
Therapeutic advancement in inflammatory bowel disease by incorporating plant-based diet
Introduction
The incidence of inflammatory bowel disease (IBD), ulcerative colitis (UC) and Crohn’s disease (CD), is associated with dietary transition from a traditional diet to the westernized diet (1). Prospective cohort studies and case-control studies indicate that the current diet is a risk factor for IBD (2-7). The current diet tends to cause gut microbial dysbiosis resulting in a pro-inflammatory state (8-14). A nutritionally balanced current diet is not able to maintain C-reactive protein (CRP) within the reference range in CD (15-18). Despite these problems, there has been no recommended diet for IBD. We assert that IBD occurs in susceptible individuals mainly due to our omnivorous (westernized) diet (19,20). Therefore, we developed and began to provide a plant-based diet (PBD), a lacto-ovo-vegetarian diet, to counter an omnivorous (westernized) diet for IBD patients in 2003 (17). We achieved far better outcomes in both UC and CD in both the induction and quiescent phases compared to the current standard therapy. We started recommending PBD for IBD since 2019 (21-23).
We have been routinely providing PBD to patients with IBD for almost 20 years. The objective of this review article is to describe the rationale for and outcomes of PBD in IBD.
Historical background of PBD for IBD
Not all but the majority of IBD physicians and gastroenterologists do not appreciate the critical role of diet in IBD. The reason for this would be the education system in medicine. Educational institutions tend to teach and study areas in the forefront in medicine. PBD was designed in 2003 (17). Therefore, it would be worthy to describe the historical background of PBD in IBD. Topics of research in IBD were like those in medicine. For the last half century, they were microbiology to identify microbial agent(s) in IBD, immunology to elucidate the mechanism of inflammation, and genetics to study susceptibility to diseases (24,25). Nowadays, multi-omics, artificial intelligence, and COVID-19 infection seem to be those among the topics in the forefront.
Resumption of hospital meals was the start of relapse in CD
Full-blown symptoms of CD such as frequent diarrhea, abdominal pain, high fever, and anorexia subsided in 1 week to 10 days of total parenteral nutrition (TPN). CRP, a sensitive biomarker of inflammation, normalized. Gradual replacement of TPN with meals was undertaken and the patient was subsequently discharged. CRP, however, gradually increased above the reference range, leading to a relapse despite adherence to the recommended balanced diet (15). This occurred in most patients with CD, while it was not so in patients with UC. Therefore, both patients with CD and gastroenterologists thought that meals per se were related to relapse (15-17). This experience led to use of elemental diet (ED) in CD. In Japan, ED was used as standard induction and maintenance therapy for adult CD patients in the pre-biologic era (26). Nowadays, it is used in selective CD patients. ED is commonly used in pediatric CD patients in Europe. Pediatricians face a similar problem of replacement of ED with meals (27) as replacement of TPN with meals. These observations indicated that an adequate diet that does not induce relapse is critically needed in CD. Therefore, an adequate diet for IBD should be screened in CD.
Research on etiology of IBD
Studies attempting to identify a sole microbial agent in IBD were ultimately unsuccessful (28,29). Immunology looks at IBD in terms of therapeutic effects of glucocorticoids and immunosuppressants. Immunology characterized a variety of pathways of inflammation that resulted in development of biologics and small molecules widely used now for IBD. Genetics identified susceptible genes in IBD. They are related to innate and acquired immunity, autophagy, as well as epithelial barrier (30). It has been calculated that disease heritability is the cause in 16% of UC patients and 23% of CD patients (30), stressing the importance of an environmental factor. The gut harbors a profuse number of microbes, and the gut is the largest lymphoid tissue. Gut microbiota maintains homeostasis of the gut milieus including nutrition, immune system, and defense against various insults. Gut microbial dysbiosis, i.e., imbalance of microbiota, was found among diseases including not only intestinal diseases but also common chronic diseases (8-14). Gut microbiota were previously thought to be fairly stable among individuals, but nowadays it is thought to be changeable due to our diet. Therefore, therapy targeting gut microbiota from the trajectory of diet and probiotics/prebiotics/synbiotics has been investigated.
Epidemiology
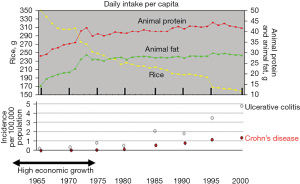
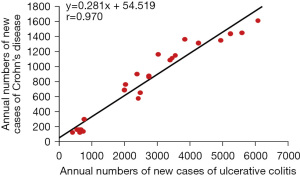
The incidence of IBD increases in immigrants who immigrate from low-IBD-prevalence (developing) countries to a high-prevalence (developed) country (31,32). This phenomenon suggests that an environmental factor(s) in developed societies contributes to the onset of IBD. This concept has been widely accepted in IBD. Economic transition induces nutritional transition (Figure 1) (33). Epidemiology of IBD in Japan showed that the incidence of IBD increased along with national economic transition (Figure 2) (1). There was a high correlation between the annual numbers of new cases of UC and CD, indicating the presence of a common environmental factor in both diseases (Figure 3) (1). IBD was common in wealthy nations, but it has occurred more frequently worldwide since the end of the last century, making it a global disease (34). The food industry became able to provide cheap food in developing countries (35). In modern society, mortality due to infectious diseases subsides, while common chronic diseases predominate due to the nutrition transition to the Western diet (35).
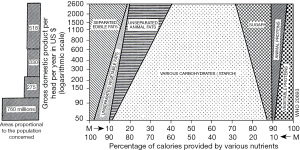
Environmental factor
Lifestyles in wealthy nations have been extensively studied (36). Representative risk and preventive factors for IBD are presented in Table 1 (20). In addition to those listed in Table 1, the hygiene hypothesis and stress were studied.
