomitant GI comorbidities, there exists a paucity of literature evaluating CF-related GI motility and subsequent consequences of neuromuscular dysfunction. Accordingly, to further emphasize the current literature and its shortcomings, the ensuing sections are separated based on segment of the GI tract, with each including background and diagnostic testing options based on research involving pwCF.
Esophagus
Background
When considering esophageal dysmotility, emphasis is placed on adequacy of lower esophageal sphincter (LES) tone and peristaltic integrity of the esophageal body. The degree to which LES tone is abnormal may influence symptoms of outflow obstruction versus GERD. Inappropriate LES relaxation may be further altered by the presence of a hiatal hernia, and accentuate GERD symptoms such as heartburn, chest pain, and regurgitation. Unfortunately, the prevalence of GERD within pwCF is exceedingly high, with some studies suggesting rates 6–8 times higher than healthy counterparts (12). Furthermore, pwCF tend to have greater proximal reach of esophageal refluxate; however, the influence of transient lower esophageal sphincter relaxations (TLESRs) secondary to higher gastroesophageal pressure gradients in pwCF as a driver of GERD rates is debated (13,14). Moreover, the pathophysiologic mechanism of increased GERD burden in pwCF is likely multifactorial including lower basal LES tone, abnormal gastric emptying (GE), and intrathoracic variables (13,15). No matter the exact mechanism, it is important to note that the presence of gastroesophageal dysmotility in pwCF increases the risk of aspiration which can subsequently worsen pulmonary dysfunction (12). Detection and treatment of pathologic GERD is especially crucial in patients who have end-stage lung disease and are being considered for transplantation, with prior data suggesting elevated pre- and post-transplant prevalence of GERD in pwCF (16,17). Following transplantation, the importance of adequately managing GERD should be emphasized given the association between refluxate (both acidic and weakly acidic), microaspiration, and development of post-transplant complications such as acute rejection and small airway inflammation eventually leading to fibrosis and bronchiolitis obliterans (17-19). Nissen fundoplication in select pwCF has been shown to slow lung deterioration and improve quality of life in those with evidence of pathologic GERD (20,21).
Diagnostic motility evaluation
Providers should be familiar with the benefits and limitations of modalities for assessing pathologic GERD in pwCF. Multichannel intraluminal pH impedance (pH/MII) can detect proximal refluxate, distinguish the directionality and presence of both acidic and weakly-acidic (thought to be biliary in nature) reflux, and provide symptom correlation to reflux episodes (12). Impedance testing requires transnasal catheter placement 5 centimeters (cm) above the esophagogastric junction (EGJ), is typically inserted under local anesthesia in most adults versus general anesthesia with endoscopic guidance in a majority of pediatric patients, and can be monitored inpatient or outpatient depending on patient age and preference. Alternatively, wireless pH monitoring (Bravo), which involves endoscopic placement of the device against the esophageal wall 6cm above the EGJ, has the capability to measure acid refluxate episodes, provide symptom correlation, and allow for ambulatory monitoring. However, wireless pH testing is unable to identify proximal reflux that could be associated with micro-aspiration events, and cannot detect weakly acidic/biliary reflux that may play a role in progression of respiratory diseases including CF (22-27). While catheter-based monitoring is only performed over a 24-hour period, wireless pH monitoring can be performed up to 96 hours and allow for a more complete understanding of a patient’s reflux burden. Either study can be performed on or off acid suppression depending on the clinical question; however, an initial diagnosis of pathologic GERD as defined by the Lyon Consensus can only be determined off acid suppression (28). As of 2023, the Lyon version 2.0 provides an updated consensus of the diagnostic algorithm that should be followed, divided between typical and atypical symptoms, in order to objectively prove or reject the presence of pathologic GERD (29).
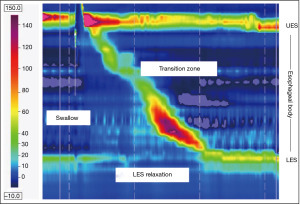
Moving beyond assessment of GERD, high-resolution esophageal manometry (HRM) remains the gold standard in evaluation of esophageal motility (Figure 2). Placement of the HRM catheter, which consists of 36 pressure transducers spaced 1 cm apart, can be completed transnasally with or without anesthesia depending on patient age and tolerance. The complete details of the protocol, including provocative maneuvers, are discussed elsewhere as part of the Chicago Classification (CC) [currently version 4.0] which was created utilizing adult norms (30,31). A diagnostic algorithm to classify measurement abnormalities on HRM between disorders of EGJ outflow (i.e., achalasia and EGJ outflow obstruction) and disorders of peristalsis (i.e., absent contractility, distal esophageal spasm, hypercontractile esophagus, and ineffective esophageal motility) is also provided in the most recent CC (30-32). There are currently no studies using HRM in pwCF. Beyond HRM, endoluminal functional luminal imaging probe (EndoFLIP) has been utilized to assess secondary esophageal peristalsis and EGJ distensibility (33). The catheter is placed transorally in an anesthetized patient, with indications ranging from evaluation of pre/post-surgical achalasia, EGJ outflow obstruction, esophageal stricture, and indeterminate HRM findings (31,34,35). Despite increasing popularity of this procedure, the literature of EndoFLIP utility in pwCF is nonexistent at this time. In areas where EndoFLIP is not readily available adjunctive esophageal testing with a timed upright barium esophagram (TBE), which utilizes barium suspension and/or tablet and includes left-posterior oblique radiographs at 1, 2 and 5 minutes post-consumption, may be useful in evaluation of transit and detection of EGJ outflow obstruction (36). A distinction should be made between TBE and a standard esophagram, the latter of which is used to evaluate anatomic abnormalities and has been found to be an inadequate diagnostic tool of esophageal dysmotility and GERD (36,37).
Except for circumstances where the impact of a medication on motility is being evaluated, in all manometric modalities discussed throughout this article medications that may influence GI motor function (i.e., anticholinergics, neuromodulators, prokinetics, opioids, antiemetics, cannabinoids) are routinely discontinued two days before testing if possible (30,38-40). Certainly, avoidance of specific medications may be difficult in pwCF; however, shared decision-making after discussion with the patient will facilitate a mutually agreed upon plan moving forward.
Stomach
Background
The stomach is crucial in the creation of chyme for further digestion and nutrient absorption in the small intestine through complex peristaltic actions and release of hydrochloric acid and proteases (41). Disturbances of gastric motility have the propensity to induce significant disruption in the lives of those affected, including symptom development (early satiety, nausea, vomiting, postprandial fullness, bloating, and abdominal discomfort), inability to tolerate oral medications with subsequent poor absorption, and the potential for nutritional compromise. Altered gastric motility in CF has been documented in the literature; however, the underlying mechanism as related to neuromuscular dysfunction is not well-defined but is likely multifactorial in etiology (38). GE in CF from the literature has been reported along the spectrum of too rapid, which has been suggested to normalize with appropriate pancreatic enzyme supplementation, to significantly delayed (14,22,25,42-46). A recent systematic review estimated that approximately one-third of pwCF had delayed GE, with a trend toward increasing frequency of gastroparesis (GP) as pwCF age (47). Vagal nerve injury, as reported in common operations involving pwCF such as lung transplantation and anti-reflux surgery, may influence acquired GP in this population. Once mechanical obstruction has been ruled out through radiologic studies and upper endoscopy, a diagnosis of GP can be made in a patient with characteristic symptoms as previously described and objective evidence of delayed GE (38,48,49).
