Long-term impact of sarcopenia in older patients undergoing gastrectomy for gastric cancer: a systematic review and meta-analysis
Highlight box
Key findings
• Preoperative sarcopenia was associated with poor survival in older patients undergoing gastrectomy.
What is known and what is new?
• A high prevalence of sarcopenia was observed particularly in older patients. Previous studies have described the negative impact of sarcopenia on short outcomes of gastrectomy for gastric cancer; however, the impact of preoperative sarcopenia on long-term outcomes has not been fully investigated in older populations.
• This analysis provided an important notion that preoperative evaluation of sarcopenia is useful for predicting overall and disease-related survivals of older patients with gastric cancer.
What is the implication, and what should change now?
• In older patients who are intended to undergo gastrectomy, the screening of sarcopenic patients, preoperative nutritional and exercise interventions may be required to obtain better survival outcomes.
Introduction
Gastric cancer is one of the most prevalent tumors and the third leading cause of cancer-related mortality worldwide (1). Recent progress in surgical skills and perioperative management, represented by enhanced recovery after surgery, have decreased postoperative mortality after gastrectomy. However, treatment strategies are still troublesome for older patients because of comorbidities, performance dysfunction, frailty, and sarcopenia. In this aging society, appropriate interventions to these perioperative factors are required for further improvements in surgical curability; therefore, studies on prediction of postoperative outcomes in older patients have great value.
Sarcopenia is described as age-related, progressive, and generalized loss of skeletal muscle mass and strength (2) and has attracted much attention in recent years. A high prevalence of sarcopenia was observed particularly in older patients (3), which is not negligible in oncology especially, because its frequency increases by up to 35% in gastric cancer (4). Several studies have described the negative impact of sarcopenia on short outcomes (5); however, the impact of preoperative sarcopenia on long-term outcomes has not been fully investigated in older populations. Especially, not only overall survival (OS) but other disease-related survival, such as relapse free survival (RFS), disease-specific survival (DSS), and cancer-specific survival (CSS), should be investigated as long-term outcomes because sarcopenia in old age might be associated with non-cancer-related death.
To investigate whether preoperative sarcopenia influences the survival outcomes of older patients undergoing gastrectomy for gastric cancer, we performed a systematic review of the literature regarding the relationship between sarcopenia and survival in older patients. We present this article in accordance with the MOOSE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-34/rc) (6).
Methods
Search strategy
The protocol has been registered on PROSPERO (CRD42023418009).
We searched articles published from January 1998 to March 2023 in the MEDLINE (PubMed), Cochrane Central Register of Controlled Trials (Cochrane Library), Web of Science, and ClinicalTrials.gov databases for ongoing or unpublished trials on April 30, 2023. We retrieved medical subject headings (Mesh) and equivalent text word terms, such as “older”, “elderly”, “sarcopenia”, “muscle atrophy”, “frail”, “gastric cancer”, and “stomach neoplasms”.
Study selection
Two investigators (Terayama M and Ohashi M) reviewed the titles and abstracts of all identified studies and judged whether these studies were eligible for review. We included both retrospective and prospective cohort studies in which patients aged 65 years and older undergoing gastrectomy were categorized into frail or sarcopenic groups. Studies were excluded if survival outcomes were not examined. Abstracts, letters, editorials and expert opinions, reviews without original data, case reports, and studies lacking control groups were excluded. If multiple publications from the same study cohort were presented, the largest and most recently updated data sets were chosen. When a study was considered relevant, we reviewed the full article. The reference lists of all relevant articles were manually scrutinized to identify potentially relevant studies. Article language was limited to English.
Data extraction and quality assessment
Two reviewers (Terayama M and Ohashi M) independently reviewed all eligible studies and extracted all relevant data. Discrepancies between the two reviewers were resolved by discussion and consensus. The following data were extracted from each eligible study: targeted age, sex, stage, type of resection, number of patients, criteria or tools used for sarcopenia, prevalence of sarcopenia, survival, and postoperative complications. We contacted the authors when relevant data were lacking. The Newcastle-Ottawa Scale was used to quantify study quality, and those achieving six or more stars (out of nine) were considered higher quality (7).