Table 1
| Environmental factor | Role in IBD | Exposure to the majority of IBD patients | Relevance to gut microbiota | ||
|---|---|---|---|---|---|
| UC | CD | Mode of role | |||
| Lifestyle | |||||
| Smoking | P | R | Divergent in IBD | No | Yes |
| Diet | Yes | Yes | |||
| Animal protein | R | R | Identical in IBD | Yes | Yes |
| Dietary fiber | N | P | – | Yes | Yes |
| Tea or coffee | P | P | Identical in IBD | Yes | Unknown |
| Low levels of vitamin D | R | R | Identical in IBD | No | Unknown |
| Breast feeding | P | P | Identical in IBD | No | Yes |
| Pharmacological agents | |||||
| NSAID | R | R | Identical in IBD | No | Unknown |
| Antibiotics in childhood | Divergent among ethnic groups | No | Yes | ||
| Oral contraceptives | R | R | Identical in IBD | No | Unknown |
| Dipeptidyl peptidase-4 inhibitors | R | N | – | No | Unknown |
| Vaccination | N | N | – | Yes | Unknown |
| Appendectomy | P | R | Divergent in IBD | No | Unknown |
| Air pollution | R | R | Identical in IBD | No | Unknown |
IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; P, protective factor; R, risk factor; N, neither protective nor risk factor; NSAID, non-steroidal anti-inflammatory drug.
These lead to the present definition of IBD. Namely, the etiology of IBD is unknown, but it occurs in a susceptible person who is triggered by an environmental factor(s) (24,25). Advances in the concept of IBD, the diagnosis, and its treatment have resulted in improvement in its clinical course and prognosis. IBD physicians and IBD researchers, however, are not able to answer dietary research questions published in 2017 (37). They are not able to answer the most common question asked by patients: “What should I eat?” (38,39).
Our current (westernized) diet is the most ubiquitous environmental factor in IBD
The biggest problem in current IBD treatment is the lack of a widely appreciated ubiquitous environmental factor in IBD. Therefore, even though some lifestyles in modern society are known to be a risk factor, no real factor has been highlighted. Gut inflammation does not occur in the absence of gut microbiota (19). Epidemiologic studies have shown that IBD increased along with the dietary transition both in developed and developing countries (1,33,35). Cohort and case-control studies, in general, showed that a westernized diet was a risk factor for IBD (2-7). Ultra-processed foods in westernized diets contain food additives (emulsifiers, thickeners, sweeteners), pesticides, and persistent organic pollutants. It has been recently reported that they increase the risk of IBD (40). Meats decrease beneficial bacteria (17). The ubiquitous environmental factor for IBD should be a risk factor for both CD and UC, should be a risk factor irrespective of geographic areas, should induce gut dysbiosis, and should be a factor to which patients with IBD are exposed. A westernized diet (high in animal fat, animal protein, and sugar, and low in carbohydrates and dietary fiber) satisfies these conditions for the ubiquitous environmental factor for IBD. Therefore, we asserted that a westernized diet was the ubiquitous environmental factor in IBD (Figure 4) (Table 1) (20).
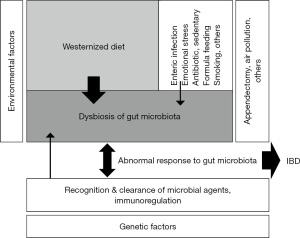
Development of PBD for IBD
Since a PBD (low in animal fat, animal protein, and sugar, and high in carbohydrates and dietary fiber) was thought to increase beneficial bacteria, PBD was designed to replace the westernized diet. PBD is a lacto-ovo-vegetarian diet. Fish and meat are served at about a half the average amount once a week and every 2 weeks, respectively. The details of PBD were previously described (17).
Results of therapeutic modality incorporating PBD in IBD
PBD is fairly different from contemporary diets. One medical doctor followed PBD based on our article, but the doctor could not achieve remission and visited us (41). We treated all newly diagnosed patients on in inpatient basis. For patients previously diagnosed with IBD, we recommended admission once to experience PBD irrespective of relapse or remission.
Efficacy of therapy must be adequately evaluated. Because disease activity decreases over time in both UC (42-45) and CD (46), and we need to know the long-term clinical course, the therapeutic effect should be evaluated for inceptive cases. Remission and response to treatment are different: the former refers to entering a quiescent phase, while the latter needs another modality to induce a remission. Clinical remission in our previous studies was defined in both diseases as the disappearance of active symptoms assessed by a clinician (17,47-51). Relapse in both diseases was defined as a change in the clinical status of the patient that required more aggressive treatment (42,43,52). There are only a few studies available in the literature on the therapeutic effect in inceptive cases. A comparison of outcomes with our modality versus those of others in the literature is given below.
Results of therapeutic modality incorporating PBD in UC
Educational hospitalization for mild UC (48)
The symptoms of mild UC are mild enough to not disrupt daily activities. Therefore, about 2-week hospital stay at a convenient time during school/company seasonal holidays was recommended for patients with mild UC. Sixty patients underwent educational hospitalization. The first author explained the followings using written information: the dietary factor in the pathogenesis of IBD, a patient’s hitherto dietary habits based on a questionnaire, future recommended dietary habits, PBD, and healthy habits (53). In 40 cases with symptoms or an abnormal fecal immunochemical test, about three-fourths (77%) experienced some improvement during hospitalization: disappearance or decrease in the amount of bloody stool, normalization or improvement in fecal immunochemical test. We already published case reports that remission of UC can be induced with PBD without medication (41,54). We have added four patients who achieved remission with PBD without medication during educational hospitalization. Relapse of UC is most common in the first 2 years after onset (42,43). There are only several reports available to compare relapse rates of initial episode cases in the last few decades. The relapse rate at 1-year follow-up in initial episode case after educational hospitalization was 4% (Figure 5) (21,42,43,48,49,55).