Diagnostic motility evaluation
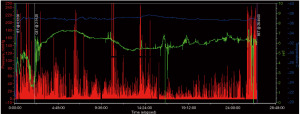
Several diagnostic tools are available for gastric motility evaluation which are further detailed below; however, scintigraphy is the current gold standard to assess GE. Radiolabeled meals to evaluate GE has been utilized since the 1960s, and is currently achieved with Technetium-99m (50). In 2008, consensus recommendations were created to standardize the protocol for measurement of GE by scintigraphy in which a low-fat solid-state meal consisting of egg whites mixed with the radiotracer is ingested (49,51). Following ingestion and baseline imaging, subsequent imaging is obtained hourly out to 4 hours which is based on several studies that demonstrated superiority in detection of delayed GE versus study cessation at 2 hours post-ingestion (49,51-55). Gastric retention of greater than 10% of the radiolabeled meal at 4 hours post-ingestion is suggestive of GP (49,51,52,54,56). An alternative test for GP in circumstances where scintigraphy cannot be performed is stable isotope (13C-spirulina or 13C-octanoic acid) breath testing, which includes ingestion of an isotope-labeled meal followed by periodic collection of exhaled air samples out to 4 hours (49,52,56). Once the study samples are collected, mass spectrometry is used to determine the ratio of exhaled 13-carbon dioxide and subsequently correlated to a GE rate (38,49,56-59). Given that GE breath testing is an indirect approximation of GE with reliance on gas exchange and small bowel absorption, this testing modality is inaccurate, and thus not recommended, in individuals with concomitant pulmonary disease, malabsorption, or pancreatic insufficiency secondary to inaccuracy (49,56-59). Beyond breath testing, the wireless motility capsule (WMC) was previously FDA approved for the evaluation of GE (Figure 3); however, has been recently discontinued from production. The current literature suggests diagnostic precision similar to the above-mentioned tests in the evaluation of GE; the abrupt change in pH as the WMC passes from the stomach to the small intestine is utilized to determine GE (40,60,61). Given that the WMC is a non-digestible solid, studies have shown that it typically exits the stomach with peristaltic waves elicited by the migratory motor complex (MMC), although in a minority of healthy individuals the capsule leaves the stomach through postprandial antral contractions (40,60-62). The MMC, which includes four phases (I–IV) and is active in the state of fasting between meals as the so-called “intestinal housekeeper”, has implications in dysmotility and subsequent development of small intestine bacterial overgrowth when dysregulated (62). Furthermore, previous studies have demonstrated that when nutritional status in CF is not compromised, MMC patterns are similar to healthy counterparts; however, regardless of nutritional state gastric mucosal IgA levels appear to be lower and duodenogastric bile acid reflux is elevated in comparison to healthy controls (24-27).
Beyond the modalities previously discussed, foregut motility can be assessed through antroduodenal manometry (ADM). High resolution sensors as utilized in esophageal manometry have been applied to ADM to facilitate evaluation of contraction coordination and pressure generation within the stomach and proximal small intestine (Figure 4). Several indications exist for the use of ADM, including evaluation of feeding intolerance, significant nausea and vomiting, rumination and retching, GP, and detection of pseudo-obstruction among others (40,52). Myopathic and neuropathic disease processes can further be subclassified based on the amplitude and pattern of contractions (40). Once the 36-pressure port catheter is positioned appropriately, typically placed transnasally under anesthesia in conjunction with upper endoscopy or through an existing gastrostomy stoma, the patient is allowed to recover overnight with testing the following day. Prior studies have demonstrated adverse influence on antroduodenal motility, as specifically related to the MMC, following the use of anesthesia (30,40). Once the patient has fully recovered from anesthesia, ADM evaluation can begin, typically lasting 6–8 hours to allow adequate observation of motility patterns in the fasting and fed states (30,40). No studies utilizing ADM in pwCF have been described in the literature to date. An alternative, non-invasive approach to evaluate myoelectrical activity of the stomach may be achieved through use of electrogastrography (EGG), which provides measurements on slow wave frequency, dominant frequency, and contraction power (38,63-65). Irregularities in EGG measurements in the pre/post-prandial states have implications on the integrity of gastric motility (38,63-65). In the available studies utilizing EGG in CF dysrhythmia was evident in a majority of patients, ranging from fasting and postprandial bradygastria (decreased frequency of normal slow waves pattern) to postprandial tachygastria (increased frequency beyond typical slow wave pattern), which has implications in gastric dysmotility (63,64).
Small bowel
Background
The small intestine plays a number of essential roles in the function of the GI tract, including mechanical digestion, propulsion of food along the GI tract, and absorption of nutrients such as iron, B9 (folic acid), and B12 (66). Thus, it is apparent that even minor alterations in small bowel function can have a large impact on a patient’s quality of life and nutritional status. With alteration of normal intestinal physiology, pwCF are predisposed to the development of several pathologic processes including SIBO, DIOS, and abnormal small bowel transit times (8,22). Bacterial overgrowth is quite prevalent in pwCF, reported in more than one-third of individuals, and presents with a variety of symptoms including diarrhea, bloating, abdominal discomfort, nutrient malabsorption, and increased flatulence (67-69). Specific to the CF-intestine, DIOS is thought to be more directly influenced by the CFTR mutation than other intestinal pathologies; however, some literature suggests non-genetic factors impacting DIOS predisposition such as dehydration, meconium ileus, and transplant surgery (70). Similar to SIBO, incidence of DIOS has also been found to be increased in patients with inadequate maintenance of pancreatic enzyme replacement therapy (PERT) (14). Under circumstances of increased intraluminal viscosity, fecal matter accumulated in the ileocecal region to cause obstruction with associated symptoms such as nausea, vomiting, and constipation or obstipation (71). Usual findings of DIOS on cross-sectional imaging include dilation of the small bowel with inspissated fecal content in the terminal ileum with a downstream decompressed colon and intestinal wall edema (72). Presence of DIOS within the adult CF population has been reported in up to one-fifth of patients (14). Consistent with the underlying mechanism that predisposes pwCF to small bowel disease, prior studies have found transit time to take up to twice as long compared to healthy controls (22,73). Furthermore, while GI transit is thought to hasten in the setting of steatorrhea, which is a common sequalae of inadequate small bowel fat absorption in pwCF, the effect on neuromuscular integrity and associated peristalsis remains unclear.
Diagnostic motility evaluation
Multiple diagnostic tools exist in the assessment of small bowel motility, including magnetic resonance imaging (MRI), WMC, and ADM. Alternatively, capsule endoscopy, which is typically employed for mucosal assessment of the small bowel, has the capability to quantify transit time; however, standard use in pwCF has not been reported. Additionally, an upper GI series with small bowel follow-through has been used to obtain a qualitative assessment of transit, with the added benefit of evaluating for any evidence of obstruction. Moreover, while small bowel aspiration is a direct test for SIBO, diagnosis is typically made with non-invasive hydrogen or methane breath testing (methane production has been shown to be increased in pwCF who have SIBO) (67,69,74,75). Similar to GE, however, breath testing within pwCF is controversial and may be of limited use given reliance on gas exchange and normal small bowel physiology (69). The process of evaluating the proximal small bowel using ADM is consistent with the indications and approach described above in relation gastric motility evaluation. Beyond ADM, WMC ingestion was previously employed to evaluate small bowel transit time (SBTT) (60,76,77). Utilizing the WMC, statistically significant differences in proximal small bowel pH and total SBTT were noted in pwCF compared to healthy controls (78). Interestingly, an extended time period was required to reach small bowel pH levels needed for PERT activation in pwCF (78). MRI has also emerged as a useful tool in evaluating small bowel function in pwCF, allowing insight into the integrity of intestinal mucosa and transit of intraluminal contents. Specifically, rapid succession (Cine) MRI scans to assess small bowel differences between pwCF and healthy controls in fasting and postprandial states has been reported (79). The study, which evaluated flow of chyme and “through-plane motion” within the small bowel, found that during fasting periods small bowel motility scores were over 50% lower in patients with CF suggesting slower transit compared to healthy controls (79). Additionally, a separate study from the Notthingham group found a statistically significant delay in oro-cecal transit time in pwCF (330 minutes) when compared to healthy controls (210 minutes) (80). Small bowel water content (SBWC) was also found to be increased on MRI in pwCF, suggesting decreased small bowel nutrient absorption as SBWC typically decreases postprandially secondary to water absorption driven by nutrient uptake (80,81).
