
Cytoreductive surgery in patients with recurrent or metastatic gastrointestinal stromal tumors sensitive to imatinib: a retrospective analysis of two Russian cancer centers
Highlight box
Key findings
• Progression-free survival and overall survival were significantly longer for patients responding to imatinib who underwent metastasectomy additionally to targeted therapy.
What is known and what is new?
• Imatinib is a standard first-line targeted therapy of gastrointestinal stromal tumor.
• This study shows the role of cytoreductive surgery during imatinib therapy.
What is the implication, and what should change now?
• Further studies should investigate outcomes in patients treated with cytoreductive surgery.
• Hepatic or peritoneal metastasectomy should be taken into consideration while imatinib therapy is effective.
Introduction
For a long period of time surgery was the only effective treatment for metastatic gastrointestinal stromal tumors (mGISTs), but the overall survival (OS) was less than one year (1). Understanding the cancerogenesis of gastrointestinal stromal tumors (GISTs)—discovery of gain-of-function mutations KIT and PDGFRA oncogenes led to the introduction of imatinib (IM) into clinical practice.
IM is a standard first line therapy for patients with mGISTs. The efficacy of IM is approximately 70% while only 4–6% of patients achieve a complete response. The median progression-free survival (PFS) of patients on IM therapy is approximately two years. In 70–80% of initially IM-sensitive GISTs mutations may be acquired in other KIT and PDGFRA exons that make them resistant to IM (2-5). The current rationale for the use of surgery in IM-sensitive patients is avoidance of the possible appearance of resistant clones by removing residual disease.
Today the role of cytoreductive surgery for patients with mGISTs responding to IM has not yet been established because of the absence of randomized trials. Two phase III trials addressing this question were begun in Europe (EORTC 62063) and in China, but both failed to recruit patients quickly enough to meet the target accrual (6,7).
All the data that we have are from single-institution and multi-institutional retrospective studies that demonstrate an increase in PFS and OS for patients responding to IM who undergo metastasectomy.
Therefore, we conducted our retrospective study of surgery in patients with mGISTs responding to IM. In this article, we provide our own experience at two Russian cancer centers [N.N. Blokhin Russian Cancer Research Center and Moscow Clinical Scientific Center named after A. S. Loginov] where patients with GISTs are treated by multidisciplinary expert teams. We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-38/rc).
Methods
A total of 235 patients were histologically diagnosed with recurrent or metastatic GISTs from November 2002 to September 2021 in N.N. Blokhin Russian Cancer Research Center and Moscow Clinical Scientific Center named after A. S. Loginov. All 222 patients with mGISTs were treated with IM. Only patients who achieved a partial response (PR) or stable disease (SD) on IM treatment according to the Response Evaluation Criteria in Solid Tumors (RECIST1.1) were included in our retrospective analysis. Fourteen of 235 patients from were excluded because they had received surgery before starting IM treatment (primary resection, peritoneal mass resection, hepatic resection, radiofrequency ablation of hepatic metastases), 20 patients were excluded because they had undergone surgery for local progression, 25 patients were excluded for general progression and 35 patients were excluded with a less than 12-month response duration to IM treatment [the duration of IM therapy was more than one year was for Group NS (not treated with surgery)]. The main inclusion criterion in our study was one site of metastasis (liver or peritoneum); therefore, ninety-seven patients were also excluded because of more than one metastatic site. Two patients in Group S had two metastatic sites but we decided not to exclude them because they had undergone R0 resection.
Finally, 44 patients were included into the study, of whom 15 had been treated surgically after IM treatment (Group S) and 29 had received IM only (Group NS).
We compared the clinical outcomes of metastasectomy after IM treatment with IM treatment alone in patients with mGISTs. After cytoreductive surgery the patients continued IM therapy until disease progression. The aim of this study was to investigate the role of metastasectomy in patients with mGISTs responding to IM.
Molecular analysis
Paraffin-embedded tumor specimens were utilized for DNA extraction. Polymerase chain reaction and sequencing were used for investigation of somatic mutations in exons 9, 11, 13, and 17 of the KIT gene and exons 12 and 18 of the PDGFR.
Statistical analysis
Baseline characteristics were compared using the Wilcoxon rank test for continuous variables and Fisher’s test for categorical variables. PFS and OS were analyzed using the Kaplan-Meier method and compared using the log-rank test.
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics committees of two Cancer Centers (Moscow Clinical Scientific Center named after A. S. Loginov, Moscow, Russian Federation, IRB No. 457/2010; N.N. Blokhin Russian Cancer Research Center, Moscow, Russian Federation, IRB No. 775698/2010) and individual consent for this retrospective analysis was waived.
Results
Baseline characteristics
The baseline characteristics were well balanced between two groups (Table 1), and several trends were identified: more patients in Group NS had peritoneal metastases (45% vs. 27%, P=0.759), which can be explained by the fact, that peritoneal metastases are difficult to resect completely; a higher proportion in Group S achieved a PR (87% vs. 55%, P=0.165).