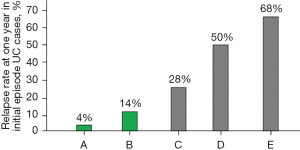
The explanation and educational materials provided during educational hospitalization were also provided to all the following inpatients with IBD.
UC treated with medication according to guidelines (49)
PBD together with medication prescribed according to UC guidelines (56) was provided during hospitalization. Ninety-two cases were studied (51 initial episode cases, 41 relapse cases; 31 mild, 48 moderate, 13 severe). The 13 severe cases were encountered before 2010 and were treated with prednisolone, not infliximab. Seventeen cases of severe UC treated with infliximab after 2010 are described below (50). The relapse rate at 1-year follow-up in initial episode cases after discharge was 14% (Figure 5). Nine of 31 mild cases in this study achieved remission without medication. This experience with four previous cases in educational hospitalization (48) convinced us that remission can be induced with PBD without medication in a considerable portion of mild cases, probably more than a third. This led to a stepwise treatment with PBD and medication for patients with mild UC (57).
Severe UC (50)
Severe UC is encountered in 10–25% of UC cases and is life-threatening with a 1% mortality rate (58). The first choice for severe UC is intravenous corticosteroid therapy. Infliximab or cyclosporine is used for non-responders to corticosteroids (16% to 34% of patients) (59-61) as rescue treatment (62). When infliximab is effective, scheduled infliximab therapy is followed (62). In the literature, remission rates in patients with moderate to severe UC naïve to infliximab, adalimumab, golimumab, and vedolizumab are 38.8%, 49%, 43.9%, and 19.2% respectively (63-67). Oh et al. (68) reported remission rates of severe UC treated with infliximab as either first-line or rescue treatment. They were 26.1% at 3 months and 45.5% at 1 year. Colectomy rates of severe UC with rescue infliximab in the induction phase, at 3 months, and at 1 year according to a meta-analysis were 11%, 16%, and 26%, respectively (62). In another recent report, the colectomy rate was at 20% in the induction phase (69). The colectomy rate at 3 months and 1 year with infliximab rescue therapy was 14.3% and 20.4%, respectively (70).
Even though induction is successful with glucocorticoid, steroid dependence or colectomy occurs in a half of such patients at 1 year (71). Once steroid hormone is used, its use lasts more than a few months, and careful observation is needed for a variety of steroid hormone side effects. Therefore, we replaced infliximab and PBD as first-line (IPF) therapy with glucocorticoid therapy for severe UC (50). In the induction phase, the remission rate was 76% (13/17) and the colectomy rate was 6% (1/17). The remission rate at 3 months and 1 year was 93% and 75%, respectively. There was no increase in colectomy rate at 3 months and 1 year. Our outcomes clearly surpass current standards. It seems that incorporation of PBD at the initiation of therapy together with infliximab contributed to the better outcomes. No increase in colectomy rate at follow-up may be explained by the improved dietary habits shown in the following (Table 2). In cases in which efficacy was insufficient to induce remission with IPF therapy, we showed that successive corticosteroid therapy induced remission (72).
Table 2
| Subject | Short term | Long term | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Base PBDS | Follow-up (months) | PBDS | P value | N | Base PBDS | Follow-up (months) | PBDS | P value | ||
| Ulcerative colitis | |||||||||||
| Mild cases underwent educational hospitalization (48) | 23 | 7.3±9.7 | 14.0 (12.0–22.0) | 21.6±10.6 | <0.0001 | 16 | 12.3±8.9 | 46.5 (39.0–54.0) | 18.7±9.8 | 0.034 | |
| Patients treated by guideline (49) | 41 | 12.0±8.7 | 12.0 (10.5–24.0) | 29.3±7.6 | <0.0001 | 41 | 11.7±8.8 | 76.5 (43.8–110.8) | 19.4±9.0 | <0.0001 | |
| Severe cases (50) | 10 | 8.8±8.3 | 12.5 (9.3–19.5) | 27.7±7.0 | <0.0001 | 8 | 9.9±8.4 | 48.5 (38.3–64.8) | 23.8±7.4 | 0.039 | |
| Crohn’s disease (51) | 23 | 8.4±8.4 | 20.0 (13.0–24.0) | 30.6±6.3 | <0.0001 | 21 | 9.2±8.3 | 60.0 (39.5–85.5) | 24.2±12.2 | <0.0001 | |
Data are presented as mean ± standard deviation or median (interquartile range). P value obtained with paired t-test or Wilcoxon test. PBDS, plant-based diet score.
Results of therapeutic modality incorporating PBD in CD
Biologics revolutionized the IBD treatment, but the optimal use of biologics is undetermined. Almost 25 years have passed since infliximab, an anti-tumor necrosis factor (TNF) antibody, was first used for IBD (73). For CD, more than a half of TREAT-registered patients have undergone infliximab treatment since 2004 (74). At entry to the I-CARE (IBD cancer and serious infections in Europe) study, rates of biological treatments were 69.7% (4,300/6,169) and 43.5% (1,757/4,037) in CD and UC, respectively (75). There is a barrier of primary nonresponders (around 30%) to biologics in both CD and UC. If we take the rate of primary nonresponders into account, biologics were used in almost all CD patients in the I-CARE study (75). Kayal et al. (76) studied the net remission rate with biologics in CD, which they calculated using an intention-to-treat analysis based on the remission rate in the induction and maintenance phases. Among anti-TNF-naïve patients, the net remission rates at around 1 year of infliximab (54 weeks), adalimumab (56 weeks), ustekinumab (44 weeks), and vedolizumab (52 weeks) were 16.7%, 28.5%, 34.8%, and 18.7%, respectively (76). All were less than 40%.