Colon
Background
The large intestine is crucial for absorbing water and electrolytes, facilitating the production of and subsequent absorption of B and K vitamins, and moving stool towards expulsion from the rectum (82). Disruption of intraluminal content forward propulsion may be a significant driver of dysfunctional bowel habits such as constipation, and cause distressing symptoms, including bloating/flatulence and crampy abdominal pain. An estimated prevalence between 32–57% for constipation in pwCF has been reported throughout the literature (3,83-89). Although not completely understood, constipation in CF appears to be multifactorial with stool impaction greatly affected by secretion of thick mucous and predisposition of the CF intestine to dysmotility; however, the influence of pancreatic insufficiency and low total fat absorption is debated (8,89,90). Distinct from healthy patients, those with CF may pass daily bowel movements despite imaging with evidence of a large stool burden and/or fecal impaction (3,83-90).
Diagnostic motility evaluation
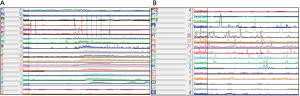
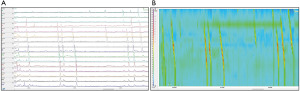
Several modalities exist in the evaluation of colonic transit, and include observation of intraluminal contents (i.e., radiopaque markers, radiolabeled isotopes, WMC) as they travel through the length of the colon; however, the validity of these tests in the CF population has not been well described. The main indication for testing is to determine whether ongoing constipation is secondary to generalized slow transit or if a particular segment of the large intestine isn’t functioning properly. Radiopaque (sitz) marker (ROM) studies have varying institutional protocols; however, involve ingestion of a specified marker quantity with interval abdominal X-rays to determine location (91,92). Based on location and interval time between X-rays, segmental and total colonic transit times can be extrapolated and have been standardized (90-94). In pediatric patients more than 3 years old, normal colonic transit times have been reported with no significant variation associated with sex or age (93,94). Furthermore, in young adult pwCF who were nutritionally optimized and receiving adequate pancreatic enzyme replacement therapy, comparison of total GI and segmental colonic transit times to healthy controls utilizing ROMs demonstrated no significant difference (95). A recent case series of pediatric pwCF highlighted prolonged total and right colon transit time using ROMs in those with comorbid constipation; however, transit time values were overall shortened compared to available literature on patients with functional constipation (90,96). Interestingly, regardless of constipation diagnosis almost two-thirds of pwCF were found to have fecal impaction, which has been previously reported in the literature on pwCF (85,90). Despite fecal impaction, there was no difference in transit time when compared to the subgroup of pwCF without evidence of impaction on imaging, suggesting pathophysiology unique from functional constipation where impaction appears to influence colonic motility (90,91). Similar to ROMs, scintigraphy has been utilized in the analysis of colonic motility and provides comparable measurement of total transit; however, segmental transit may be more easily delineated with scintigraphy (91,92,97-99). Furthermore, colonic scintigraphy involves ingestion of a insoluble radioisotope with interval measurement of the geometric center (i.e., fraction of radioisotope in each anatomic region of interest) and requires an experienced institution for reliable interpretation (91,92,97-100). With the involvement of specialized centers, scintigraphy has the ability to discern regional colon transit abnormalities that ultimately alter the course of management in pediatric patients (91,97,100,101). As described in the text above, WMC also has the ability to capture colonic transit measurements through pH changes (decline entering the cecum), temperature (decline exiting the rectum) and pressure recordings (lost exiting the rectum) (40,60,61,97,102). Transit with WMC has been validated against ROMs for constipated patients and demonstrates 87% agreement in detection of slow versus normal transit constipation (103). Although the above modalities are useful in determining colonic transit, evaluation of intrinsic colonic motor function is best achieved through colonic manometry (CM). Recent work has highlighted the paucity of literature utilizing CM in CF-related constipation (104). A full understanding of indications and protocol are described elsewhere; however, common reasons to perform CM includes refractory constipation despite optimal medical management, to evaluate Hirschsprung disease patients who remain symptomatic despite aganglionosis resection, and to evaluate function prior to surgical reconnection or creation of an anterograde enema option (40). Through CM, detection of high amplitude propagating contractions (HAPCs), as dictated by distance and pressure measurements, is sought as evidence of intact colonic neuromuscular function (Figure 5) (40,91,104). Forward propulsion of fecal matter is thought to occur secondary to HAPCs, which increase in frequency while awake and following meals but are diminished in slow transit constipation (40,91,97,105).
Anorectum
Background
Once fecal matter reaches the rectum, incoordination of the complex process of defecation (i.e., pelvic floor dyssynergia or outleft dysfunction) may further exacerbate constipation and contribute to undesirable complications such as rectal prolapse. In undiagnosed pwCF, early studies suggested that almost one-half had episodes of rectal prolapse preceding diagnosis; however, a recent longitudinal review estimated this value to be closer to 3% which is partly attributed to earlier detection from newborn screening and subsequent treatment optimization of CF comorbidities (106,107). Interestingly, there is some evidence suggesting significantly increased GI symptoms in those pwCF with history of rectal prolapse (3).
Diagnostic motility evaluation
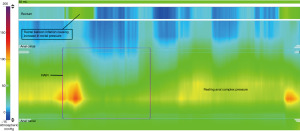
As stool leaves the sigmoid colon, the intricate interaction between the rectum and anal canal allows defecation to occur. Dysfunction in the process may exacerbate GI symptoms, including constipation, and can be further evaluated with defecography and/or anorectal manometry (ARM). Balloon expulsion testing may also be performed separately or in conjunction with the above-mentioned testing to evaluate for dyssynergia, in which a 50mL water-filled balloon is positioned in the rectum while the patient is sitting on a toilet and they are asked to subsequently pass the balloon in a set amount of time (108). Defecography, which may be performed with fluoroscopy or MRI while the patient is asked to expel instilled rectal contrast gel, provides real-time observation of the complex evacuation process including a functional assessment of pelvic floor muscle contractions (109-111). The most common indications for defecography include evaluation for outlet obstruction, which can be secondary to altered anatomy or pelvic floor dyssynergia, fecal incontinence, and rectal prolapse (109,111). Pelvic floor dyssynergia, also known as outlet dysfunction or dyssynergic defecation, is related to difficulty passing stools secondary to incoordination of the pelvic floor musculature (109,112). Beyond defecography, dyssynergia can be identified through ARM (39,112,113). The detailed protocol and interpretation of findings is published elsewhere; however, the utility of ARM ranges from detection of the rectoanal inhibitory reflex and disorders of rectal sensation to disorders of anal tone/contractility and anorectal coordination (Figure 6) (39,112,113). Similar to other manometric procedures, a high resolution solid-state catheter system is utilized in ARM; however, unlike other modalities an inflatable balloon is fastened to the catheter tip to allow for rectoanal inhibitory reflex and sensation to be assessed during the procedure. There are currently no studies evaluating the utility of defecography or ARM in pwCF or findings in comparison to healthy controls.
