
Image enhanced colonoscopy: updates and prospects—a review
Introduction
Colonoscopy is an effective tool for detecting and preventing colorectal cancer, but its success depends on reliably detecting colorectal neoplasia (1-3). A successful diagnostic endoscopy requires accurate detection of various gastrointestinal (GI) lesions and proper characterization of these lesions, including differentiation between neoplastic and non-neoplastic types, and determination of their lateral borders and depth.
In order to improve accuracy, not only examination techniques and bowel preparation need improvement, but incorporating advanced imaging technologies such as image-enhanced endoscopy (IEE) to characterize lesions in real time diagnosis play an important role (1). IEE improves visualization of GI lesion patterns and microvasculature, even in precancerous stages, through different dyes and technologies (4,5).
IEE can be either conventional dye-based chromoendoscopy (CE) or electronic (virtual CE). In dye-based CE (DCE), a dye is applied to the mucosa while the endoscope is withdrawn, which enhances the mucosal surface and aids in lesion resection or biopsies. However, this method is operator-dependent, time-consuming and requires technical expertise (6). Equipment-based methods, such as narrow band imaging (NBI) (Olympus Medical Systems, Tokyo, Japan), linked-color imaging (LCI) (Fujinon, Fujifilm Medical Co, Saitama, Japan), flexible spectral imaging color enhancement (FICE) (Fujinon), i-SCAN (Pentax Endoscopy, Tokyo, Japan), and blue light imaging (BLI) (Fujinon), provide detailed contrast images through optical filtering or software processing. These methods have an advantage of having a shorter overall procedural time compared to dye-based methods, and no special assembly or dye is required (7) (Table 1).
Table 1
| Name | Underlying mechanism | Date launched |
|---|---|---|
| Chromoendoscopy | Utilizes the dyes/stains to enhances the appearance of mucosal surfaces | 2003 |
| Narrow band imaging | Uses blue and green spectrum of visible light to improve the visualization of capillary patterns and mucosal surfaces | |
| 1st generation: creates images by using a filter mechanism for separating the blue and green light, however, the images were not adequately bright | First generation: 2006 | |
| 2nd generation: used a xenon lamp, in addition to eliminating the filter mechanism to produce sharp brighter images | Second generation: 2012 | |
| 3rd generation: uses color LEDs for better illumination and bypasses the use of filters. It also includes an expanded depth of field functionality to provide a broad focus. In addition, it can allow for up to 90× magnification | Third generation: 2020 | |
| Confocal laser endomicroscopy | A low-power laser is used to better visualize cellular and subcellular structures | 2004 |
| Flexible spectral imaging color enhancement | Images are created using a specific spectrum of visible light; images are later processed to enhances mucosal surfaces and microvascular patterns | 2005 |
| Blue-laser imaging | Uses a blue laser light source to create brighter images which enhance the visibility of colonic lesions | 2012 |
| Linked-color imaging | A pre- and post-processing technology is used, in addition to improved red color enhancement, to differentiate between normal and abnormal colon mucosa | 2014 |
| i-SCAN | Uses a digital post-processing technology, which generates high-definition images using three functions: contrast enhancement, surface enhancement, and tone enhancement | 2016 |
| Autofluorescence endoscopy | Highlights endogenous fluorescence generated by the molecules in the tissue; differentiates colonic lesions from normal colon by relative amount of fluorescence produced by each type of tissue | 2001 |
| Endocytoscopy | Utilizes dyes along with a contact-type optical endoscope to create ultra-high-resolution images of up to 520× magnification power; a method for “virtual histology” | 2003 |
| Full spectrum endoscopy | Uses an endoscope mounted with multiple cameras and light emitting diodes to allow a panoramic view of 330 degrees of the gastrointestinal tract | 2008 (EndoChoice)† |
†, EndoChoice is the parent company which developed full spectrum endoscopy. LED, light-emitting diode.