Statistical analysis
The primary outcome of this analysis was to evaluate the association between sarcopenia and OS in older patients. The disease-related survival, such as RFS, DSS, and CSS and postoperative complications in older patients undergoing gastrectomy were secondary outcomes. Dichotomous variables were analyzed using estimation of odds ratios (ORs) with 95% confidence intervals (CIs), and hazard ratios (HRs) and 95% CIs were used to analyze postoperative OS. Heterogeneity across studies was evaluated by I2. We considered heterogeneity to be present if the I2 statistic was >50%. The fixed effect model was used for meta-analysis in cases of non-significant heterogeneity. If there was significant heterogeneity, the random model was used. These results were visualized with forest plot. Publication bias was assessed with a funnel plot. All statistical tests were two-sided, and P<0.05 was considered statistically significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study characteristics
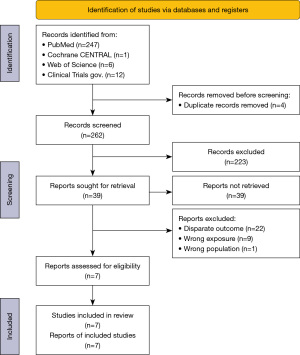
The search strategy initially identified 262 studies. After exclusion of irrelevant studies, 39 potentially relevant articles were obtained for assessment. We further excluded 32 studies because of disparate outcomes (n=22) and wrong exposure (n=9) and focused on other cancers (n=1). Finally, seven studies (four from Japan, two from China, and one from South Korea) were included (8-14). The PRISMA flowchart of literature assessment is shown in Figure 1.
The characteristics of seven studies are shown in Table 1. A total of 1,831 patients was included. Patients categorized as older patients were more than 65 years old in four studies (8,12-14), more than 75 years old in two studies (10,11), and more than 80 years old in one study (9). Four studies adopted the skeletal muscle index (SMI) for evaluating skeletal muscle mass according to the algorithm of the European Working Group on Sarcopenia in Older Persons (EWGSOP) (8,10-12). In the remaining three studies, psoas muscle index (PMI) (12), study of osteoporotic fractures (SOF) index (14), and clinical frailty scale (CFS) score (9) were used. The prevalence of sarcopenia ranged from 13.6% to 52.1%.
Table 1
| Study author, year | Type of study, country | Number of patients | Age, years | Sarcopenia criteria | Prevalence of sarcopenia, % | Survival outcomes (sarcopenia vs. non-sarcopenia), HR (95% CI) |
|---|---|---|---|---|---|---|
| Huang, 2016 (8) | Prospective, China | 173 | ≥65 | SMI | 30.1 | 1-year mortality: 3.62 (1.46–8.95) |
| Tanaka, 2019 (9) | Retrospective, Japan | 96 | ≥80 | CFS score | 17.1 | OS: 3.43 (1.43–8.22) |
| DSS: 4.00 (1.20–13.3) | ||||||
| Yamamoto, 2019 (10) | Retrospective, Japan | 90 | ≥75 | SMI | 21.2 | OS: 2.92 (1.10–7.75) |
| Taki, 2021 (11) | Retrospective, Japan | 257 | ≥75 | SMI | 52.1 | OS: 1.78 (1.12–2.83) |
| Watanabe, 2021 (12) | Retrospective, Japan | 242 | ≥65 | PMI | 13.6 | OS: 4.01 (1.75–9.22) |
| Non-CSS: 3.27 (1.61–6.67) | ||||||
| Chen, 2022 (13) | Prospective, China | 742 | ≥65 | SMI | 16.4 | OS: 1.08 (0.82–1.42) |
| DFS: 1.03 (0.78–1.35) | ||||||
| Jeong, 2022 (14) | Prospective, South Korea | 231 | ≥65 | SOF index | 15.2 | OS: 3.33 (1.16–9.55) |
HR, hazard ratio; CI, confidence interval; SMI, skeletal muscle index; CFS, clinical frailty scale; OS, overall survival; DSS, disease-specific survival; PMI, psoas muscle index; CSS, cancer-specific survival; DFS, disease-free survival; SOF, study of osteoporotic fractures.
Table 2 showed the characteristics of sarcopenic patients in the included studies. A total of 412 patients were diagnosed as sarcopenic. Patients with sarcopenia were relatively old and a high proportion had advanced tumor-node-metastasis (TNM) stage, with stage ≥II patients accounting for 76.1% of patients in a study by Chen et al. (13). All seven studies were methodologically sound, with no less than six stars.