Motility testing difficulties and limitations
Throughout all segments of the GI tract, diagnostic imaging and manometry options exist in the evaluation of dysmotility; however, most assessment tools require a specialized center for optimal performance and results. A majority of the adjunctive imaging modalities available (i.e., X-ray, gamma camera scintillation, fluoroscopy) expose the patient to varying degrees of radiation, which is of particular importance in pwCF where cumulative radiation exposure is elevated proportional to longevity (114). Radiation exposure in the pediatric CF population is not trivial either, especially with the advancement in highly effective modulator therapies and their impact on long term outcomes and quality of life, and should be ordered with a targeted clinical question in mind. Beyond radiation, some patients may be unwilling or unable to participate in certain imaging tests based on ability to follow protocolized directions, anxiety surrounding the test, intolerance of the contrast carrying medium, or discomfort. Similar obstacles arise in the evaluation of neuromuscular integrity through manometry (Table 2), in which unsedated catheter placement may be poorly tolerated and ultimately require general anesthesia for proper positioning. Furthermore, anatomic variants or patient comorbidities may require the protocol to be individualized to ensure the best chance of manometric success. Nevertheless, catheter migration or dislodgement may occur and requires an experienced provider to recognize and appropriately make adjustments to enhance interpretation of findings. Anesthesia may influence manometric measurements, especially for antroduodenal and CM, and most centers defer evaluation to the following day to avoid uninterpretable results (30,39,115). While most esophageal and anorectal manometry studies can be performed on an outpatient basis, given the general anesthesia need for endoscopic catheter placement and potentially a preceding bowel cleanout, most patients can expect a 2–3-day inpatient stay if undergoing antroduodenal or CM. Moreover, even with an experienced provider, manometric interpretation may be challenging in the pediatric population given paucity of normative data and need for extrapolation from the robust adult literature. The clinical context should be considered when interpreting manometry results, as an atypical finding does not always correspond to the patient’s ongoing symptomatology. Similarly, these shortcomings exist in the CF population where minimal literature exists on normative data within the disease spectrum and in comparison to healthy controls with analogous GI comorbidities (Table 3).
Table 2
| Manometry | Indications | Limitations |
|---|---|---|
| High-resolution esophageal manometry | (I) Evaluation of EGJ obstructive pathology (i.e., achalasia, EGJOO) | (I) May require general anesthesia for placement if participant is unable to tolerate while awake |
| (II) To evaluate the association between symptoms and esophageal motor dysfunction | (II) Cooperation with study protocol may be difficult depending on age | |
| (III) Pre-surgical evaluation in patients undergoing foregut surgery | (III) Crying/coughing/laughing during the procedure can make interpretation difficult due to artifacts | |
| (IV) Evaluation of post-surgical esophageal outflow obstruction | (IV) Pediatric norms have not been established | |
| (V) Evaluation of rumination and retching when the clinical diagnosis is not clear | (V) Requires specialized providers for accurate interpretation | |
| (VI) Equipment can be costly | ||
| Antroduodenal manometry | (I) Feeding intolerance | (I) General anesthesia for catheter placement is typically required |
| (II) Persistent and chronic nausea, vomiting, and abdominal pain of unknown etiology | (II) Given that the manometry tracing may be influenced by anesthesia, inpatient admission is required to defer the study to the following day | |
| (III) Rumination and retching | (III) Catheter dislodgement/migration may occur | |
| (IV) Gastroparesis | (IV) Pediatric norms have not been established | |
| (V) Suspicion of pseudo-obstruction | (V) Requires specialized providers for accurate interpretation | |
| (VI) Equipment can be costly | ||
| Colonic manometry | (I) Refractory constipation despite optimal medical management | (I) Pre-procedural bowel cleanout is required |
| (II) Pre-surgical planning when considering antegrade enema placement, intestinal diversion, or bowel reconnection | (II) General anesthesia for catheter placement is typically required | |
| (III) Evaluation of post-surgical disordered defecation in patients with Hirschsprung disease or anorectal malformations | (III) Given that the manometry tracing may be influenced by anesthesia, inpatient admission is required to defer the study to the following day | |
| (IV) Pediatric norms have not been established | ||
| (V) Low repository of studies in healthy volunteers to develop valid norms in adults | ||
| (VI) Requires specialized providers for accurate interpretation | ||
| (VII) Equipment can be costly | ||
| Anorectal manometry | (I) Evaluation of the RAIR | (I) May be uncomfortable for some patients, limiting willingness to participate |
| (II) Evaluation of post-surgical disordered defecation in patients with Hirschsprung disease or anorectal malformations | (II) Cooperation with the study protocol may be difficult for some patients | |
| (III) Evaluation of dyssynergic defecation in constipated patients | (III) Crying/coughing/laughing during the procedure can make interpretation difficult | |
| (IV) Evaluation of rectal sensation and anal tone in patients with constipation and/or fecal incontinence | (IV) Requires specialized providers for accurate interpretation | |
| (V) Equipment can be costly |
Please note that manometric evaluation is not typically included in routine diagnostic testing for presenting symptomatology, and is usually utilized is cases of severe, refractory symptoms not amenable to conservative management. EGJ, esophagogastric junction; EGJOO, esophagogastric junction outflow obstruction; RAIR, recto-anal inhibitory reflex.