Various societies recommend incorporating IEE in routine colonoscopy practice (5). American Society for Gastrointestinal Endoscopy (ASGE) Technology Committee, in a systemic review and meta-analysis, concluded that the histology of diminutive (<5 mm) colorectal polyps can be predicted in vivo during colonoscopy by using various endoscopic techniques available currently, which is sometimes referred to as “optical biopsy”. This can allow the use of “diagnose-and-leave” strategy for diminutive polyps in rectosigmoid colon, based on the in vivo real-time prediction by these advanced endoscopic techniques. In vivo real-time assessment of the histology of diminutive (≤5 mm) colorectal polyps detected at colonoscopy can be achieved by means of an “optical biopsy” by using currently available endoscopic technologies, supporting a “diagnose-and-leave” strategy for diminutive predicted non neoplastic polyps in the rectosigmoid colon (8). The European Society of Gastrointestinal Endoscopy (ESGE) recommends using advanced imaging technologies to improve mucosal visualization and details (9). The Asian professional group ANBI2G also endorses using IEE for better endoscopic diagnosis and early detection of colorectal neoplasia and polyps, as well as in patients with inflammatory bowel disease (IBD) to improve detection of dysplasia (10). Furthermore, British Society of Gastroenterology (BSG) and ESGE suggests that surveillance in IBD patients can be done using CE with targeted biopsies (11,12), while the American Gastroenterology Association (AGA) and ASGE endorse using IEE with high-definition (HD) colonoscopy for long-term colonic IBD patients (13,14).
This article reviews the current and recent advancements in the field of IEE and their applications with colonoscopy.
CE
The use of DCE helps the endoscopist identify and assess abnormalities in the digestive tract by using dyes or stains. This method enhances the color patterns and appearance of the mucosal surfaces, and thereby improving the differentiation of lesions (15). The dye is usually delivered through a spraying catheter that produces a fine mist on the surface of the digestive tract (16). The dye is sprayed through the tip of endoscope in a spiral motion as the endoscope is being withdrawn slowly. Only small sections, about 20 cm, should be stained at a time and then examined carefully, as the effect of the dye is temporary. The dyes can be sprayed through either whole colon CE or they can be applied specifically on the areas of visible abnormalities (15). It is necessary to have an excellent preparation of bowel when using DCE in the distal part of the digestive tract (5).
The dyes used for evaluating lesions in colon can be divided into two main types: contrast dyes and absorptive dyes. Absorptive dyes provide clear details of cell surfaces as they are taken up by specific cells (16). An example of this type of dye is crystal violet, which stains the epithelial cells of the glands in colon mucosa and makes the pits appear white after staining. The staining of colon mucosa roughly takes 3 minutes after the dye is sprayed (5). Another example of an absorptive dye is methylene blue, which stains the epithelial cells of glands in small or large intestine as blue, while dysplastic lesions remain unstained. It has shown to increase detection of smaller polyps (17). As an alternative, methylene blue can be consumed in oral tablet form, taken with agents used for bowel preparation. This has shown to increase the detection rate of adenomas compared to placebo in a phase 3 trial (18).
Contrast dyes reveal the shape of the mucosal surface by flowing into the spaces between cells without coloring cellular surfaces. Indigo carmine, as an example, improves the detection of neoplastic lesions by highlighting the surface topography. It helps differentiate benign from malignant lesions, outline early-stage neoplastic lesions, and determine cancer depth of invasion (4). The way contrast dyes stain pit patterns can be categorized using systems such as the Kudo pit pattern classification (19).
A meta-analysis of 4 randomized controlled trials (RCTs) showed a significant difference in favor of CE compared to conventional endoscopy for all detection outcomes, particularly, higher yield in patients with at least one neoplastic lesion [odds ratio (OR) 1.61; 95% confidence interval (CI): 1.24–2.09] and significantly more patients with three or more neoplastic lesions (OR 2.55; 95% CI: 1.49–4.36) (20). CE group had significantly slower withdrawal times.
With regards to lateral spreading tumors (LST), Tamai et al. reported improved visualization of non-granular LSTs with DCE for both expert and novice group, whereas similar improvement with electronic IEE was only seen in the expert group (21).
DCE has been demonstrated in several trials to have higher detection of dysplasia compared to standard definition white light endoscopy (WLE) (17,22-24). However, since the invention of HD endoscopy, a number of meta-analyses of studies in patients with IBD (endorsed by SCENIC international consensus) have shown that use of HD endoscopes, with or without DCE, have improved the visibility of dysplasia, such that 90% of dysplastic lesions are now visible compared to 80% with standard-definition endoscopes (25). Therefore, both AGA and European guidelines consider virtual CE as a suitable alternative for DCE when using HD endoscopy in patients with IBD (14,26).
