
Preoperative antibiotic prophylaxis in acute cholecystectomy: a systematic review and meta-analysis of randomised controlled trials
Highlight box
Key findings
• Preoperative prophylactic antibiotics for mild to moderate acute cholecystitis does not offer extra benefit to reduce infective complications during acute cholecystectomy.
What is known and what is new?
• Existing guidelines advocate the use of pre-operative antibiotic prophylaxis for acute cholecystitis.
• In random effect model, use of single does preoperative prophylactic antibiotics in patients undergoing acute cholecystectomy for mild to moderate cholecystitis fails to demonstrate any advantage of reducing the risk of infective complications.
What is the implication, and what should change now?
• This systematic review has shown that there is no benefit of using preoperative antibiotics in emergency laparoscopic cholecystectomy for grades 1 and 2 of acute cholecystitis.
Introduction
Acute cholecystitis is one of the most common presentations for emergency admission and surgery in any hospital. It is defined as acute inflammation of the gall bladder with or without underlying gallstones usually associated with a critical illness (1). It is diagnosed in about 200,000 individuals in the USA (1) every year. In the world, prevalence of gallstones is around 10–15% and 10–15% of these patients initially present with acute cholecystitis (2). There is also a significant cumulative mortality (0.9%) and morbidity (17.8%) associated with cholecystectomy (3). It is globally acceptable that acute cholecystitis should be managed with emergency cholecystectomy (4).
The use of preoperative antibiotics in cholecystitis has been a topic of debate for a long. There are existing guidelines advocating the use of pre-operative antibiotic prophylaxis for patients undergoing laparoscopic cholecystectomy (LC) in acute cholecystitis (5,6), but the evidence used in these guidelines seems to be inadequate because recently published randomized control trials (RCTs) are contradictory (7-9). Colling et al. have also recommended the use of perioperative antibiotic prophylaxis for patients undergoing LC for acute cholecystitis (10). The risk of developing post-operative infective complications after grades 1 and 2 acute cholecystitis is around 17% (11). The major pathogenesis thought to be behind this is thought to be due to contamination with the infected bile intra-operatively. In a normal individual biliary tree is supposed to be sterile (12). The outflow obstruction of the biliary tree leads to an inflammatory process which eventually is the cause hypothesized for bacterial colonisation. In acute cholecystitis, bile becomes colonized in about 35–60% of the patients (13). The most common microbes leading to this colonization are gram-negative bacteria and enterococci species (6,14).
Therefore, it is imperative to analyze the recently published data about the use of pre-operative antibiotic prophylaxis in patients undergoing acute cholecystectomy and evaluate the effectiveness of prophylactic antibiotics in reducing perioperative infective complications. The objective of this study is to evaluate the role of single-dose pre-operative prophylactic antibiotics in acute cholecystectomy for mild to moderate acute cholecystitis. We present this article in accordance with the PRISMA reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-48/rc).
Methods
Data sources and literature search technique
Literature exploration was systematically carried out from electronic databases like MEDLINE, EMBASE, PubMed and Cochrane Library using the MeSH search terms. Boolean operators (AND, OR, NOT) were used for protracted search results. The titles were carefully examined for study selection. Moreover, references from selected articles were analyzed to find any further relevant trials.
Trial selection
The inclusion criteria were the combined analysis of RCTs, reporting the effectiveness of preoperative antibiotic prophylaxis versus placebo for patients undergoing acute cholecystectomy.
Data collection and management
Reported data were collected from the included trials by independent researchers on a standard data extraction sheet. The collected dataset was matched and found to be in satisfactory inter-researcher agreement. The extracted data consisted of a list of the authors, title of the published study, journal of publication, country and year of the publication, testing sample size, the number of patients in each group of antibiotics and placebo and development of any postoperative infective complications. Researchers discussed the results following the data extraction and if a disagreement was reached, then mutual consensus was used for resolution.
Quality of analysis
The methodological quality of the included trials was initially assessed using the published guidelines of Jadad et al., Chalmers et al. and Rangel et al. (15-17). A comprehensive table for the assessment of quality among the included trials is given in Table 1.