Table 3
| GI tract location | Author | Article year | Study type | Population | Modality | Variables | Results |
|---|---|---|---|---|---|---|---|
| Esophagus | Pauwels et al. (13) | 2012 | Case control | Adult | HRM-MII | Basal LES pressure, GEPG, TLESRs, total reflux, proximal extent of reflux | Lower basal LES pressure in pwCF; no difference in total TLESRs, but more frequent reflux in pwCF; higher proportion of reflux episodes with greater proximal reach in pwCF |
| Esophagus, stomach | Pauwels et al. (23) | 2011 | Case series | Adult | pH/MII, Bilitec, GEBT (13C-octanoic acid) | Bile acid, DGER, GE | One-third of pwCF had DGE; correlation noted between increased bile acid DGER and DGE |
| Esophagus, stomach | Hauser et al. (42) | 2016 | Case series | Pediatric | pH/MII, GEBT (13C-acetate/octanoic acid) | GER, GE | A majority of pwCF with GERD symptoms had abnormal acid exposure and 1/4 had delayed GE |
| Stomach | Kuo et al. (44) | 2011 | RCT | Adult | Scintigraphy | GE, blood glucose level, GLP-1 and GIP secretion | pwCF had inadequate GLP-1/GIP secretion and faster GE; GE normalized with PERT |
| Stomach | Collins et al. (43) | 1997 | Case control | Pediatric | Scintigraphy | GE | Faster solid-phase GE in pwCF |
| Stomach | Schäppi et al. (63) | 2004 | Case control | Children | EGG | Gastric rhythm, tachygastria, dominant frequency instability coefficient, power ratio | pwCF with higher percentage of post-prandial tachygastria, which is suggestive of stomach hypomotility |
| Stomach | Hallberg et al. (24) | 2001 | Case control | Adult | ADM | MMC phases, gastric IgA secretion | Normal MMC in pwCF, but decreased levels of gastric IgA secretion |
| Stomach | Hallberg et al. (25) | 2001 | Case control | Adult | ADM | MMC phases, gastric secretions, bilirubin reflux | Normal MMC and interdigestive acid/bicarbonate secretion in pwCF; lower total gastric secretion volume with increased bilirubin concentration in pwCF |
| Stomach | Nazareth et al. (45) | 2019 | Case control | Adult | US | GE | DGE regardless of meal type in pwCF |
| Stomach | Bodet-Milin et al. (46) | 2006 | Case control | Pediatric and adult | Scintigraphy | GE | DGE in 2/3 pwCF scheduled for lung transplant; Higher solid retention at hours 2 and 3 after surgery |
| Stomach | Bentur et al. (64) | 2006 | Case control | Pediatric and adult | EGG, scintigraphy | Gastric rhythm, GE | Dysrhythmia was found in a majority of pwCF with most EGG findings (normal versus abnormal) corresponding to scintigraphy results |
| Stomach, small bowel | Rovner et al. (73) | 2013 | Case control | Pediatric | Scintigraphy | GE, small bowel transit time | In pwCF there was normal DGE, but SBT was slowed |
| Small bowel | Gelfond et al. (78) | 2013 | Case control | Adult | WMC | Total GI transit time | Diminished SBT in pwCF |
| Small bowel | Dellschaft et al. (79) | 2022 | Case control | Pediatric and adult | Cine MRI | Small bowel motility, chyme flow | pwCF had diminished fasting small bowel motility scores and less defined texture change between small bowel and colon contents |
| Small bowel | Ng et al. (80) | 2021 | Case control | Pediatric and adult | Cine MRI | Oro-cecal transit time, small bowel water content | Longer oro-cecal transit time and higher small bowel water content in pwCF |
| Stomach, small bowel, colon | Hedsund et al. (95) | 2012 | Case control | Adult | ROM, MTS-1 | Total GI transit time, segmental colonic transit time, GE, SBT | No difference in GE, GITT, right or left SCTT in pwCF; Delayed overall SBT with faster upper SBT in pwCF |
| Colon | de Sillos et al. (90) | 2021 | Case series | Pediatric | ROM | Total colonic transit time, segmental right colon transit time, fecal impaction | Longer total and segmental colonic transit time was observed in constipated pwCF; There was no difference in transit time in those with or without fecal impaction |
GI, gastrointestinal; HRM, high-resolution manometry; MII, multichannel intraluminal impedance; LES, lower esophageal sphincter; GEPG, Gastroesophageal pressure gradient; TLESR, transient lower esophageal sphincter relaxation; pwCF, patients with cystic fibrosis; GEBT, gastric emptying breath test; DGER, duodenogastroesophageal reflux; GE, gastric emptying; DGE, delayed gastric emptying; pH/MII, multichannel intraluminal pH impedance; GLP-1, glucagon-like peptide-1; GIP, gastric inhibitory polypeptide; PERT, pancreatic enzyme replacement therapy; EGG, electrogastrography; ADM, antroduodenal manometry; MMC, migratory motor complex; US, ultrasound; SBT, small bowel transit; WMC, wireless motility capsule; MRI, magnetic resonance imaging; ROM, radiopaque marker; MTS-1, magnet-based motility tracking system; GITT, gastrointestinal transit time; SCTT, segmental colonic transit time.
The importance of standardized baseline protocol in motility testing
As evident throughout this review, the literature is scarce on defining a consistent path the clinician should follow when pursuing a diagnostic evaluation of GI motility in CF. A standardized protocol would ensure that each particular GI complaint within the CF population has the appropriate assessment in order to facilitate timely diagnoses and subsequent treatments. Further utility of such protocols would be in environments where a neurogastroenterologist is not readily available to allow the clinician to anticipate next steps in diagnostic workup before referring the patient elsewhere. Standardization is particularly important when one considers that dysmotility disorders heavily overlap in symptom characteristics with disorders of gut-brain interaction (DGBI). Noticeable examples include functional dyspepsia and GP, as well as slow transit constipation and irritable bowel syndrome. Beyond clinical practice, protocol standardization will provide the foundation on which research and field advancement in CF-related motility can be built. Certainly, baseline protocols will continue to be tailored to the individual patient to answer the clinical question in mind, but even these alterations offer meaningful insight into the utility of each modality within the CF population. Once diagnostic modalities for dysmotility in CF have been optimized, individualized treatment can be designed. Nevertheless, establishment of standardized guidelines will allow the clinician to efficiently address common GI complaints and preserve quality of life within the CF population.
Future direction
Despite advancement in our understanding of GI motility and the rise of high resolution manometry in diagnostic assessment, the applicability to CF remains largely unexplored. Although it is widely recognized that our focused efforts are required to address the rising number of GI comorbidities in CF, relevant literature on CF-specific norms and diagnostic utility for common GI complaints is lacking. Longitudinal cohort data, potentially included as part of the CF Foundation Patient Registry, would be invaluable in furthering our understanding of GI disease and motility disorders within CF. The GALAXY Study Group has begun to address the overlap in CF-related GI symptomatology; however, this could be further strengthened with the use of motility testing parameters that have attained CF-specific validation (116). Moreover, motility testing would ideally be studied in CF populations with and without GI complaints to understand whether evidence of abnormal motility exists in CF regardless of symptom presence. This understanding would likely influence the development of innovative, individualized diagnostic and therapeutic options for the CF population, and facilitate the creation of standardized protocols and objective diagnostic guidance to complement the current recommendations which are predominantly expert consensus driven. Furthermore, there would be an opportunity to better define and validate GI motility-related outcomes in the CF population, and how these outcomes ultimately influence improvements in GI symptoms and quality of life. Moreover, while pwCF have an organ-based/structural reason for chronic GI complaints (CFTR mutation), it remains unknown whether DGBI-specific interventions are applicable. It would be reasonable, using a standardized motility test protocol, to consider employing DGBI therapies (including neuromodulators, behavioral therapy, and complementary/alternative treatment) when motility results are normal or if symptoms appear disproportionate to motility findings. The application of DGBI diagnostic protocols, patient-reported outcome measures, and treatment in pwCF with refractory GI symptoms and normal/minimally abnormal motility testing should be further studied.
Conclusions
Several diagnostic imaging and manometry options exist in the evaluation of dysmotility; however, the literature is lacking in high-quality, prospective studies to validate such testing in CF despite the significant number of GI comorbidities that occur within this population. We envisage this literature review as a guide for the clinician in settings where a neurogastroenterologist is not readily available to initiate a diagnostic workup for pwCF presenting with GI complaints. Evaluation should be tailored to the patient’s symptoms, with risk-benefit profile in mind based on patient age and other comorbidities. Furthermore, this review may serve as a compliment to the recent publication by Henen et al. in order to further emphasize the discrepancy of research in CF-related intestinal motility, which has the potential to influence the development of new individualized diagnostic and therapeutic options in this population (104).
Acknowledgments
The authors would like to thank Jesús Bazán Villicaña in association with InPrint at Washington University in St. Louis for creation of the illustration in Figure 1.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-59/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-59/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-59/coif). C.H. reports funding from the CF Foundation evaluating colon cancer screening in CF with donation of stool kits from Exact Sciences and Polymedco, honoraria for lectures through the ACG, and support for attending North American CF Conference. C.H. is also an ACG Governor and committee member. D.P. reports consultation with Renexxion Pharmaceuticals and receiving a DIGEST grant from the CF Foundation. C.V. reports funding from the CF Foundation, participation in the CF Foundation Data Safety Monitoring Board, presentation at the 2022 North American Cystic Fibrosis Conference on Motility and Functional GI Disorders, and support to attend various CF Foundation-related meetings. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farrell PM, White TB, Ren CL, et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr 2017;181S:S4-S15.e1.