In general, DCE is effective for both detection and characterization of polyps, but it is not widely used due to longer time needed for applying the dye with a spray and removing any excess dye, as well as the challenge of identifying lesions when there is severe inflammation or when the view is obstructed by the accumulation of solution in sunken-type lesions (4). Furthermore, some dyes, such as crystal violet, are not widely available in some countries (27).
Electronic enhancing techniques (virtual chromoendoscopy)
NBI
NBI was the first narrow-image technology (developed by Sano et al. at the National Cancer Center Hospital East in 1999) that filters the light used for illumination (28). It removes the red component of the standard red, green, blue (RGB) filters and centers the bandwidth of blue and green light on a narrowed spectrum of 415 and 540 nm to 20–30 nm. The CCD signals are processed to create a false-color image. Hemoglobin absorbs blue light due to its absorption peak at 415 nm and this short wavelength also penetrates the mucosa less deeply than red light. This leads to improved contrast for small blood vessels close to the surface and better visibility of the structures on the mucosal surface, for differentiation between abnormal and normal tissue (29). Sano et al., for the first time, reported the usefulness of pit pattern and vascular classification using NBI in 2004, and subsequently the first classification of the capillary pattern using NBI magnifying colonoscopy (28). Hirata et al. showed that NBI could be used to assess the histological grade and invasion depth of colorectal tumors by measuring vessel thickness (30).
First generation NBI was launched in 2006 as a part of two endoscopic systems, EVIS LUCERA SPECTRUM (in Japan and UK) and EXERA II (elsewhere) (31). Images generated by first-generation NBI were not ideal due to low brightness, and studies indicated that the adenoma detection rate (ADR) was comparable between NBI and WLE (32,33). The second generation NBI was subsequently launched in 2012 as a built-in functionality in two more endoscopic models, including EVIS LUCERA ELITE (in Japan and UK) and EXERA III (elsewhere). These models used a stronger xenon lamp and a new signal processor which worked by elimination of filter mechanism as well as reduction of image noise, thereby producing brighter sharp images. It also allowed an optical magnification of ×45 power with a simple press of a button (27,31). A recent meta-analysis of 11 RCTs involving 4,491 patients found that ADR for second-generation NBI was better compared to HD-WLE (OR: 1.28; 95% CI: 1.05–1.56; P=0.02) (34). Overall, the ADR for NBI (first and second generations of NBI combined) was 45.2% compared to 42.3% for HD-WLE (OR for adenoma detection of 1.14 NBI vs. HD-WLE; 95% CI: 1.01–1.29; P=0.02). Third generation NBI has recently been introduced in 2020 as a part of novel endoscopic system (EVIS X-1). This allows system uses five-color light-emitting diodes (LEDs) as a light source and in contrast to previous NBI generation, it directly uses violet and blue LEDs for illumination instead of using an NBI filter. This allows for brighter images at a high frame rate. Some other notable improvements of this novel endoscopic model include expanded depth of field, which provides a continuous broad focus and an improved dual-focus functionality, which allows for ×90 magnification power. This system has been referred to as a “Universal Model” since it is compatible with all magnifying scopes including some specialized scopes such as power spiral motorized enteroscope and endocytoscope (31,35). ENDO-AID (OIP-1) is a computer-aided detection (CAD) device which works with EVIS X-1 endoscopic system. This allows for real-time polyp detection. Multiple studies have demonstrated the efficacy of ENDO-AID in improvement of ADR compared to standard colonoscopy (36,37). Gimeno-García et al. conducted an RCT comparing ENDO-AID with conventional colonoscopy and results showed ADR of 55.1% (ENDO-AID) vs. 43.8% (standard colonoscopy); P=0.029 (38).
Wanders et al. in a meta-analysis of 91 studies revealed that all virtual techniques, including NBI, FICE, i-SCAN, and confocal laser endomicroscopy (CLE), but not autofluorescence imaging (AFI), can accurately diagnose polyps as adenomatous or hyperplastic, when used by well-trained endoscopists with an overall sensitivity of 91%, specificity of 85.6%, negative predictive value (NPV) of 82.5% for NBI (39). In another meta-analysis of 28 studies (6,280 polyps in 4,053 patients), NBI for diagnosis of colorectal polyps showed a sensitivity and NPV >90% (40).