Table 1
| Study | Randomization technique | Concealment | Blinding | Intention to treat analysis | Ethical approval | Registration number | Power calculation |
|---|---|---|---|---|---|---|---|
| Jaafar 2020 (7) | Manually | Sealed envelope | Double | Reported | Reported | NCT02619149 | Reported, power not achieved |
| Park 2023 (8) | Computer generated | Serial opaque sealed envelope | Double | Reported | Not reported | NCT04661371 | Reported, power achieved |
| van Braak 2022 (9) | Computer generated | Independent programmer | Not reported | Reported | Reported | NTR5802 | Reported, power achieved |
Statistical analysis
RevMan 5.4 (18) statistical analysis tool was used in this analysis (Review Manager 5.4, The Nordic Cochrane Centre, Copenhagen, Denmark). The random-effects model analysis was used for both the continuous and dichotomous variables and risk ratio (RR) with a confidence interval (CI) of 95% was used for the binary data analysis (19,20). A forest plot was used for calculating the heterogeneity and computing Chi2, significance was set at P<0.05 and the I2 test was used for identifying the heterogeneity, with a maximum value of 30% (21). Under the random effect model, RR was used for calculation as per the Mantel-Haenszel method (22). For the sensitivity analysis, in each cell frequency, 0.5 was added in the studies where no event occurred in either the treatment or control group, as per the guidelines recommended by Deeks et al. (23). In the event of unavailability of the standard deviation, Cochrane collaborations guidelines were used for the risk of bias calculation (19). In this event, it was assumed that variance was the same in both groups, which is not true in every case. In this case, variance was estimated either from the P value or range. Both techniques were used for pooling the estimate of difference, depending upon the effect weights in results determined by each trial estimate variance. Results were graphically displayed as a forest plot. The horizontal line represented the 95% CI and the square around the estimate stood for the accuracy of the estimation (sample size).
Endpoint
Post-operative occurrence or absence of infective complication was considered as the primary endpoint in this meta-analysis.
Results
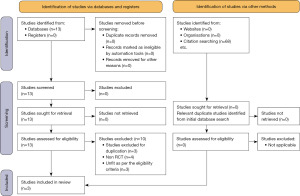
The initial database search generated 13 studies. After assessment of the studies for duplication, study type and inclusion criteria, 10 were excluded. Three RCTs were included in the final meta-analysis (Figure 1).
Characteristics and demographics of included studies
This meta-analysis is done in accordance with the guidelines provided by the Cochrane Collaboration, it includes three RCTs on 781 patients. A flow chart in accordance with the PRISMA guidelines is given in Figure 1. The included trials were conducted in Sweden (7), Korea (8) and The Netherlands (9). The demographic characteristics from the studies included are given in Table 2. A detailed review of the treatment protocol among the included studies is given in Table 3. The quality of the included trials is included in Table 1.
Table 2
| Study | Country | Antibiotic group | No antibiotic group | Follow-up (days) | |||||
|---|---|---|---|---|---|---|---|---|---|
| N value | Men (%) | Age (years) | N value | Men (%) | Age (years) | ||||
| Jaafar 2020 (7) | Sweden | 42 | 18.0 | 48.5 (median) | 48 | 23.0 | 49 (median) | 30 | |
| Park 2023 (8) | Korea | 116 | 47.4 | 50.9±15.28 (mean ± standard deviation) | 118 | 36.4 | 52.2±13.64 (mean ± standard deviation) | 30 | |
| van Braak 2022 (9) | The Netherlands | 226 | 47.3 | 58 (median) | 231 | 50.6 | 57.5 (median) | 30 | |
Table 3
| Study | Cholecystitis grade (Tokyo guidelines) | Antibiotics/dose | N value (antibiotic group) | No antibiotic | N value (no antibiotic group) | Time between surgery and antibiotic dose | Single dose | Type of cholecystectomy |
|---|---|---|---|---|---|---|---|---|
| Jaafar 2020 (7) | Grades 1 & 2 | Piperacillin-tazobactam/4,000 mg | 42 | Nil | 48 | 1–72 h | 79% | Laparoscopic/converted/open |
| Park 2023 (8) | Grades 1 & 2 | First generation cephalosporin/1,000 mg | 116 | 10 mL saline | 118 | Within 24 h | All | 3 or 4 port laparoscopic |
| van Braak 2022 (9) | Grades 1 & 2 | First generation cephalosporin/2,000 mg | 226 | Nil | 231 | 15–30 min | All | 4 port laparoscopic |
Outcome of the primary variable
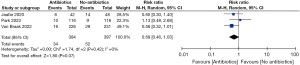
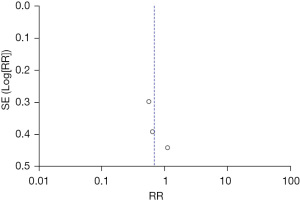
There were 384 patients in single dose pre-operative antibiotics group whereas 397 patients were recruited in the no-antibiotics group. In the random effects model analysis, the use of single-dose preoperative prophylactic antibiotics in patients undergoing acute cholecystectomy for mild to moderate cholecystitis failed to demonstrate any advantage of reducing the risk of [RR =0.69; 95% CI: 0.46–1.03; Z=1.80; P=0.07] infective complications. There was no heterogeneity [Tau2 =0; Chi2 =1.74, df =2 (P=0.42; I2=0%)] (Figure 2) among included studies. Also, the similarity of the included studies is presented as a funnel plot (Figure 3).