It is known that early use of biologics (the top-down approach) is more effective than late use (the step-up approach) (73). Therefore, earlier use of biologics than before is prevailing, although most guidelines in the world recommend step-up use. One of the reasons for step-up use is the high cost of biologics. Jongsma et al. (77) compared the efficacy of first-line use of infliximab versus conventional exclusive enteral nutrition or prednisolone in therapy-naïve, new-onset pediatric patients with CD. As for infliximab, the standard induction regimen of infliximab (5 mg/kg at 0, 2, and 6 week) was administered. They reconfirmed the superiority of infliximab therapy over conventional therapy in terms of clinical remission rate. The clinical remission rate at week 10, however, was 59% (77). Even first-line use of infliximab in therapy-naïve patients did not break the barrier of primary nonresponders (Figure 6) (47,77-81). A new and highly effective medical therapy including biologics for CD is eagerly needed.
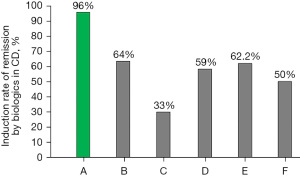
Induction phase (47)
Ideal treatment is the initiation of therapy before irreversible damage occurs, namely during the window of opportunity (82). Considering that most CD patients exhibit a chronic progressive disabling course (83-86) and that there is no way to identify patients who exhibit a long-term indolent course (87), all patients with CD, even mild cases, were given IPF therapy (47). PBD and infliximab infusion were initiated on the same day. Our modality, IPF therapy, did not accompany an immunosuppressive agent. Our 44 CD patients could be divided into three groups: newly diagnosed adults (n=24), newly diagnosed children (n=11), and relapsed adults (n=9); median age 27.5, 16.0, and 24.0 years old, respectively; median disease duration 4.5, 6.0, and 72.0 months, respectively. The number of cases by Crohn’s Disease Activity Index score ≤150, 151–220, 221–450, and ≥451 were 8, 7, 19, and 10 cases, respectively. The remission rate according to intention-to-treat and per-protocol analyses was 96% (44/46) and 100% (44/44), respectively (47,88). We did not encounter any primary nonresponders among 46 consecutive cases. Figure 6 shows CD remission rates, including the highest one in the literature, of three biologics (infliximab, adalimumab, and ustekinumab) with/without azathioprine (47,77-81).
Overcoming the hurdle of primary nonresponders lead to a favorable clinical course. The net remission rate at one year was 79% with our modality in new-onset adult CD cases (51). We believe that PBD provides sufficient dietary fiber to gut microbiota, resulting in production of butyrate, which improves gut homeostasis to suppress inflammation (89).
Quiescent phase (51)
Remitted newly diagnosed adult patients with CD treated with IPF therapy (n=22) were advised to adhere to PBD. Infliximab was used only in the induction phase. Patients were followed without biologics, immunosuppressants, or corticosteroids. A relapse-free course at 10 years after IPF therapy in CD was achieved in nearly a half of patients (52%) (Figure 7) (Table 3) (46,51,52,90). This is an unprecedented relapse-free course in CD (Figure 8) (51,52).
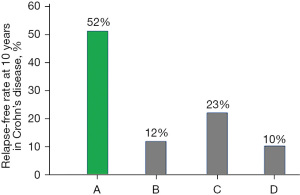
Table 3
| Author | Country | Subjects & year | Number of cases | Cumulative relapse-free rate | Cumulative surgical rate | |||
|---|---|---|---|---|---|---|---|---|
| 5 years | 10 years | 5 years | 10 years | |||||
| Munkholm et al., 1995 (46) | Denmark | PBIC 1962–1987 | 373 | 22% | 12% | n.d. | n.d. | |
| Wolters et al., 2006 (90) | European countries | PBIC 1991–1993 | 358 | 31% | 23% | 21% | 29% | |
| Solberg et al., 2007 (52) | Norway | PBIC 1990–1994 | 237 | 15% | 10% | 27% | 38% | |
| Chiba et al., 2022 (51) | Japan | 2003–2017 | 26 | 52% | 52% | 12% | 19% | |
PBIC, population-based inception cohort; n.d., not described.

The limited use of infliximab in IPF therapy releases patients from anxiety about side effects of medication and lowers medical costs. A long-term relapse-free outcome obviously keeps the patient free from disability objectively and provides a high quality of life subjectively. IPF therapy will provide a great benefit to CD patients.
Plant-based diet score (PBDS)
We developed a simple way to evaluate the adherence to a PBD for Japanese patients with IBD (91). The PBDS was evaluated on admission (baseline PBDS) and again within or beyond 2 years after discharge, referred to as short-term and long-term PBDS, respectively. In our previous study, the mean baseline PBDS in UC (n=159) and CD (n=70) was 10.9 and 8.2, respectively (91). PBDSs in short term follow-up were significantly increased relative to baseline in all studies, i.e., three studies of UC and one study of CD (P<0.0001) (Table 2) (48-51). Although sustained dietary modification is desired, the majority of patients tended to lose their determination once they had been in remission for a few to several years. However, they still consumed more of the recommended foods and consumed less of the discouraged foods compared with baseline. Long-term PBDS was significantly higher than baseline PBDS even after 6 years (Table 2). Considering that successful weight reduction or improvement in hemoglobin A1c as a result of dietary intervention subsequently reverted back toward baseline in 1 year (92,93), significant adherence to PBD compared with baseline several years later in our studies was remarkable.