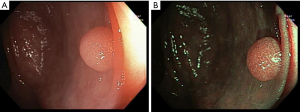
The accuracy of using NBI to characterize colonic polyps through microvascular density is similar to magnified chromoendoscopic assessment (41). However, there’s subjective variability between both the lesion color and vessel thickness, leading to the development of the NBI International Colorectal Endoscopic (NICE) classification system, which helps in in vivo diagnosis of small polyps in colon by utilizing a criterion based on color, surface pattern, and vessels (42). This classification system can be used with or without optical magnification and has been validated with a sensitivity of 98%, accuracy of 89%, and NPV of 95%, for real-time diagnoses during colonoscopy (43). Despite its excellent diagnostic accuracy, NICE classification was only useful in a fraction of cases, and therefore Japanese NBI Expert Team (JNET) classification was later developed that further subdivides adenomatous lesions (NICE II) into type 2A (Figure 1), for low grade adenomas, and type 2B, for high grade adenomas including and superficial submucosal invasive cancer to unify and facilitate a globally recognized classification (28).
Sessile serrated lesions (SSL) are precursor lesions to colorectal cancer, which are not specifically described in NICE and JNET classifications (44). “Workgroup serrated polyps and Polyposis” (WASP) classification was developed to identify these lesions with the help of IEE, based on at least two criteria of four SSL-like characteristics: cloudy surface, dark spots within the crypts, irregular shape, and indistinct border (5). This classification system has been validated with high accuracy (84%) after 6 months and a high NPV of 91% for small neoplastic lesions (adenomas and SSL) (45). Despite their usefulness, the utility of these classifications in differentiating SSLs from hypoplastic polyps has been questioned in recent studies. In a multicenter prospective study of 217 JNET type 1 lesions from 162 patients, the sensitivity of magnifying colonoscopy in detecting SSLs was only 79.8% (95% CI: 74.7–84.4%) (46). In another prospective study of artificial intelligence (AI)-assisted CADx system developed for NBI that analyzes still NBI images and outputs a histopathological prediction, the sensitivity and specificity of differentiating SSL from hyperplastic polyps (HP) were 80.9% and 62.1% respectively (47).
Both ASGE and ESGE have endorsed the use of i-SCAN, NBI, and FICE for diagnosis of polyps ≤5 mm (8,48).
In an analysis of 2 previous studies (total of 1,658 patients) at 5 medical centers, three “resect and discard” strategies were compared: optimal strategy (using NBI examination), location-based strategy (classified all polypoid lesions proximal to rectosigmoid colon as neoplastic and all recto-sigmoid diminutive polyps as hyperplastic), and a simplified optical strategy (all recto-sigmoid diminutive polyps were classified as hyperplastic unless confidently assessed as neoplastic) (49). The simplified optical and location-based resect and discard strategy generated surveillance suggestions that concurred with pathology results for at least 90% of average-risk patients.
The use of NBI and other electronic IEE were studied in multiple studies in patients with IBD and was not found to improve neoplasia detection (50-54). One study even showed higher dysplasia rate (55). A meta-analysis of 4 studies on surveillance and management of dysplasia in IBD showed no significant advantage of NBI over chromoendoscopy, with a slight preference for chromoendoscopy (25). The prevalence of dysplasia detection was 0.1% to 22% higher with chromoendoscopy compared to NBI, however the difference was not significant. The study also did not demonstrate a significant difference with a risk ratio of 1.3 (0.8–2.1) and an absolute risk difference of 6% (−1% to 14%). Similar results were found in a large multi-center prospective RCT of 131 patients (56). All things considered, NBI is not recommended for IBD surveillance (57).
In order to enhance the accuracy of in vivo diagnosis using NBI, various training modules have been developed. Improving accuracy and modest agreement boost (κ=0.56 for novices, κ=0.70 for trainees, and κ=0.54 for experienced colonoscopists) has been seen using still images in training sessions led by experts or a validated PowerPoint presentation (58). Short video clips of polyps were found to be more effective in improving diagnostic accuracy for non-academic and community-based gastroenterologists (59). Several studies have shown improvement of diagnostic performance of CAD systems using NBI (60-62).