Discussion
Key findings
The debate on using preoperative antibiotic prophylaxis for acute cholecystitis has been a topic of discussion for a very long time. Different antibiotics have been used by surgeons to minimize the risk of post-operative infective complications. In this systematic review 781 patients were studied from 3 RCTs, 384 patients in single dose pre-operative antibiotics group and 397 patients were recruited in the no antibiotic or placebo group. A preoperative single dose of prophylactic antibiotics in patients undergoing emergency LC for mild to moderate acute cholecystitis does not offer the benefit of reducing post-operative infective complications.
Comparison with existing literature
In the literature review, there have not been any published systematic reviews on this topic for comparison. There have been multiple systematic reviews comparing the efficacy of preoperative antibiotic prophylaxis for elective LC. There have been studies favouring the use of preoperative antibiotic prophylaxis for elective LC (24,25) while there have been systematic reviews showing no added benefits of preoperative antibiotic prophylaxis (26). Two RCTs used in the review showed no significant benefit of antibiotic use in the prevention of post-operative infective complications in grades 1 and 2 of acute cholecystitis (7,8). One RCT concluded that it is not possible to select a subset of patients based on pre- and post-operative characteristics that will benefit from antibiotic prophylaxis (9).
Strength and limitations
Two of the RCTs used computer-generated randomization (8,9) and all three and the intention to treat analysis were given. There was double blinding in two RCTs (7,8) and concealment with sealed envelopes was also used in them. Therefore, the three RCTs which were used in this meta-analysis were of solid strength. Also, there was no heterogeneity among the trials used in this systematic review.
The primary limitation of this systematic review was the lack of evidence for the use of pre-operative antibiotics in moderate and severe acute cholecystitis (as per Tokyo guidelines) (6). Also, the lack of the presence of manual randomization (7) and the presence of independent programmer-generated concealment (9) also limits the RCTs used in this meta-analysis. Another limitation of this systematic review is paucity of the RCTs and the number of patients. This can be overcome in the future by conducting a major multicentre RCT with adequate power calculation.
Implications
This systematic review has shown that there is no benefit of using preoperative antibiotics in grades 1 and 2 of acute cholecystitis. Nonetheless, the use of pre-operative antibiotics can be limited to exceptional cases as per the surgeon’s judgment, especially in the case of grades 1 and 2 of acute cholecystitis.
Conclusions
A preoperative single dose of prophylactic antibiotics in patients undergoing acute cholecystectomy for mild to moderate acute cholecystitis does not offer extra benefits to reduce infective complications. A further major multicentric RCT is needed to confirm the findings of this systematic review.