Onset of IBD after deteriorated dietary habits
Focusing on diet, we published several case reports in which IBD occurred after the patient adopted worse dietary habits. Such typical cases occur in the latter teenage years in Japan. He/she graduates a high school in March and moves to Tokyo for a new life of company/college in April. Soft/loose stool appears in June/July. He/she thinks that the change in stool must be due to a change of environment, and that it will gradually improve. The change in stool, however, becomes worse, and he/she visits a doctor in autumn/winter. IBD is ultimately diagnosed (72,94). Our PBDS analysis showed that the score before the patients departed for Tokyo clearly decreased after living in Tokyo (72,94). We experienced two cases of pregnancy-onset UC. The onset was in the second trimester after emesis gravidarum in one case (54), and at 20 weeks postpartum in the other case (95). In both cases, the dietary pattern changed toward unhealthy ones. The patient was seeking relief from the emesis in the former, and the patient wanted sweets after 1 year of tension due to pregnancy and labor in the latter (54,95). UC occurred and recurred in a diabetic when glycemic control deteriorated (96).
Supporting evidence for PBD
Recent developments in epidemiology, microbiology, nutrition, and clinical studies support our notion. Data from Japan showed an increased incidence of IBD in association with dietary westernization (1). Current global consumption consists of an excess of unhealthy foods and a shortage of healthy foods (97,98). This is also true in patients with IBD (2-7). These unhealthy dietary habits are related to a global increase in the incidence of diet-related obesity and chronic diseases including coronary heart disease, stroke, and diabetes mellitus (97). Basic research has revealed the interplay between diet, microbiota/its metabolites and health/disease, indicating that a westernized diet (high in fat, animal protein, and sugar, low in dietary fiber) tends to be pro-inflammatory, while a PBD (low in fat, animal protein, and sugar, high in dietary fiber) tends to be anti-inflammatory (8-13). Therefore, the current diet is problematic and should be changed to a suitable one in IBD. Even nutritionally balanced current meals cannot prevent relapse in CD (15-18). PBDs are recommended to the public as healthy diets to prevent common chronic diseases and are more environmentally sustainable than meat-based diets (97).
Qualified diet for IBD
As described above, adequate diet is critically needed in CD. There are several conditions for qualification as a suitable diet for IBD (21). The diet should be rationally designed based on the pathogenesis of IBD. Otherwise, foods exclusion tends to be more excessive than needed. We should be aware of absence of IBD before dietary westernization when milk and eggs were usually consumed. The diet should be clinically effective. The diet together with medication should induce remission in the active phase, and the diet with/without medication should prevent relapse in the quiescent phase. The diet should be accepted by the majority of IBD patients. Naming of the diet is also important. A simple name that conjures up an image of the content of the diet is preferable. The diet should be available worldwide. Diets in trials published to date in the literature are far from the satisfying these conditions except for PBD. PBD satisfies the above criteria. Therefore, we recommended PBD for IBD (21-23).
Orthodox avenue in medicine—lifestyle medicine and self-management skills
Patients are told by their gastroenterologists that they have to take medication for life to prevent relapse of IBD as long as your life. It would be reasonable because guidelines for IBD recommend continuous adherence to medication. Such patients are relieved by our modality. If remission lasts for a few years and you are able to maintain a healthy diet, you will be free from medication. In mild UC, we first provide only PBD. Medication is provided when remission is not achieved with PBD alone (57). A healthy lifestyle is fundamental in medicine. There are similar proverbs in the west and the east: we are what we eat, and proper diet and Chinese herbal medicine share the same principles/a balanced diet leads to a healthy body, respectively. If we want to improve what we are, we need to improve what we eat (12,99). It is PBD that IBD patients eat. The experience of PBD during hospitalization seemed to contribute to increased adherence to PBD (Table 2). It seemed that it increased self-management skills, which in turn decreased the risk of relapse.
Steps toward PBD for IBD
There are steps to achieve the same results we achieved or to reproduce our results. First of all, appreciation of our current diet as a ubiquitous environmental factor in IBD is key. Without this belief, nothing advances. Second, PBD is specifically designed. Neither a balanced diet nor a proximate diet to PBD is enough. A lacto-ovo-semi-vegetarian diet is needed. Third, hospitalization during which the patient can experience the semi-vegetarian diet is desirable. Fourth, all patients with active CD are to be treated with IPF therapy. This indication will change when we can reliably identify patients who do not relapse. Infliximab will be replaced with the other when we have better biologics or medication than infliximab.
The present authors were fortunate to be able to provide therapy we thought was best without worrying about the patient’s economic burden thanks to medical aid for IBD patients from the Japanese government. We are sure, however, our modality improved the clinical course and cut medical costs eventually.
Discussion
We published our view in Japanese for the first time in 2002, i.e., that IBD is thought to be a lifestyle disease mediated by a westernized diet based on Japanese epidemiologic data (100). The data showed an increased incidence of IBD in association with dietary westernization. We tried in vain to publish our view in leading journals in IBD, gastroenterology, and internal medicine. We began to provide a PBD for patients with IBD in 2003. Basic medicine established the interplay between diet, gut microbiota, microbial metabolites, and health/disease. It shows that the current westernized diet is pro-inflammatory, while PBD is anti-inflammatory (8-13). Our therapeutic outcomes in CD and UC in the induction and quiescent phases are the best in the world. Therefore, we believe our assertion that our current diet is a ubiquitous environmental factor is correct. Needless to say, we answer PBD to the most common question asked by patients: “What should I eat?” (38,39) based on our studies (47-51). Our PBD can be modified among nations, races, and individuals based on their culture and tradition.