CLE
CLE is a technique used to image cells and structures within 250 micrometers of a mucosal surface (63,64). It uses a low-power laser that is focused on a single point to create an optical section image with gray tones. There are two types of CLE systems: integrated CLE (iCLE, Pentax), which is no longer commercially available, and probe-based CLE (pCLE), which is still commercially available. A pCLE (Cellvizio Endomicroscopy System, Paris, France) system consists of a flexible mini-probe that can be inserted through a standard endoscope working channel (65,66). Fluorescence dyes can be applied topically or intravenously to improve the visualization of cellular, subcellular, and vessel structure.
Polyp detection and characterization improvement has been shown in multiple studies. In the meta-analysis of 91 studies mentioned previously, overall sensitivity of CLE was 93.3%, specificity was 89.90%, with negative predictive value of 94.8% (39).
The Mainz classification was the first system to categorize colonic polyps for iCLE based on normal, regenerative, and dysplastic epithelium, and showed overall accuracy of 85.6%, 95.6%, and 92.2%, respectively for each observer, and κ values of the intra-observer agreement were 0.68, 0.84, and 0.77, respectively for each observer, and k=0.73 for interobserver agreement (67). The Miami classification for pCLE, used for both upper and lower GI tracts, identifies dysplasia with irregular, dark and thickened epithelium (68).
An improvement in accuracy was observed with pCLE training to correctly identify benign and neoplastic colorectal lesions, increasing from 63% (lesions 1–20) to 86% for the final set (lesions 61–76) (69). Several studies have demonstrated increase in diagnostic yield in the assessment of polyps in colon and identification of lesions in IBD, thereby reducing the need for biopsies (22,63). CAD-based systems have been developed to automate classification of colonic polyps using intravenous fluorescein pCLE with high performance comparable to expert endoscopists (70).
FICE
FICE is superior to NBI and Chromoendoscopy with dyes, as it provides better visualization without exposing the patient to the dyes. Most studies are directed towards establishing the efficacy of FICE in in vivo differentiation between neoplastic and non-neoplastic polyps (71-73). This differentiation is mainly done based on the surface capillary pattern of the adenoma using classification given by Teixeira (74).
Longcroft-Wheaton et al. conducted a large prospective study on surveillance population in UK and the results indicated that FICE could predict in vivo histology of polyps (<10 mm in size) with 86% accuracy compared to WLE which predicted the in vivo histology with 71% accuracy (71). A more recent study conducted in Vietnam tested the diagnostic capabilities of FICE in differentiating neoplastic from non-neoplastic polyps. This study showed that the FICE was 92.1% sensitive, 68.5% specific and 88.3% accurate (72). This can be utilized as a basis for polyp resection and discard policy, thereby decreasing overall healthcare burden and cost.
Even though FICE is useful for endoscopic differentiation between neoplastic/non-neoplastic lesions, the ADR of FICE is comparable to WLE (75,76). One of the studies utilized FICE for early detection of colon dysplasia in patients with longstanding ulcerative colitis (UC) and concluded that FICE is not better than white light colonoscopy for screening of dysplasia (77). Compared to other imaging techniques, FICE is limited in predicting the extent of submucosal invasion of neoplastic polyps (72).
BLI
NBI can allow the detailing of micro-vessel pattern on the surface of a GI mucosal lesion, but it does not allow a clear visualization of microstructural architecture of the lesions. Also, the images produced by NBI are darker and therefore do not allow the observation of wide mucosal area. To overcome this limitation, FICE was introduced which allows for brighter images but the microvascular pattern on the tumor surface is not as sharp as NBI. BLI compensates for the limitations of both these techniques and produce bright detailed images of microvasculature and microstructure of these tumors (7). The light source used in BLI is laser instead of xenon lamps used in NBI and FICE. This makes BLI more cost effective as it used less energy and requires relatively less frequent replacements (78).
Yoshida et al. conducted various studies in multiple tertiary care centers in Japan. One of their studies concluded that BLI can accurately predict the histological diagnosis and therefore can differentiate between neoplastic and non-neoplastic polyps (78,79). The accuracy of the results was found to be 99.3% in this study (79) which is relatively similar to FICE (72). They also showed that depth of invasion by colorectal cancers can also be predicted with high accuracy using the same Hiroshima classification (80), which is also used for NBI (78). JNET classification for NBI was also found to be equally predictive for in vivo diagnosis of colorectal lesions using BLI (81).