Acknowledgments
The provisional abstract of this systematic review has been presented at the annual conference of “The Association of Surgeons of Great Britain and Ireland” on 17th–19th May 2023 at Harrogate, United Kingdom.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-48/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-48/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gallaher JR, Charles A. Acute Cholecystitis: A Review. JAMA 2022;327:965-75. [Crossref] [PubMed]
- Pisano M, Allievi N, Gurusamy K, et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J Emerg Surg 2020;15:61. [Crossref] [PubMed]
- Papi C, Catarci M, D'Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol 2004;99:147-55. [Crossref] [PubMed]
- Coccolini F, Solaini L, Binda C, et al. Laparoscopic Cholecystectomy in Acute Cholecystitis: Refining the Best Surgical Timing Through Network Meta-Analysis of Randomized Trials. Surg Laparosc Endosc Percutan Tech 2022;32:755-63. [Crossref] [PubMed]
- Mazuski JE, Tessier JM, May AK, et al. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg Infect (Larchmt) 2017;18:1-76. [Crossref] [PubMed]
- Gomi H, Solomkin JS, Schlossberg D, et al. Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 2018;25:3-16. [Crossref] [PubMed]
- Jaafar G, Sandblom G, Lundell L, et al. Antibiotic prophylaxis in acute cholecystectomy revisited: results of a double-blind randomised controlled trial. Langenbecks Arch Surg 2020;405:1201-7. [Crossref] [PubMed]
- Park SE, Choi HJ, You YK, et al. Clinical significance of preoperative antibiotic use in mild to moderate acute inflammatory gallbladder disease: A randomized controlled trial. J Hepatobiliary Pancreat Sci 2023;30:482-92. [Crossref] [PubMed]
- van Braak WG, Ponten JEH, Loozen CS, et al. Antibiotic prophylaxis for acute cholecystectomy: PEANUTS II multicentre randomized non-inferiority clinical trial. Br J Surg 2022;109:267-73. [Crossref] [PubMed]
- Colling KP, Besshoff KE, Forrester JD, et al. Surgical Infection Society Guidelines for Antibiotic Use in Patients Undergoing Cholecystectomy for Gallbladder Disease. Surg Infect (Larchmt) 2022;23:339-50. [Crossref] [PubMed]
- Regimbeau JM, Fuks D, Pautrat K, et al. Effect of postoperative antibiotic administration on postoperative infection following cholecystectomy for acute calculous cholecystitis: a randomized clinical trial. JAMA 2014;312:145-54. [Crossref] [PubMed]
- Csendes A, Burdiles P, Maluenda F, et al. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg 1996;131:389-94. [Crossref] [PubMed]
- Nitzan O, Brodsky Y, Edelstein H, et al. Microbiologic Data in Acute Cholecystitis: Ten Years' Experience from Bile Cultures Obtained during Percutaneous Cholecystostomy. Surg Infect (Larchmt) 2017;18:345-9. [Crossref] [PubMed]
- Armiñanzas C, Tigera T, Ferrer D, et al. Role of bacteriobilia in postoperative complications. Rev Esp Quimioter 2016;29:123-9. [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Chalmers TC, Smith H Jr, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49. [Crossref] [PubMed]
- Rangel SJ, Kelsey J, Colby CE, et al. Development of a quality assessment scale for retrospective clinical studies in pediatric surgery. J Pediatr Surg 2003;38:390-6. [Crossref] [PubMed]
- Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341-50. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Matthias E, Davey SG, Altman DG. editors. Systematic reviews in health care: meta-analysis in context. 2nd edition. London: BMJ Publishing Group; 2001:487.
- Deeks JJ, Altman DG, Bradburn MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta-Analysis. In: Egger M, Smith GD, Altman DG. editors. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: John Wiley & Sons, Ltd.; 2001:285-312.
- Matsui Y, Satoi S, Hirooka S, et al. Reappraisal of previously reported meta-analyses on antibiotic prophylaxis for low-risk laparoscopic cholecystectomy: an overview of systematic reviews. BMJ Open 2018;8:e016666. [Crossref] [PubMed]
- Liang B, Dai M, Zou Z. Safety and efficacy of antibiotic prophylaxis in patients undergoing elective laparoscopic cholecystectomy: A systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:921-8. [Crossref] [PubMed]
- Zhou H, Zhang J, Wang Q, et al. Meta-analysis: Antibiotic prophylaxis in elective laparoscopic cholecystectomy. Aliment Pharmacol Ther 2009;29:1086-95. [Crossref] [PubMed]
Cite this article as: Singh A, Kaur M, Swaminathan C, Subramanian A, Singh KK, Sajid MS. Preoperative antibiotic prophylaxis in acute cholecystectomy: a systematic review and meta-analysis of randomised controlled trials. Transl Gastroenterol Hepatol 2023;8:37.