Nowadays, gastroenterologists and IBD physicians provide treatment based on therapeutic guidelines. It will take a few decades to accept PBD for IBD in the guidelines. As described above, the current westernized diet has not yet been established as a ubiquitous environmental factor. Reproduction of our outcomes for PBD versus a control diet is not easy. It is a pity that the majority of patients are not told of PBD and are suffering from the disease. The present authors are confident and hope that PBD will bring great benefit to IBD patients.
Conclusions
By replacing a westernized diet with PBD, our studies achieved far better outcomes in both CD and UC and in both the active and quiescent phases.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-6/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-6/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chiba M, Morita N, Nakamura A, et al. Increased incidence of inflammatory bowel disease in association with dietary transition (Westernization) in Japan. JMA J 2021;4:347-57. [PubMed]
- Amre DK, D’Souza S, Morgan K, et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am J Gastroenterol 2007;102:2016-25. Erratum in: Am J Gastroenterol 2007;102:2614. [Crossref] [PubMed]
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 2011;106:563-73. [Crossref] [PubMed]
- Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013;145:970-7. [Crossref] [PubMed]
- Ananthakrishnan AN, Khalili H, Konijeti GG, et al. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014;63:776-84. [Crossref] [PubMed]
- Dolan KT, Chang EB. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol Nutr Food Res 2017;61: [Crossref] [PubMed]
- Khalili H, Chan SSM, Lochhead P, et al. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2018;15:525-35. [Crossref] [PubMed]
- De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010;107:14691-6. [Crossref] [PubMed]
- Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105-8. [Crossref] [PubMed]
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559-63. [Crossref] [PubMed]
- Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 2014;20:779-86. [Crossref] [PubMed]
- Tilg H, Moschen AR. Food, immunity, and the microbiome. Gastroenterology 2015;148:1107-19. [Crossref] [PubMed]
- Bolte LA, Vich Vila A, Imhann F, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021;70:1287-98. [Crossref] [PubMed]
- Pittayanon R, Lau JT, Leontiadis GI, et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterology 2020;158:930-46.e1. [Crossref] [PubMed]
- Chiba M, Suzuki T, Naganuma H, et al. A case of child Crohn’s disease markedly responded to TPN, ED, and HEEH treatment. J Jpn Soc Colo-Proctol 1991;44:235-42. [Crossref]
- Chiba M, Iizuka M, Horie Y, et al. Long remission in Crohn’s disease by polymeric enteral diet. Akita J Med 1996;22:131-8.
- Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol 2010;16:2484-95. [Crossref] [PubMed]
- Limketkai BN, Hamideh M, Shah R, et al. Dietary patterns and their association with symptoms activity in inflammatory bowel diseases. Inflamm Bowel Dis 2022;28:1627-36. [Crossref] [PubMed]
- Chiba M, Tsuda H, Abe T, et al. Missing environmental factor in inflammatory bowel disease: diet-associated gut microflora. Inflamm Bowel Dis 2011;17:E82-3. [Crossref] [PubMed]
- Chiba M, Nakane K, Komatsu M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm J 2019;23:18-107. [Crossref] [PubMed]
- Chiba M, Ishii H, Komatsu M. Recommendation of plant-based diets for inflammatory bowel disease. Transl Pediatr 2019;8:23-7. [Crossref] [PubMed]
- Chiba M, Hosoba M, Yamada K. Plant-based diet recommended for inflammatory bowel disease. Inflamm Bowel Dis 2023;29:e17-8. [Crossref] [PubMed]
- Chiba M, Morita N. Incorporation of plant-based diet surpasses current standards in therapeutic outcomes in inflammatory bowel disease. Metabolites 2023;13:332. [Crossref] [PubMed]
- Kirsner JB. Crohn’s disease: yesterday, today, and tomorrow. Gastroenterology 1997;112:1028-30. [Crossref] [PubMed]
- Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol 2007;42:16-25. [Crossref] [PubMed]
- Hirakawa H, Fukuda Y, Tanida N, et al. Home elemental enteral hyperalimentation (HEEH) for the maintenance of remission in patients with Crohn’s disease. Gastroenterol Jpn 1993;28:379-84. [Crossref] [PubMed]
- Sigall Boneh R, Van Limbergen J, Wine E, et al. Dietary therapies induce rapid response and remission in pediatric patients with active Crohn’s disease. Clin Gastroenterol Hepatol 2021;19:752-9. [Crossref] [PubMed]
- Iizuka M, Nakagomi O, Chiba M, et al. Absence of measles virus in Crohn’s disease. Lancet 1995;345:199. [Crossref] [PubMed]
- Chiba M, Komatsu M, Iizuka M, et al. Microbiology of the intestinal lymph follicle: a clue to elucidate causative microbial agent(s) in Crohn’s disease. Med Hypotheses 1998;51:421-7. [Crossref] [PubMed]
- Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut 2011;60:1739-53. [Crossref] [PubMed]
- Probert CS, Jayanthi V, Pinder D, et al. Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population of Leicestershire. Gut 1992;33:687-93. [Crossref] [PubMed]
- Benchimol EI, Mack DR, Guttmann A, et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol 2015;110:553-63. [Crossref] [PubMed]
- Fejfar Z. Prevention against ischaemic heart disease: a critical review. In: Oliver MF. editor. Modern trends in cardiology—3. London: Butterworths; 1974:465-99.