Table 2
| Study author, year | Number of sarcopenic patients | Age, years | Sex, M/F | Stage, I/II/III/IV | Type of resection, TG/non-TG | Postoperative severe complications, n (%) | NOS score |
|---|---|---|---|---|---|---|---|
| Huang, 2016 (8) | 52 | 76 [69–83]† | 14/38 | 8/12/32/0 | 25/27 | N/A | 6 |
| Tanaka, 2019 (9) | 17 | 83 [80–92]† | 10/7 | 6/6/5/0 | 2/15 | 3 (17.6) | 8 |
| Yamamoto, 2019 (10) | 19 | 76 [67–83]† | 17/2 | N/A | 6/13 | 6 (31.6) | 7 |
| Taki, 2021 (11) | 134 | N/A | N/A | N/A | N/A | N/A | 8 |
| Watanabe, 2021 (12) | 33 | 77.6±5.2‡ | 17/16 | 18/6/9/0 | 5/28 | 4 (12.1) | 7 |
| Chen, 2022 (13) | 122 | 74 [65–83]† | 99/23 | 34/32/56/0 | 51/71 | 11 (9.0) | 8 |
| Jeong, 2022 (14) | 35 | 70.0±4.82‡ | 22/13 | 22/7/5/1 | 27/8 | N/A | 7 |
†, values are represented as median with range; ‡, values are represented as mean ± standard deviation. TG, total gastrectomy; NOS, Newcastle-Ottawa scale, N/A, not applicable.
Primary outcome
OS
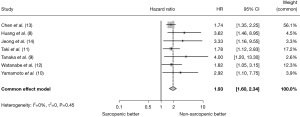
All included studies investigated OS after gastrectomy in sarcopenic and non-sarcopenic patients. In these studies, all the patients underwent R0 resection for gastric cancer. The meta-analysis results suggested that sarcopenic patients showed worse survival than non-sarcopenic patients (HR =1.93; 95% CI:1.60–2.34; P<0.001) (Figure 2).
Secondary outcomes
Disease-related survival
Three studies analyzed the relationship between preoperative sarcopenia and disease-related survival: DSS, DFS, or CSS, as described in each study. We extracted HRs for disease-related survival from these three studies involving 1,080 patients. Chen et al. revealed no association between preoperative sarcopenia and DFS (HR =1.03; 95% CI: 0.78–1.35; P=0.857) (13), while Watanabe et al. showed a significant correlation between preoperative sarcopenia and non-CSS (HR =3.27; 95% CI: 1.61–6.67; P=0.001) (12). In contrast, Tanaka et al. noted that patients with preoperative sarcopenia showed significantly worse DSS than non-sarcopenic patients (HR =4.00; 95% CI: 1.20–13.3; P=0.024) (9).
Postoperative complications
Four studies further analyzed the relationship between preoperative sarcopenia and postoperative severe complications (Clavien-Dindo classification ≥III). The analysis showed that sarcopenic patients experienced more severe complications than non-sarcopenic patients (OR =1.80; 95% CI: 1.10–2.95; P=0.019) (Figure 3).

Publication bias
Publication bias was not assessed because of the small number of included studies (fewer than 10).
Discussion
The present analysis clearly revealed that older gastric cancer patients with sarcopenia showed poorer survival than those without sarcopenia. Several systematic reviews and meta-analyses have reported the prognostic value of evaluating sarcopenia for postoperative complications and survival in patients undergoing gastrectomy (15-18). These reviews are for all generations; however, the impact of sarcopenia on postoperative outcomes should be completely different in clinical practice between older patients and the other generations because the physical dysfunctions caused by sarcopenia are more frequently observed in older patients. A review targeted to older patients is in demand by surgeons, but no such reviews have been found thus far. Although only one review suggested a relationship between sarcopenia and postoperative complications in older patients (19), to the best of our knowledge, this is the first review and meta-analysis to focus on the impact of sarcopenia in predicting long-term outcomes in older patients undergoing gastrectomy for gastric cancer. From our study, preoperative assessment of sarcopenia is required for older patients with gastric cancer to determine adequate treatment strategies and improve survival outcomes in patients undergoing gastrectomy.