- Sabharwal S. Gastrointestinal Manifestations of Cystic Fibrosis. Gastroenterol Hepatol (N Y) 2016;12:43-7. [PubMed]
- Tabori H, Arnold C, Jaudszus A, et al. Abdominal symptoms in cystic fibrosis and their relation to genotype, history, clinical and laboratory findings. PLoS One 2017;12:e0174463. [Crossref] [PubMed]
- McBennett KA, Davis PB, Konstan MW. Increasing life expectancy in cystic fibrosis: Advances and challenges. Pediatr Pulmonol 2022;57:S5-S12. [Crossref] [PubMed]
- Nichols DP, Paynter AC, Heltshe SL, et al. Clinical Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor in People with Cystic Fibrosis: A Clinical Trial. Am J Respir Crit Care Med 2022;205:529-39. [Crossref] [PubMed]
- Nichols DP, Donaldson SH, Frederick CA, et al. PROMISE: Working with the CF community to understand emerging clinical and research needs for those treated with highly effective CFTR modulator therapy. J Cyst Fibros 2021;20:205-12. [Crossref] [PubMed]
- Freeman AJ, Sathe M, Aliaj E, et al. Designing the GALAXY study: Partnering with the cystic fibrosis community to optimize assessment of gastrointestinal symptoms. J Cyst Fibros 2021;20:598-604. [Crossref] [PubMed]
- Assis DN, Freedman SD. Gastrointestinal Disorders in Cystic Fibrosis. Clin Chest Med 2016;37:109-18. [Crossref] [PubMed]
- Schwarzenberg SJ, Vu PT, Skalland M, et al. Elexacaftor/tezacaftor/ivacaftor and gastrointestinal outcomes in cystic fibrosis: Report of promise-GI. J Cyst Fibros 2023;22:282-9. [Crossref] [PubMed]
- Smith S, Rowbotham N, Davies G, et al. How can we relieve gastrointestinal symptoms in people with cystic fibrosis? An international qualitative survey. BMJ Open Respir Res 2020;7:e000614. [Crossref] [PubMed]
- Jaudszus A, Pfeifer E, Lorenz M, et al. Abdominal Symptoms Assessed With the CFAbd-Score are Associated With Intestinal Inflammation in Patients With Cystic Fibrosis. J Pediatr Gastroenterol Nutr 2022;74:355-60. [Crossref] [PubMed]
- Mousa HM, Woodley FW. Gastroesophageal reflux in cystic fibrosis: current understandings of mechanisms and management. Curr Gastroenterol Rep 2012;14:226-35. [Crossref] [PubMed]
- Pauwels A, Blondeau K, Dupont LJ, et al. Mechanisms of increased gastroesophageal reflux in patients with cystic fibrosis. Am J Gastroenterol 2012;107:1346-53. [Crossref] [PubMed]
- Gabel ME, Galante GJ, Freedman SD. Gastrointestinal and Hepatobiliary Disease in Cystic Fibrosis. Semin Respir Crit Care Med 2019;40:825-41. [Crossref] [PubMed]
- Maqbool A, Pauwels A. Cystic Fibrosis and gastroesophageal reflux disease. J Cyst Fibros 2017;16:S2-S13. [Crossref] [PubMed]
- Button BM, Roberts S, Kotsimbos TC, et al. Gastroesophageal reflux (symptomatic and silent): a potentially significant problem in patients with cystic fibrosis before and after lung transplantation. J Heart Lung Transplant 2005;24:1522-9. [Crossref] [PubMed]
- Lo WK, Flanagan R, Sharma N, et al. Pre-Lung transplant reflux testing demonstrates high prevalence of gastroesophageal reflux in cystic fibrosis and reduces chronic rejection risk. World J Transplant 2023;13:138-46. [Crossref] [PubMed]
- Hathorn KE, Chan WW, Lo WK. Role of gastroesophageal reflux disease in lung transplantation. World J Transplant 2017;7:103-16. [Crossref] [PubMed]
- Robinson NB, DiMango E. Prevalence of gastroesophageal reflux in cystic fibrosis and implications for lung disease. Ann Am Thorac Soc 2014;11:964-8. [Crossref] [PubMed]
- Sheikh SI, Ryan-Wenger NA, McCoy KS. Outcomes of surgical management of severe GERD in patients with cystic fibrosis. Pediatr Pulmonol 2013;48:556-62. [Crossref] [PubMed]
- Robertson AG, Krishnan A, Ward C, et al. Anti-reflux surgery in lung transplant recipients: outcomes and effects on quality of life. Eur Respir J 2012;39:691-7. [Crossref] [PubMed]
- Sathe MN, Freeman AJ. Gastrointestinal, Pancreatic, and Hepatobiliary Manifestations of Cystic Fibrosis. Pediatr Clin North Am 2016;63:679-98. [Crossref] [PubMed]
- Pauwels A, Blondeau K, Mertens V, et al. Gastric emptying and different types of reflux in adult patients with cystic fibrosis. Aliment Pharmacol Ther 2011;34:799-807. [Crossref] [PubMed]
- Hallberg K, Mattsson-Rydberg A, Fändriks L, et al. Gastric IgA in cystic fibrosis in relation to the migrating motor complex. Scand J Gastroenterol 2001;36:843-8. [Crossref] [PubMed]
- Hallberg K, Abrahamsson H, Dalenbäck J, et al. Gastric secretion in cystic fibrosis in relation to the migrating motor complex. Scand J Gastroenterol 2001;36:121-7. [Crossref] [PubMed]
- Blondeau K, Dupont LJ, Mertens V, et al. Gastro-oesophageal reflux and aspiration of gastric contents in adult patients with cystic fibrosis. Gut 2008;57:1049-55. [Crossref] [PubMed]
- Pauwels A, Decraene A, Blondeau K, et al. Bile acids in sputum and increased airway inflammation in patients with cystic fibrosis. Chest 2012;141:1568-74. [Crossref] [PubMed]
- Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351-62. [Crossref] [PubMed]
- Gyawali CP, Yadlapati R, Fass R, et al. Update to the modern diagnosis of GERD: Lyon Consensus 2.0. Gut 2024;73:361-71. [Crossref] [PubMed]
- Rosen R, Garza JM, Tipnis N, et al. An ANMS-NASPGHAN consensus document on esophageal and antroduodenal manometry in children. Neurogastroenterol Motil 2018;30: [Crossref] [PubMed]
- Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0 Neurogastroenterol Motil 2021;33:e14058. [Crossref] [PubMed]
- Fox MR, Sweis R, Yadlapati R, et al. Chicago classification version 4.0(©) technical review: Update on standard high-resolution manometry protocol for the assessment of esophageal motility. Neurogastroenterol Motil 2021;33:e14120. [Crossref] [PubMed]
- Donnan EN, Pandolfino JE. EndoFLIP in the Esophagus: Assessing Sphincter Function, Wall Stiffness, and Motility to Guide Treatment. Gastroenterol Clin North Am 2020;49:427-35. [Crossref] [PubMed]
- Ata-Lawenko RM, Lee YY. Emerging Roles of the Endolumenal Functional Lumen Imaging Probe in Gastrointestinal Motility Disorders. J Neurogastroenterol Motil 2017;23:164-70. [Crossref] [PubMed]
- Ahuja NK, Agnihotri A, Lynch KL, et al. Esophageal distensibility measurement: impact on clinical management and procedure length. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Neyaz Z, Gupta M, Ghoshal UC. How to perform and interpret timed barium esophagogram. J Neurogastroenterol Motil 2013;19:251-6. [Crossref] [PubMed]
- O’Rourke AK, Lazar A, Murphy B, et al. Utility of Esophagram versus High-Resolution Manometry in the Detection of Esophageal Dysmotility. Otolaryngol Head Neck Surg 2016;154:888-91. [Crossref] [PubMed]
- Camilleri M, Kuo B, Nguyen L, et al. ACG Clinical Guideline: Gastroparesis. Am J Gastroenterol 2022;117:1197-220. [Crossref] [PubMed]
- Rodriguez L, Sood M, Di Lorenzo C, et al. An ANMS-NASPGHAN consensus document on anorectal and colonic manometry in children. Neurogastroenterol Motil 2017;29: [Crossref] [PubMed]