Table 1
| Baseline characteristics | Group S (N=15) | Group NS (N=29) | P value |
|---|---|---|---|
| Sex | 0.348 | ||
| Female | 10 (67%) | 15 (52%) | |
| Male | 5 (33%) | 14 (48%) | |
| Median of age (years) | 63 | 54 | |
| Primary tumor site | |||
| Stomach | 7 (47%) | 13 (45%) | 0.337 |
| Small bowel | 8 (53%) | 12 (41%) | |
| Colon | – | 2 (7%) | |
| Other | – | 2 (7%) | |
| Disease status | |||
| Metastatic de novo | 8 (53%) | 17 (59%) | 0.740 |
| Recurrent | 7 (47%) | 12 (41%) | |
| Metastatic organ | |||
| Liver | 9 (60%) | 16 (55%) | 0.246 |
| Peritoneum | 4 (27%) | 13 (45%) | 0.759 |
| Liver and peritoneum | 2 (13%) | – | – |
| Genotype of the primary tumor | |||
| KIT exon 11 mutation | 13 (86%) | 9 (31%) | |
| KIT exon 9 mutation | 1 (7%) | 2 (7%) | |
| Wild type | 1 (7%) | 5 (17%) | |
| Unknown | – | 13 (45%) | |
| Best response to IM | |||
| Complete response | – | 1 (3%) | |
| Partial response | 13 (87%) | 16 (55%) | 0.165 |
| Stable disease | 2 (13%) | 11 (38%) | |
| Not evaluated | – | 1 (3%) | |
Group S, treated with surgery group; Group NS, not treated with surgery group; IM, imatinib.
Complete macroscopic surgical resection was possible in the majority of cases (13 patients), and incomplete resection was possible in 2 patients (Table 2).
Table 2
| Description of surgery | Number |
|---|---|
| Outcome of surgery | |
| Complete resection R0 | 13 (87%) |
| Incomplete resection R2 | 2 (13%) |
| Type of intervention | |
| Peritoneal resection | 7 (46%) |
| Hepatic resection | 5 (33%) |
| Hepatic and peritoneal resection | 1 (7%) |
| Hepatic resection and primary tumor resection | 1 (7%) |
| RFA of hepatic metastases | 1 (7%) |
RFA, radiofrequency ablation.
Survival outcomes
Cutoff data was obtained in January 2022. The median PFS for Group S and NS was 78 and 35 months, respectively (P=0.088) (Figure 1A). The median time to surgery from the initiation of IM in Group S was 8 months (range, 3–23 months). There were 11 events in Group S and 23 events in Group NS. The median OS for Group S and NS was 141 and 80 months, respectively (P=0.154) (Figure 1B). There were 9 events in Group S group and 12 events in Group NS.
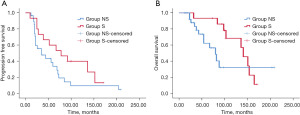
Cytoreductive surgery in IM-responding patients led to an increase in PFS of more than three years and OS of more than five years.
Discussion
IM is the most effective option in patients with mGISTs. After progression on IM, we have only two drugs: sunitinib and regorafenib (ripretinib and avapritinib are not approved in Russia). Meanwhile booth drugs have limited efficacy and high toxicity. PFS on sunitinib is only 6 months; on regorafenib 5 months (8,9). Surgery of residual disease in IM sensitive patients prevents secondary mutations as a result prolongs duration of IM and delays need to switch to sunitinib and regorafenib. Our data suggest that cytoreductive surgery in IM sensitive patients with mGISTs may improve the results of treatment. The median PFS in Group S in our study was 78 months, which was more than two times longer than that in Group NS (35 months). However, the PFS difference did not reach statistical significance (P=0.088), which that can be explained by the small number of patients included in our trial.
Our study has similar design with two retrospective trials—Park et al.’s study (10) and Rubió-Casadevall et al.’s study (11).
Park et al.’s study (10) included 92 patients in Group NS group, and 42 in Group S. The median PFS in Groups NS and S was 43 and 88 months, respectively (Р=0.001). Rubió-Casadevall et al. (11) published the results of treatment patients with mGISTs in 14 centers in Spain, 144 patients received IM only, and 27 patients received IM in combination with cytoreductive surgery. The median PFS was 45 months in Group NS group and 73 months in Group S group (Р=0.41). These results are consistent with the data in our study.
Two randomized phase III trials that began in Europe and China to evaluate the role of cytoreductive surgery for patients with mGISTs responding to IM has failed to recruit quickly, but Du et al. published the results of phase III trials that were conducted in China: the 2-year PFS was 88.4% in the surgery arm and 57.7% in the IM-alone arm (Р=0.089) (7). Du et al. concluded that while no significant differences were observed in two arms, the surgical removal of metastases may improve the results of treatment in patients with mGIST who have achieved tumor control with IM.