Sarcopenia is an inevitable problem in older patients. Muscle strength declines by 3% after approximately 60 years, and 11–50% of older people aged around 80 years are affected by sarcopenia (20,21). In patients with malignant tumors, sarcopenia accelerates because of metabolic changes such as increased energy expenditure, excess catabolism, and chronic inflammation, with a prevalence of 39.6% in the curative setting and 49.2% in the palliative setting (4). In gastric cancer patients, sarcopenia significantly affected long-term outcomes in curative settings like other tumors (22), although the prognostic role of sarcopenia is limited in palliative settings (23). Older patients with gastric cancer are easy to fall into malnutrition because they often experience inadequate dietary intake, leading to poor protein synthesis and subsequent skeletal muscle loss. Additionally, the low skeletal muscle mass increases chemotherapeutic toxicity, decreases chemotherapeutic efficacy and reduces quality of life (24), which become obstacles to adjuvant chemotherapy administration. A rehabilitation program is also initiated after gastrectomy, but postoperative pain and decreased activity during hospitalization interrupt effective exercise. These specific situations in older patients after gastrectomy make it difficult to promote postoperative nutritional and exercise support for muscle maintenance. Moreover, because older patients have less functional and energy reserves, the more severe the preoperative sarcopenia is, the more difficult their functional recovery is. Thus, extracting preoperative sarcopenia is very important for older patients with gastric cancer. A recent study showed the possibility that preoperative exercise and a nutritional support program reduced sarcopenia in aged sarcopenic patients with gastric cancer (25).
The prevalence of preoperative sarcopenia ranged from 13.6% (14) to 52.1% (11) in the present review. This wide prevalent range of sarcopenia may be mainly due to the different diagnosed criteria used to assess muscle decline. In the present review, four criteria were used for the diagnosis of sarcopenia: SMI, PMI, CFS score, and SOF index. These indicators are divided into two types: CT-based objective methods (SMI and PMI) and person-based subjective methods (CFS score and SOF index). However, in terms of accuracy and reproducibility, SMI and PMI might be reliable indicators. The EWGSOP (26) and the Asian Working Group for Sarcopenia (AWGS) (27) recommend the use of SMI, although both indicators are reported to reflect the skeletal muscle mass of the whole body (28) and be associated with postoperative outcomes after gastrectomy (29). Thus, SMI is used worldwide and is a more informative indicator than PMI.
The negative impact of sarcopenia on postoperative outcomes has recently been debated. A recent meta-analysis of 7,843 patients with solid tumors from 38 studies concluded that sarcopenia was associated with poor OS (3). A systematic review conducted by Wagner et al. showed that frailty and sarcopenia predicted poor outcomes among patients undergoing gastrointestinal surgery (30). In gastrectomy, the association between sarcopenia and postoperative outcomes was noted in a systematic review by Yang et al., in which preoperative sarcopenia increased the risk of severe complications as well as poorer OS (31). Consistent with these previous studies, our analysis showed that preoperative sarcopenia was significantly associated with severe postoperative complications if limited to older patients. However, in this aging era, a strategy focused on older patients is required. Although only one meta-analysis by Shen et al. showed the negative impact of sarcopenia on short outcomes in older patients undergoing gastrectomy (19), there has been no systematic review focused on older patients reporting the predictive value of sarcopenia on long-term outcomes, not perioperative outcomes, after gastrectomy.
In our all-included studies, six studies reported that preoperative sarcopenia was independently associated with poor survival. Although the other study reported by Chen et al. showed no association between sarcopenia and survival outcomes (OS and DFS) (13), this difference might depend on their large population at a highly advanced stage. TNM stage is a well-known risk factor for worse survival and might mask the negative effect of sarcopenia on survival. A recent study focused on sarcopenic patients suggested that they have significantly poorer OS and DFS than non-sarcopenic patients at TNM stages II and III, but not stage I (32). As well as OS, two studies in our analysis by Tanaka et al. (9) and Watanabe et al. (12) revealed the adverse impact of sarcopenia on disease-related survival. This analysis indicated that preoperative sarcopenia in older patients was related not only to OS but also to disease-related survival.