- Camilleri M, Bharucha AE, di Lorenzo C, et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil 2008;20:1269-82. [Crossref] [PubMed]
- Cheng LK, O’Grady G, Du P, et al. Gastrointestinal system. Wiley Interdiscip Rev Syst Biol Med 2010;2:65-79. [Crossref] [PubMed]
- Hauser B, De Schepper J, Malfroot A, et al. Gastric emptying and gastro-oesophageal reflux in children with cystic fibrosis. J Cyst Fibros 2016;15:540-7. [Crossref] [PubMed]
- Collins CE, Francis JL, Thomas P, et al. Gastric emptying time is faster in cystic fibrosis. J Pediatr Gastroenterol Nutr 1997;25:492-8. [Crossref] [PubMed]
- Kuo P, Stevens JE, Russo A, et al. Gastric emptying, incretin hormone secretion, and postprandial glycemia in cystic fibrosis--effects of pancreatic enzyme supplementation. J Clin Endocrinol Metab 2011;96:E851-5. [Crossref] [PubMed]
- Nazareth D, Mohan K, Fewins H, et al. Evaluation of Gastric Emptying in Cystic Fibrosis Using Bedside Ultrasonography. J Ultrasound Med 2019;38:2955-62. [Crossref] [PubMed]
- Bodet-Milin C, Querellou S, Oudoux A, et al. Delayed gastric emptying scintigraphy in cystic fibrosis patients before and after lung transplantation. J Heart Lung Transplant 2006;25:1077-83. [Crossref] [PubMed]
- Corral JE, Dye CW, Mascarenhas MR, et al. Is Gastroparesis Found More Frequently in Patients with Cystic Fibrosis? A Systematic Review. Scientifica (Cairo) 2016;2016:2918139. [Crossref] [PubMed]
- Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013;108:18-37; quiz 38. [Crossref] [PubMed]
- Usai-Satta P, Bellini M, Morelli O, et al. Gastroparesis: New insights into an old disease. World J Gastroenterol 2020;26:2333-48. [Crossref] [PubMed]
- Griffith GH, Owen GM, Kirkman S, et al. Measurement of rate of gastric emptying using chromium-51. Lancet 1966;1:1244-5. [Crossref] [PubMed]
- Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol 2008;103:753-63. [Crossref] [PubMed]
- Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers 2018;4:41. [Crossref] [PubMed]
- Ziessman HA, Bonta DV, Goetze S, et al. Experience with a simplified, standardized 4-hour gastric-emptying protocol. J Nucl Med 2007;48:568-72. [Crossref] [PubMed]
- Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 2000;95:1456-62. [Crossref] [PubMed]
- Guo JP, Maurer AH, Fisher RS, et al. Extending gastric emptying scintigraphy from two to four hours detects more patients with gastroparesis. Dig Dis Sci 2001;46:24-9. [Crossref] [PubMed]
- Szarka LA, Camilleri M. Evaluation of Patients with Suspected Gastroparesis. Gastrointest Endosc Clin N Am 2019;29:39-54. [Crossref] [PubMed]
- Lee JS, Camilleri M, Zinsmeister AR, et al. A valid, accurate, office based non-radioactive test for gastric emptying of solids. Gut 2000;46:768-73. [Crossref] [PubMed]
- Szarka LA, Camilleri M, Vella A, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol 2008;6:635-643.e1. [Crossref] [PubMed]
- Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 1993;104:1640-7. [Crossref] [PubMed]
- Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 2008;27:186-96. [Crossref] [PubMed]
- Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 2008;20:311-9. [Crossref] [PubMed]
- Deloose E, Janssen P, Depoortere I, et al. The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol 2012;9:271-85. [Crossref] [PubMed]
- Schäppi MG, Roulet M, Rochat T, et al. Electrogastrography reveals post-prandial gastric dysmotility in children with cystic fibrosis. J Pediatr Gastroenterol Nutr 2004;39:253-6. [Crossref] [PubMed]
- Bentur L, Hino B, Shamir R, et al. Impaired gastric myolectrical activity in patients with cystic fibrosis. J Cyst Fibros 2006;5:187-91. [Crossref] [PubMed]
- Chen JD, Richards RD, McCallum RW. Identification of gastric contractions from the cutaneous electrogastrogram. Am J Gastroenterol 1994;89:79-85. [PubMed]
- Fish EM, Burns B. Physiology, Small Bowel. Treasure Island (FL): StatPearls Publishing; 2023.
- Fridge JL, Conrad C, Gerson L, et al. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J Pediatr Gastroenterol Nutr 2007;44:212-8. [Crossref] [PubMed]
- Dorsey J, Gonska T. Bacterial overgrowth, dysbiosis, inflammation, and dysmotility in the Cystic Fibrosis intestine. J Cyst Fibros 2017;16:S14-23. [Crossref] [PubMed]
- Furnari M, De Alessandri A, Cresta F, et al. The role of small intestinal bacterial overgrowth in cystic fibrosis: a randomized case-controlled clinical trial with rifaximin. J Gastroenterol 2019;54:261-70. [Crossref] [PubMed]
- Green J, Gilchrist FJ, Carroll W. Interventions for preventing distal intestinal obstruction syndrome (DIOS) in cystic fibrosis. Cochrane Database Syst Rev 2018;6:CD012619. [Crossref] [PubMed]
- Sandy NS, Massabki LHP, Gonçalves AC, et al. Distal intestinal obstruction syndrome: a diagnostic and therapeutic challenge in cystic fibrosis. J Pediatr (Rio J) 2020;96:732-40. [Crossref] [PubMed]
- Nassenstein K, Schweiger B, Kamler M, et al. Distal intestinal obstruction syndrome in the early postoperative period after lung transplantation in a patient with cystic fibrosis: morphological findings on computed tomography. Gut 2005;54:1662-3. [Crossref] [PubMed]
- Rovner AJ, Schall JI, Mondick JT, et al. Delayed small bowel transit in children with cystic fibrosis and pancreatic insufficiency. J Pediatr Gastroenterol Nutr 2013;57:81-4. [Crossref] [PubMed]
- Lisowska A, Wójtowicz J, Walkowiak J. Small intestine bacterial overgrowth is frequent in cystic fibrosis: combined hydrogen and methane measurements are required for its detection. Acta Biochim Pol 2009;56:631-4. [PubMed]
- Shah SC, Day LW, Somsouk M, et al. Meta-analysis: antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2013;38:925-34. [Crossref] [PubMed]
- Farmer AD, Scott SM, Hobson AR. Gastrointestinal motility revisited: The wireless motility capsule. United European Gastroenterol J 2013;1:413-21. [Crossref] [PubMed]
- Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci 2009;54:2167-74. [Crossref] [PubMed]
- Gelfond D, Ma C, Semler J, et al. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci 2013;58:2275-81. [Crossref] [PubMed]
- Dellschaft NS, Ng C, Hoad C, et al. Magnetic resonance imaging of the gastrointestinal tract shows reduced small bowel motility and altered chyme in cystic fibrosis compared to controls. J Cyst Fibros 2022;21:502-5. [Crossref] [PubMed]
- Ng C, Dellschaft NS, Hoad CL, et al. Postprandial changes in gastrointestinal function and transit in cystic fibrosis assessed by Magnetic Resonance Imaging. J Cyst Fibros 2021;20:591-7. [Crossref] [PubMed]
- Marciani L, Cox EF, Hoad CL, et al. Postprandial changes in small bowel water content in healthy subjects and patients with irritable bowel syndrome. Gastroenterology 2010;138:469-77, 477.e1.