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017;152:313-21.e2. [Crossref] [PubMed]
- Popkin BM. The nutrition transition in low-income countries: an emerging crisis. Nutr Rev 1994;52:285-98. [Crossref] [PubMed]
- Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol 2018;15:39-49. [Crossref] [PubMed]
- Hart AL, Lomer M, Verjee A, et al. What are the top 10 research questions in the treatment of inflammatory bowel disease? A priority setting partnership with the James Lind Alliance. J Crohns Colitis 2017;11:204-11. [Crossref] [PubMed]
- Lewis JD, Abreu MT. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 2017;152:398-414.e6. [Crossref] [PubMed]
- Sasson AN, Ananthakrishnan AN, Raman M. Diet in treatment of inflammatory bowel diseases. Clin Gastroenterol Hepatol 2021;19:425-35.e3. [Crossref] [PubMed]
- Sandys O, Te Velde A. Raising the alarm: environmental factors in the onset and maintenance of chronic (low-grade) inflammation in the gastrointestinal tract. Dig Dis Sci 2022;67:4355-68. [Crossref] [PubMed]
- Chiba M, Tsuda S, Komatsu M, et al. Onset of ulcerative colitis during a low-carbohydrate weight-loss diet and treatment with a plant-based diet: a case report. Perm J 2016;20:80-4. [Crossref] [PubMed]
- Höie O, Wolters F, Riis L, et al. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol 2007;102:1692-701. [Crossref] [PubMed]
- Moum B, Ekbom A, Vatn MH, et al. Clinical course during the 1st year after diagnosis in ulcerative colitis and Crohn’s disease. Results of a large, prospective population-based study in southeastern Norway, 1990-93. Scand J Gastroenterol 1997;32:1005-12. [Crossref] [PubMed]
- Magro F, Rodrigues A, Vieira AI, et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis 2012;18:573-83. [Crossref] [PubMed]
- Prosberg MV, Vester-Andersen MK, Andersson M, et al. Long-term compliance with oral 5-aminosalicylic acid therapy and risk of disease recurrence in patients with ulcerative colitis: a population-based cohort study. Inflamm Bowel Dis 2016;22:925-32. [Crossref] [PubMed]
- Munkholm P, Langholz E, Davidsen M, et al. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol 1995;30:699-706. [Crossref] [PubMed]
- Chiba M, Tsuji T, Nakane K, et al. Induction with infliximab and a plant-based diet as first-line (IPF) therapy for Crohn disease: a single-group trial. Perm J 2017;21:17-009. [Crossref] [PubMed]
- Chiba M, Nakane K, Tsuji T, et al. Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: a single-group trial. Perm J 2018;22:17-167. [Crossref] [PubMed]
- Chiba M, Nakane K, Tsuji T, et al. Relapse prevention by plant-based diet incorporated into induction therapy for ulcerative colitis: a single-group trial. Perm J 2019;23:18-220. [Crossref] [PubMed]
- Chiba M, Tsuji T, Nakane K, et al. High remission rate with infliximab and plant-based diet as first-line (IPF) therapy for severe ulcerative colitis: single-group trial. Perm J 2020;24:1-10. [Crossref] [PubMed]
- Chiba M, Tsuji T, Nakane K, et al. Relapse-free course in nearly half of Crohn’s disease patients with infliximab and plant-based diet as first-line therapy: a single-group trial. Perm J 2022;26:40-53. [Crossref] [PubMed]
- Solberg IC, Vatn MH, Høie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007;5:1430-8. [Crossref] [PubMed]
- Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Prev Med 1980;9:469-83. [Crossref] [PubMed]
- Chiba M, Sugawara T, Komatsu M, et al. Onset of ulcerative colitis in the second trimester after emesis gravidarum: treatment with plant-based diet. Inflamm Bowel Dis 2018;24:e8-9. [Crossref] [PubMed]
- Kitano A, Okawa K, Nakamura S, et al. The long-term assessment of the patients with ulcerative colitis (> 10 years follow-up, mean follow-up 21.7 years). J New Rem & Clin 2011;60:1347-55.
- Ko CW, Singh S, Feuerstein JD, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology 2019;156:748-64. [Crossref] [PubMed]
- Chiba M, Tsuji T, Ohno H, et al. Stepwise treatment with plant-based diet and medication for patient with mild ulcerative colitis. Perm J 2021;25:21.052.
- McClements D, Probert C. Managing acute severe ulcerative colitis in the hosptialised setting. Frontline Gastroenterol 2015;6:241-5. [Crossref] [PubMed]
- Lindgren SC, Flood LM, Kilander AF, et al. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol 1998;10:831-5. [Crossref] [PubMed]
- Oshitani N, Matsumoto T, Jinno Y, et al. Prediction of short-term outcome for patients with active ulcerative colitis. Dig Dis Sci 2000;45:982-6. [Crossref] [PubMed]
- Turner D, Walsh CM, Steinhart AH, et al. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol 2007;5:103-10. [Crossref] [PubMed]
- Choy MC, Seah D, Faleck DM, et al. Systematic review and meta-analysis: optimal salvage therapy in acute severe ulcerative colitis. Inflamm Bowel Dis 2019;25:1169-86. [Crossref] [PubMed]
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462-76. [Crossref] [PubMed]
- Travis S, Feagan BG, Peyrin-Biroulet L, et al. Effect of adalimumab on clinical outcomes and health-related quality of life among patients with ulcerative colitis in a clinical practice setting: results from InspirADA. J Crohns Colitis 2017;11:1317-25. [Crossref] [PubMed]
- Taxonera C, Rodríguez C, Bertoletti F, et al. Clinical outcomes of golimumab as first, second or third anti-TNF agent in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis 2017;23:1394-402. [Crossref] [PubMed]
- Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699-710. [Crossref] [PubMed]
- Danese S, Fiorino G, Peyrin-Biroulet L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med 2014;160:704-11. [Crossref] [PubMed]