There are possible explanations for the negative impact of sarcopenia on the survival outcomes of older gastric cancer patients. First, sarcopenic patients have impaired tolerance for adjuvant therapy after gastrectomy. Because malignancy is a cause of sarcopenia (18), sarcopenic patients are likely to have a more advanced TNM stage and require adjuvant chemotherapy. However, the induction and compliance rate of postoperative adjuvant chemotherapy after gastrectomy tends to be lower in sarcopenic patients (33). Consequently, in the included studies, a high recurrence rate was observed in the older sarcopenic group compared with the findings in the non-sarcopenic group, as shown by Tanaka et al. (sarcopenic group 23.5% vs. non-sarcopenic group 13.9%) (9). This poor locoregional control might lead to early death and worse DSS. Second, sarcopenia is also associated with non-cancer-related death and will harm the benefits of surgical curability. Under the condition of cancer cachexia, an involuntary loss of skeletal muscle mass is caused by simulating systemic inflammation and cytokine networks, which increase secondary comorbidities and lead to non-cancer-related death (34). For instance, postoperative pneumonia is a representative adverse impact of sarcopenia caused by impaired functioning of respiratory and swallowing muscles (19) and is a serious problem in older patients, leading to poor long-term survival (35). In this analysis, the association between preoperative sarcopenia and disease-related survival could not be conducted because of limited studies; therefore, further research is required.
There were several limitations of this analysis. All included studies were conducted in an Asian population, and thus the findings are not generalizable to non-Asian populations. Second, all included studies in this analysis were retrospective and uncontrolled, which might potentially have introduced selection bias. Third, all included studies missed information about adjuvant therapy. Chemotherapy data between sarcopenic and non-sarcopenic patients affect survival outcomes because the number of patients who received chemotherapy might be small because of old age. Fourth, sarcopenic patients were at more advanced TNM stages than non-sarcopenic patients, which might have affected the survival outcomes. Fifth, even though we applied random-effects models and performed subgroup analyses, the heterogeneity still could not be ignored. Sixth, we could not undertake analyses for DSS, CSS, and DFS because multiple studies were not conducted. Thus, further research about these outcomes is required. Finally, the various criteria used for muscle evaluation in each study may be an issue. Although each criterion is reliable, the heterogeneity of evaluation indicators might affect the outcomes.
Conclusions
In conclusion, this analysis provided an important notion that preoperative evaluation of sarcopenia is useful for predicting not only OS but disease-related survival. According to our analysis, the selection of sarcopenic patients and preoperative nutritional and exercise interventions are required for the better prognosis of older patients undergoing gastrectomy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-34/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-34/coif). MO serves as an unpaid editorial board member of Translational Gastroenterology and Hepatology from November 2022 to October 2024. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. [Crossref] [PubMed]
- Shachar SS, Williams GR, Muss HB, et al. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer 2016;57:58-67. [Crossref] [PubMed]
- Surov A, Wienke A. Prevalence of sarcopenia in patients with solid tumors: A meta-analysis based on 81,814 patients. JPEN J Parenter Enteral Nutr 2022;46:1761-8. [Crossref] [PubMed]
- Fukuda Y, Yamamoto K, Hirao M, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer 2016;19:986-93. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Huang DD, Chen XX, Chen XY, et al. Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: a prospective study. J Cancer Res Clin Oncol 2016;142:2347-56. [Crossref] [PubMed]
- Tanaka T, Suda K, Inaba K, et al. Impact of Frailty on Postoperative Outcomes for Laparoscopic Gastrectomy in Patients Older than 80 Years. Ann Surg Oncol 2019;26:4016-26. [Crossref] [PubMed]