A more recent study indicated that BLI bright mode, compared to WLE, can increase the visibility of colorectal polyps especially regarding polyp location, size and morphology (82). However, ADR with BLI was found to be comparable to WLE with multiple studies, including a large multicenter randomized control trial (83-85). Koehn et al. reported that BLI can be taught to trainee endoscopists with online module and the improvement in accuracy of polyp characterization seemed to correlate with the years in training. Overall diagnostic accuracy improved from 74.7% (before the training) to 85.4% (after the training), P<0.01 (86).
Even though BLI has high efficacy in characterizing and differentiating neoplastic polyps but there are no studies using BLI for other colorectal diseases. There is a need for more studies employing BLI for diagnosis and surveillance of other diseases as well.
LCI
LCI is a novel technology, introduced by Fujifilm Co., Tokyo, Japan. LCI involves pre-processing of narrow band radiation and a specific color technology during post-processing which separates the colors into blue, green and red. These separated colors are reallocated to improve the visible color contrast. This combination of pre- and post-processing enhances the color difference between colorectal lesions and surrounding normal mucosa. LCI shows polyps and inflammation as red while the surrounding normal mucosa appears white.
Yoshida et al. conducted a study which showed that LCI improved visibility scores of polyps compared to WLE and BLI-bright mode (87). However, another multicenter study conducted by the same group reported that LCI was not significantly better than BLI-bright with regards to mean polyp visibility scores (3.1±0.9) vs. (3.0±1.0) (P=0.19), however, it was significantly better than WLE (2.5±1.0, P<0.001) in mean polyp visibility scores (88). Both these studies were performed by examining pre-recorded videos and pictures. There is a need for more studies involving active utilization of LCI during colonoscopies to establish the superiority of LCI over BLI-bright in mean polyp visibility scores.
A study conducted on still images compared LCI with WLE and BLI-bright mode reported that LCI improved the visibility of non-granular flat colorectal lesions. The visibility scores reported as mean ± standard deviation for LCI, BLI-bright mode and white light were 3.36±0.72, 2.94±0.97 and 2.74±1.08, respectively (89).
Suzuki et al. recently published the results of a large multicenter randomized control trial from Asia. It showed that LCI improves the ADR compared to WLE (58.7% vs. 46.7%; P<0.01) (90). This has been consistently reported by many studies previously including multicenter, randomized control trials (91-94). This increase in ADR is especially dependent on increased detection of adenomas in right sided colon where adenomas adenoma miss rate (AMR) is higher (94). There is also an increased detection of diminutive polyps with LCI which is also a contributing factor to the increased ADR with this technique (95,96).
Sessile serrated adenomas/polyps (SSA/P) found in the right colon are missed frequently because they are flat, and they are usually precursor lesions to colorectal cancer. The previously mentioned large international RCT also reported increased detection of SSL with LCI compared to WLE (4.8% vs. 2.8%; P<0.01) (90). Various other studies have reported similar results (87,95,97). This is thought to be due to the ability of LCI to differentiate between normal and abnormal mucosa based on their color. As the vascular pattern of colorectal mucosa is enhanced with LCI, the main finding to detect SSA/P is a discontinuation of vascular pattern (93).
Residual fluid in the colon interferes with the detection of polyps in the colon with NBI and BLI as they change the color of this fluid to dark red which interferes with the visibility. LCI keeps the color of residual to yellow, similar to WLE, and therefore enhances the visibility and detection of the polyps (93). Underwater endoscopic mucosal resection is becoming a new technique for polyp resection and LCI can aid in this by allowing more visibility under cloudy residual fluid (98).
Based on the efficacy of LCI in enhancing the visibility of polyps, improving the ADR and detection of SSA/P, many studies have recommended the use of LCI during routine colonoscopies (95) because it can contribute towards a decreased rate of interval cancer (93). Also, because of the increased detection of adenomas and polyps, LCI was found to shorten the recommended surveillance schedules leading to more frequent colonoscopies (90). There is a need for more extensive studies focusing on the outcome measures of such increased detection rates. LCI has also shown to improve the detection of any residual inflammation in UC and non-relapse rates can be strongly predicted with LCI classification of mucosal damage (99).
i-SCAN
i-SCAN was introduced by Pentax Medical, Tokyo, Japan. It involves image enhancement by using 3 modes: surface enhancement (SE), contrast enhancement (CE) and tone enhancement (TE). The utility of these modes is different. SE is better for visualizing polyps as it differentiates between normal and abnormal mucosa. CE can aid in determining the depth of mucosa involvement. TE augments microvasculature abnormalities (100). Standardized factory settings currently available in i-SCAN processor are i-SCAN 1 (includes SE), i-SCAN 2 (includes combination of SE and TE) and i-SCAN 3 (includes combination of SE, CE and TE), these are recommended for lesion detection, characterization and localization, respectively (9).