- Oh SJ, Shin GY, Soh H, et al. Long-term outcomes of infliximab in a real-world multicenter cohort of patients with acute severe ulcerative colitis. Intest Res 2021;19:323-31. [Crossref] [PubMed]
- Moore AC, Bressler B. Acute severe ulcerative colitis: the Oxford criteria no longer predict in-hospital colectomy rates. Dig Dis Sci 2020;65:576-80. [Crossref] [PubMed]
- Syal G, Robbins L, Kashani A, et al. Hypoalbuminemia and bandemia predict failure of infliximab rescue therapy in acute severe ulcerative colitis. Dig Dis Sci 2021;66:199-205. [Crossref] [PubMed]
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology 2001;121:255-60. [Crossref] [PubMed]
- Chiba M, Tsuji T, Masai R, et al. Infliximab and plant-based diet as first-line therapy followed by corticosteroid therapy for severe ulcerative colitis: a case report. Gastrointest Disord 2022;4:230-6. [Crossref]
- D’Haens GR, van Deventer S. 25 years of anti-TNF treatment for inflammatory bowel disease: lessons from the past and a look to the future. Gut 2021;70:1396-405. [Crossref] [PubMed]
- Lichtenstein GR, Feagan BG, Cohen RD, et al. Infliximab for Crohn’s disease: more than 13 years of real-world experience. Inflamm Bowel Dis 2018;24:490-501. [Crossref] [PubMed]
- Peyrin-Biroulet L, Rahier JF, Kirchgesner J, et al. I-CARE, a European prospective cohort study assessing safety and effectiveness of biologics in inflammatory bowel disease. Clin Gastroenterol Hepatol 2023;21:771-88.e10. [Crossref] [PubMed]
- Kayal M, Ungaro RC, Bader G, et al. Net remission rates with biologic treatment in Crohn’s disease: a reappraisal of the clinical trial data. Clin Gastroenterol Hepatol 2023;21:1348-50. [Crossref] [PubMed]
- Jongsma MME, Aardoom MA, Cozijnsen MA, et al. First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn's disease: an open-label multicentre randomised controlled trial. Gut 2022;71:34-42. [Crossref] [PubMed]
- D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 2008;371:660-7. [Crossref] [PubMed]
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383-95. [Crossref] [PubMed]
- Miyoshi J, Hisamatsu T, Matsuoka K, et al. Early intervention with adalimumab may contribute to favorable clinical efficacy in patients with Crohn’s disease. Digestion 2014;90:130-6. [Crossref] [PubMed]
- Sands BE, Irving PM, Hoops T, et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn's disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 2022;399:2200-11. [Crossref] [PubMed]
- O’Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum 2002;46:283-5. [Crossref] [PubMed]
- Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis 2011;17:1415-22. [Crossref] [PubMed]
- Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg 2000;231:38-45. [Crossref] [PubMed]
- Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn’s disease. Gastroenterology 2006;130:650-6. [Crossref] [PubMed]
- Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology 2017;152:351-61.e5. [Crossref] [PubMed]
- Siegel CA, Bernstein CN. Identifying patients with inflammatory bowel diseases at high vs low risk of complications. Clin Gastroenterol Hepatol 2020;18:1261-7. [Crossref] [PubMed]
- Chiba M, Tanaka Y, Ono I. Early intestinal obstruction after infliximab therapy in Crohn’s disease. Autops Case Rep 2019;9:e2018068. [Crossref] [PubMed]
- Chiba M, Tsuji T, Komatsu M. How to optimize effects of infliximab in inflammatory bowel disease: incorporation of a plant-based diet. Gastroenterology 2020;158:1512. [Crossref] [PubMed]
- Wolters FL, Russel MG, Sijbrandij J, et al. Phenotype at diagnosis predicts recurrence rates in Crohn's disease. Gut 2006;55:1124-30. [Crossref] [PubMed]
- Chiba M, Nakane K, Takayama Y, et al. Development and application of a plant-based diet scoring system for Japanese patients with inflammatory bowel disease. Perm J 2016;20:16-019. [Crossref] [PubMed]
- Atallah R, Filion KB, Wakil SM, et al. Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: a systematic review of randomized controlled trials. Circ Cardiovasc Qual Outcomes 2014;7:815-27. [Crossref] [PubMed]
- Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588S-96S. [Crossref] [PubMed]
- Chiba M, Sugawara T, Morikawa Y, et al. Onset of Crohn’s disease after moving to Tokyo -maintenance of remission by semi-vegetarian diet: a case report. The Japanese Society of Digestion and Absorption 2006;29:92-6.
- Chiba M, Tsuji T, Komatsu M, et al. Ulcerative colitis in the postpartum period. Autops Case Rep 2020;10:e2020187. [Crossref] [PubMed]
- Chiba M, Komatsu M, Hosoba M, et al. Onset of ulcerative colitis in a patient with type 2 diabetes: efficacy of a plant-based diet for both diseases. Gastrointest Disord 2022;4:223-9. [Crossref]
- Willett W, Rockström J, Loken B, et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019;393:447-92. [Crossref] [PubMed]
- Dietary Guidelines Advisory Committee. Key elements of healthy eating patterns. Dietary Guidelines for Americans 2015-2020. 8th edition. Washington, DC, USA: US Department of Health and Human Services and US Department of Agriculture; 2015:13-36.
- Barritt AS 4th. We are what we eat: food as medicine in health and disease. Clin Ther 2022;44:642-3. [Crossref] [PubMed]
- Chiba M, Morita N, Nakamura A, et al. Inflammatory bowel disease (Crohn’s disease and ulcerative colitis) is thought to be lifestyle disease mediated mainly by diet. Annual report of the Research Committee of Inflammatory Bowel Disease. The Ministry of Health and Welfare of Japan; 2002:126-30.
Cite this article as: Chiba M, Tsuji T, Komatsu M. Therapeutic advancement in inflammatory bowel disease by incorporating plant-based diet. Transl Gastroenterol Hepatol 2023;8:38.