- Azzouz LL, Sharma S. Physiology, Large Intestine. Treasure Island (FL): StatPearls Publishing; 2023.
- Dos Santos ALM, de Melo Santos H, Nogueira MB, et al. Cystic Fibrosis: Clinical Phenotypes in Children and Adolescents. Pediatr Gastroenterol Hepatol Nutr 2018;21:306-14. [Crossref] [PubMed]
- Rubinstein S, Moss R, Lewiston N. Constipation and meconium ileus equivalent in patients with cystic fibrosis. Pediatrics 1986;78:473-9. [Crossref] [PubMed]
- van der Doef HP, Kokke FT, Beek FJ, et al. Constipation in pediatric cystic fibrosis patients: an underestimated medical condition. J Cyst Fibros 2010;9:59-63. [Crossref] [PubMed]
- Munck A, Alberti C, Colombo C, et al. International prospective study of distal intestinal obstruction syndrome in cystic fibrosis: Associated factors and outcome. J Cyst Fibros 2016;15:531-9. [Crossref] [PubMed]
- Gelfond D, Borowitz D. Gastrointestinal complications of cystic fibrosis. Clin Gastroenterol Hepatol 2013;11:333-42; quiz e30-1. [Crossref] [PubMed]
- Stefano MA, Poderoso RE, Mainz JG, et al. Prevalence of constipation in cystic fibrosis patients: a systematic review of observational studies. J Pediatr (Rio J) 2020;96:686-92. [Crossref] [PubMed]
- Baker SS, Borowitz D, Duffy L, et al. Pancreatic enzyme therapy and clinical outcomes in patients with cystic fibrosis. J Pediatr 2005;146:189-93. [Crossref] [PubMed]
- de Sillos MD, Chiba SM, Soares ACF, et al. Colonic Transit Time and Fecal Impaction in Children and Adolescents With Cystic Fibrosis-associated Constipation. J Pediatr Gastroenterol Nutr 2021;73:319-24. [Crossref] [PubMed]
- Vriesman MH, Koppen IJN, Camilleri M, et al. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol 2020;17:21-39. [Crossref] [PubMed]
- Solnes LB, Sheikhbahaei S, Ziessman HA. Nuclear Scintigraphy in Practice: Gastrointestinal Motility. AJR Am J Roentgenol 2018;211:260-6. [Crossref] [PubMed]
- Velde SV, Notebaert A, Meersschaut V, et al. Colon transit time in healthy children and adolescents. Int J Colorectal Dis 2013;28:1721-4. [Crossref] [PubMed]
- Arhan P, Devroede G, Jehannin B, et al. Segmental colonic transit time. Dis Colon Rectum 1981;24:625-9. [Crossref] [PubMed]
- Hedsund C, Gregersen T, Joensson IM, et al. Gastrointestinal transit times and motility in patients with cystic fibrosis. Scand J Gastroenterol 2012;47:920-6. [Crossref] [PubMed]
- Benninga MA, Büller HA, Tytgat GN, et al. Colonic transit time in constipated children: does pediatric slow-transit constipation exist? J Pediatr Gastroenterol Nutr 1996;23:241-51. [Crossref] [PubMed]
- Maurer AH. Gastrointestinal Motility, Part 2: Small-Bowel and Colon Transit. J Nucl Med 2015;56:1395-400. [PubMed]
- Southwell BR, Clarke MC, Sutcliffe J, et al. Colonic transit studies: normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatr Surg Int 2009;25:559-72. [Crossref] [PubMed]
- Lundin E, Graf W, Garske U, et al. Segmental colonic transit studies: comparison of a radiological and a scintigraphic method. Colorectal Dis 2007;9:344-51. [Crossref] [PubMed]
- Cook BJ, Lim E, Cook D, et al. Radionuclear transit to assess sites of delay in large bowel transit in children with chronic idiopathic constipation. J Pediatr Surg 2005;40:478-83. [Crossref] [PubMed]
- Medaer E, Hoffman I, Van Laere K, et al. Colon transit scintigraphy: high impact on clinical decision making in severely constipated children. J Nucl Med 2019;60:155.
- Stein E, Berger Z, Hutfless S, et al. Wireless Motility Capsule Versus Other Diagnostic Technologies for Evaluating Gastroparesis and Constipation: A Comparative Effectiveness Review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013.
- Camilleri M, Thorne NK, Ringel Y, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil 2010;22:874-82, e233.
- Henen S, Denton C, Teckman J, et al. Review of Gastrointestinal Motility in Cystic Fibrosis. J Cyst Fibros 2021;20:578-85. [Crossref] [PubMed]
- Bharucha AE. High amplitude propagated contractions. Neurogastroenterol Motil 2012;24:977-82. [Crossref] [PubMed]
- El-Chammas KI, Rumman N, Goh VL, et al. Rectal prolapse and cystic fibrosis. J Pediatr Gastroenterol Nutr 2015;60:110-2. [Crossref] [PubMed]
- Stern RC, Izant RJ Jr, Boat TF, et al. Treatment and prognosis of rectal prolapse in cystic fibrosis. Gastroenterology 1982;82:707-10. [Crossref] [PubMed]
- Lee BE, Kim GH. How to perform and interpret balloon expulsion test. J Neurogastroenterol Motil 2014;20:407-9. [Crossref] [PubMed]
- Patcharatrakul T, Rao SSC. Update on the Pathophysiology and Management of Anorectal Disorders. Gut Liver 2018;12:375-84. [Crossref] [PubMed]
- Kanmaniraja D, Arif-Tiwari H, Palmer SL, et al. MR defecography review. Abdom Radiol (NY) 2021;46:1334-50. [Crossref] [PubMed]
- Lalwani N, Khatri G, El Sayed RF, et al. MR defecography technique: recommendations of the society of abdominal radiology’s disease-focused panel on pelvic floor imaging. Abdom Radiol (NY) 2021;46:1351-61. [Crossref] [PubMed]
- Rao SSC, Tetangco EP. Anorectal Disorders: An Update. J Clin Gastroenterol 2020;54:606-13. [Crossref] [PubMed]
- Carrington EV, Heinrich H, Knowles CH, et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil 2020;32:e13679. [Crossref] [PubMed]
- O’Reilly R, Ryan S, Donoghue V, et al. Cumulative radiation exposure in children with cystic fibrosis. Ir Med J 2010;103:43-6. [PubMed]
- Arbizu RA, Nurko S, Heinz N, et al. Prospective evaluation of same day versus next day colon manometry results in children with medical refractory constipation. Neurogastroenterol Motil 2017;29: [Crossref] [PubMed]
- Moshiree B, Freeman AJ, Vu PT, et al. Multicenter prospective study showing a high gastrointestinal symptom burden in cystic fibrosis. J Cyst Fibros 2023;22:266-74. [Crossref] [PubMed]
Cite this article as: Davis TA, Miller A, Hachem C, Velez C, Patel D. The current state of gastrointestinal motility evaluation in cystic fibrosis: a comprehensive literature review. Transl Gastroenterol Hepatol 2024;9:10.