Fairweather et al. reported the largest series of patients with mGISTs treated with tyrosine kinase inhibitors and cytoreductive surgery, with the experience of two centers (Memorial Sloan Kettering Cancer Center, New York and Dana-Farber/Brigham and Women’s Cancer Center, Boston, MA) (12). As opposed to the trials that we mentioned above Fairweather et al. studied the influence of surgery not only on IM therapy but also sunitinib and regorafenib treatment. A total of 323 patients with mGISTs were enrolled in this trial, including 234 patients who underwent surgery for IM treatment. The median of PFS was 71 months in patients who responded to IM with surgery. Patients who underwent surgery while receiving IM had a significantly longer PFS and OS. Despite the small number of patients, other trials also demonstrated an improvement in clinical outcomes for PFS and OS. Gronchi et al. (13) published the results of 27 patients treated with surgery for stable or responsive disease: 4-year PFS was 72%; DeMatteo et al.—20 patients, 2-year PFS was 61% (14); Raut et al.—1-year PFS was 80% (15); Mussi et al.—49 patients, 2-year PFS was 64.4% (16).
Concerning the limitations of this study, the major problem with retrospective analysis is the bias in selecting patients. Many authors suggest that patients in the combination treatment group have a potentially better prognosis: they are more sensitive to IM, have a higher response rate, and have longer PFS. Trying to avoid this bias, we included patients in Group NS group with a favorable prognosis: duration of efficacy of IM therapy for more than 12 months and one metastatic site. In the results, the median PFS was 35 months in the Group NS group which is longer than that in randomized trials (EORTC trial median: PFS 20 months, S0033 18 months) (2,3,6).
Conclusions
Our study suggests that resection benefit patients with responsive mGISTs and prolong the median PFS from 35 to 78 months. Moreover, our data are consistent with the results of other trials. We believe that cytoreductive surgery increases PFS and OS and will continue to discuss surgical options with IM-responding patients.
Acknowledgments
The abstract was presented at ESMO 24th World Congress on Gastrointestinal Cancer 2022, Barcelona, Spain, 29 June-2 July 2022 and has been published in Annals of Oncology Volume 33 Supplement 4S239-S382.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-38/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-38/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-38/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-38/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics committees of two Cancer Centers (Moscow Clinical Scientific Center named after A. S. Loginov, Moscow, Russian Federation, IRB No. 457/2010; N.N. Blokhin Russian Cancer Research Center, Moscow, Russian Federation, IRB No. 775698/2010) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gold JS, van der Zwan SM, Gönen M, et al. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann Surg Oncol 2007;14:134-42. [Crossref] [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [Crossref] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Demetri GD, Rankin CJ, Benjamin RS, et al. Long-term disease control of advanced gastrointestinal stromal tumors (GIST) with imatinib (IM): 10-year outcomes from SWOG phase III intergroup trial S0033. J Clin Oncol 2014;32:10508. [Crossref]
- ClinicalTrials.gov Identifier: NCT00956072. Available online: https://clinicaltrials.gov/study/NCT00956072
- Du CY, Zhou Y, Song C, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer 2014;50:1772-8. [Crossref] [PubMed]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. [Crossref] [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [Crossref] [PubMed]
- Park SJ, Ryu MH, Ryoo BY, et al. The role of surgical resection following imatinib treatment in patients with recurrent or metastatic gastrointestinal stromal tumors: results of propensity score analyses. Ann Surg Oncol 2014;21:4211-7. [Crossref] [PubMed]
- Rubió-Casadevall J, Martinez-Trufero J, Garcia-Albeniz X, et al. Role of surgery in patients with recurrent, metastatic, or unresectable locally advanced gastrointestinal stromal tumors sensitive to imatinib: a retrospective analysis of the Spanish Group for Research on Sarcoma (GEIS). Ann Surg Oncol 2015;22:2948-57. [Crossref] [PubMed]
- Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated With Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann Surg 2018;268:296-302. [Crossref] [PubMed]
- Gronchi A, Fiore M, Miselli F, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 2007;245:341-6. [Crossref] [PubMed]
- DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg 2007;245:347-52. [Crossref] [PubMed]
- Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006;24:2325-31. [Crossref] [PubMed]
- Mussi C, Ronellenfitsch U, Jakob J, et al. Post-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients? Ann Oncol 2010;21:403-8. [Crossref] [PubMed]
Cite this article as: Filonenko D, Arkhiri P, Nikulin M, Sagaidak I, Yugai V, Zhukova L, Meshcheryakov A. Cytoreductive surgery in patients with recurrent or metastatic gastrointestinal stromal tumors sensitive to imatinib: a retrospective analysis of two Russian cancer centers. Transl Gastroenterol Hepatol 2024;9:7.