- Yamamoto K, Hirao M, Nishikawa K, et al. Sarcopenia Is Associated With Impaired Overall Survival After Gastrectomy for Elderly Gastric Cancer. Anticancer Res 2019;39:4297-303. [Crossref] [PubMed]
- Taki Y, Sato S, Nakatani E, et al. Preoperative skeletal muscle index and visceral-to-subcutaneous fat area ratio are associated with long-term outcomes of elderly gastric cancer patients after gastrectomy. Langenbecks Arch Surg 2021;406:463-71. [Crossref] [PubMed]
- Watanabe J, Osaki T, Ueyama T, et al. The Combination of Preoperative Skeletal Muscle Quantity and Quality is an Important Indicator of Survival in Elderly Patients Undergoing Curative Gastrectomy for Gastric Cancer. World J Surg 2021;45:2868-77. [Crossref] [PubMed]
- Chen WZ, Zhang XZ, Zhang FM, et al. Coexistence of GLIM-defined malnutrition and sarcopenia have negative effect on the clinical outcomes in the elderly gastric cancer patients after radical gastrectomy. Front Nutr 2022;9:960670. [Crossref] [PubMed]
- Jeong JR, Choi JW, Ryu SY, et al. Relationship between frailty and mortality after gastrectomy in older patients with gastric cancer. J Geriatr Oncol 2022;13:67-73. [Crossref] [PubMed]
- Chen F, Chi J, Liu Y, et al. Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: A meta-analysis of cohort studies. Arch Gerontol Geriatr 2022;98:104534. [Crossref] [PubMed]
- Borggreve AS, den Boer RB, van Boxel GI, et al. The Predictive Value of Low Muscle Mass as Measured on CT Scans for Postoperative Complications and Mortality in Gastric Cancer Patients: A Systematic Review and Meta-Analysis. J Clin Med 2020;9:199. [Crossref] [PubMed]
- Rinninella E, Cintoni M, Raoul P, et al. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin Nutr 2020;39:2045-54. [Crossref] [PubMed]
- Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2019;22:10-22. [Crossref] [PubMed]
- Shen Y, Hao Q, Zhou J, et al. The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: a systematic review and meta-analysis. BMC Geriatr 2017;17:188. [Crossref] [PubMed]
- Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249-56. [Crossref] [PubMed]
- von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 2010;1:129-33. [Crossref] [PubMed]
- Surov A, Wienke A, Gutzmer R, et al. Time to include sarcopenia into the oncological routine. Eur J Cancer 2023;190:112939. [Crossref] [PubMed]
- Meyer HJ, Wienke A, Surov A. Sarcopenia as a Prognostic Marker for Survival in Gastric Cancer Patients Undergoing Palliative Chemotherapy. A Systematic Review and Meta Analysis. Nutr Cancer 2022;74:3518-26. [Crossref] [PubMed]
- Pin F, Couch ME, Bonetto A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr Opin Support Palliat Care 2018;12:420-6. [Crossref] [PubMed]
- Yamamoto K, Nagatsuma Y, Fukuda Y, et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 2017;20:913-8. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101. [Crossref] [PubMed]
- Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016;32:1200-5. [Crossref] [PubMed]
- Wakabayashi H, Matsushima M, Uwano R, et al. Skeletal muscle mass is associated with severe dysphagia in cancer patients. J Cachexia Sarcopenia Muscle 2015;6:351-7. [Crossref] [PubMed]
- Wagner D, DeMarco MM, Amini N, et al. Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg 2016;8:27-40. [Crossref] [PubMed]
- Yang Z, Zhou X, Ma B, et al. Predictive Value of Preoperative Sarcopenia in Patients with Gastric Cancer: a Meta-analysis and Systematic Review. J Gastrointest Surg 2018;22:1890-902. [Crossref] [PubMed]
- Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine (Baltimore) 2016;95:e3164. [Crossref] [PubMed]
- Fujihata S, Sakuramoto S, Morimoto Y, et al. Impact of loss of skeletal muscle mass within 6-12 months after gastrectomy and S1 adjuvant chemotherapy on the survival prognosis of elderly patients with gastric cancer. Surg Today 2022;52:1472-83. [Crossref] [PubMed]
- Webster JM, Kempen LJAP, Hardy RS, et al. Inflammation and Skeletal Muscle Wasting During Cachexia. Front Physiol 2020;11:597675. [Crossref] [PubMed]
- Suzuki S, Kanaji S, Matsuda Y, et al. Long-term impact of postoperative pneumonia after curative gastrectomy for elderly gastric cancer patients. Ann Gastroenterol Surg 2018;2:72-8. [Crossref] [PubMed]
Cite this article as: Terayama M, Ohashi M, Ri M, Makuuchi R, Hayami M, Ida S, Kumagai K, Sano T, Nunobe S. Long-term impact of sarcopenia in older patients undergoing gastrectomy for gastric cancer: a systematic review and meta-analysis. Transl Gastroenterol Hepatol 2023;8:35.