The data on reporting ADR for i-SCAN compared to WLE has shown mixed results. One recent meta-analysis published by Aziz et al. reported increased ADR with i-SCAN compared to HD colonoscopy (RR: 1.20; 95% CI: 1.06–1.34, P =0.003) (101). Multiple other studies (102,103) have also reported an increased ADR compared to HD-WLE, including a randomized control trial. However, another prospective, randomized trial compared various i-SCAN modes to HD-WLE colonoscopy and reported no improvement in ADR (104). i-SCAN was also found to have more efficacy in predicting right-sided diminutive polyps compared to HD-WLE colonoscopy (103).
There have been consistent reports of i-SCAN predicting the histology of colorectal polyps with relatively high accuracy (105,106). Multiple studies have also shown that i-SCAN can predict histology of diminutive colorectal polyps with high accuracy in real-time (107-109). Lee et al. compared the diagnostic accuracies of NBI and i-SCAN in predicting in vivo histology of intermediate to large colorectal polyps. The results were comparable for both the techniques 73.7% for NBI vs. 75.8% for i-SCAN, P=0.744 (110). Another study conducted in South Korea reported similar results for diminutive polyps (108).
Neumann et al. (111) reported that i-SCAN can improve the diagnosis regarding the activity and extent of disease in patient with IBD while Iacucci et al. (112) reported that mucosal inflammation detected by i-SCAN correlated with histological scores of acute and chronic inflammatory changes. Currently, DCE is used for detection of neoplasia in IBD and the studies comparing i-SCAN with DCE for surveillance of dysplasia in IBD report similar results (113,114). However, both studies reported shorter withdrawal times compared to DCE. Another study by Iacucci et al. reported that in patients with Mayo Endoscopic Scores (MES) of 0 for UC were found to have subtle mucosal changes which can be detected by i-SCAN (115). This can aid in stratifying patients with MES 0 for their risk of disease, without any biopsy.
There is a need for more large studies employing the use of i-SCAN for detection of other colorectal lesions such as SSP/A and right sided lesions, and their effects on the patient outcomes. The development of standardized histological classification for in vivo diagnosis of polyps with i-SCAN is also essential because most of the studies utilized the histological classification developed for NBI for in vivo histological diagnosis with i-SCAN.
Autofluorescence endoscopy
AFI detects changes in the colonic mucosa based on color differences generated by the normal and abnormal tissue. The molecules present in the tissues such as collagen, flavins and porphyrins called fluorophores emit fluorescence of varied colors depending on their relative concentrations within the tissue, and these color differences between the tissues allows for the detection and characterization of colonic lesions (116). Normal mucosa usually appears green with AFI, while mucosal thickening due to neoplasm or inflammation appears magenta (117).
ADR with AFI has shown inconsistent results across multiple studies. A large meta-analysis including six studies has shown that ADR was not significantly different from WLE (OR 1.01; 95% CI: 0.74 – 1.37) (118). Moriichi et al. reported that AFI dramatically improved ADR in less experienced endoscopists compared to WLE (30.3% vs. 7.7%) (119). However, these results were not replicated in experienced endoscopists. Both studies also showed that duration of AFI was longer than conventional endoscopy (118,119). A recent large, multicenter, RCT demonstrated that AFI can detect significantly higher number of flat neoplastic lesions compared to white light imaging (120).
Multiple studies report reduction in AMR with AFI compared to WLE (118,119,121). However, a study conducted by van den Broek et al. reported no significant reduction in AMR with AFI when comparing it to high resolution endoscopy (122).
Rotondano et al. reported that AFI had a poor accuracy of 63% in differentiating neoplastic from non-neoplastic adenomas. However, the accuracy seemed to improve to 84% by using AFI with NBI (123). Another study conducted in Netherlands showed similar accuracy of 62%, while the sensitivity and specificity for differentiating colonic lesions were 90% and 37%, respectively (124). A study utilizing AFI to distinguish adenoma from HPs reported 84.9% accuracy, while the conventional endoscopy had 75.9% accuracy (125). However, another study reported only 65% accuracy with AFI in differentiating adenoma from HPs and 55% accuracy in differentiating sessile serrated adenomas from HPs (116). AFI color intensity was also correlated with grade of dysplasia in colorectal adenomas, and it can be used to predict degree of dysplasia in vivo (126).
The utilization of quantified AFI in assessing the severity of mucosal inflammation in UC resulted in a diagnostic accuracy of 84.7% vs. conventional endoscopy 78.5%, P<0.01 (127). Osada et al. showed that MES correlated with quantified green color during AFI. They also found an associated between green color and polymorphonuclear cell infiltration in patients who had mucosal inflammation classified as MES 0 (128). Van den Broek et al. also reported that AFI increased the detection of neoplasia in patients with long-standing UC (129).
There has also been a study utilizing AFI for differentiating lymphoma from lymphoid hyperplasia. The study used a visual classification system based on the color intensities generated by AFI and this resulted in an overall accuracy of 91.5% in diagnosing lymphoma (130).
Endocytoscopy (EC)
It is a contact type optical endoscope used to visualize living cells in GI tract mucosa with the optical magnification of up to 520×, providing white light images of ultra-high resolution. Dye staining is essential for better visualization during EC, and for this purpose, methylene blue, toluidine blue and crystal violet are used. For detection of squamous cell dysplasia/cancer, 1% methylene blue is preferred. However, for the detection of colonic adenomas, 1% toluidine blue is preferred. After staining, EC allows detailed visualization of cellular and tissue features such as cell nuclei size and shape, polarity, nuclear dye intensity as well as papillae, crypt and gland shapes and sizes, their integrity. This allows for in vivo histological diagnosis, commonly referred to as “optical biopsy” or “virtual histology” (131-133).
Multiple studies have reported high accuracy of EC for differentiating non-neoplastic colorectal lesions from neoplastic lesions. Kudo et al. developed a classification for in vivo histological diagnosis based on endocytoscopic appearance of the lesions. They reported 100% specificity and sensitivity in differentiating non-neoplastic lesions from neoplastic lesions (134). Several other studies reported similar efficacy with EC (131,135). A recent study utilized EC for differentiating diminutive polyps, the accuracy, sensitivity, specificity, positive predictive value and negative predictive value were all more than 90% in differentiating neoplastic diminutive polyps from non-neoplastic (136).
SSA/Ps are frequently misdiagnosed because of their morphological appearance. EC can efficiently differentiate between HPs and SSA/P in vivo. The main characteristic used to detect SSA/Ps was oval glandular lumens (137,138). Ogawa et al. further quantified the size of lumen and reported that SSA/Ps have lumens which are twice as big as HPs lumens (mean luminal areas 4,152 vs. 2,117 µm2, P< 0.001) (139).
Mori et al. compared the diagnostic accuracies of EC with standard biopsy for diagnosing colorectal neoplasia. Results showed diagnostic accuracy of 94.1% with EC vs. 96.0% with standard biopsy. They established that EC is non-inferior to standard biopsy and could be used as an alternative for diagnosing colorectal neoplasia during routine colonoscopies (140). Several studies have utilized EC for predicting depth of invasion of colorectal cancer using endocytoscopic classification (based on the shape of the glands and lumens) (135,141), endocytoscopic-vascular pattern (EC-V) classification (based on the appearance surface micro-vessels) (142) or detecting desmoplastic reaction in tumor surface (143). All of them found EC to be highly accurate in predicting depth of invasion.
Multiple computer-aided systems have been developed based on EC and they have shown high accuracy, sensitivity and specificity which were comparable with expert endoscopists, but better than non-experts (144-147). This shows that CAD can be used for facilitation of diagnosis in non-expert settings in conjunction with EC.
Bessho et al. found a relationship between Matts’ histologic score used for stratifying patients with UC and EC findings. Based on their findings, they developed the EC system score (ECSS). They reported a strong correlation between ECSS and Matts’ histologic score (148,149). Another study reported that EC can precisely identify the different inflammatory cells (e.g., basophils, eosinophils, and neutrophils) in the mucosa of patients with UC (150).
Full spectrum endoscopy (FUSE)
FUSE (developed by EndoChoice Inc., Alpharetta, GA, USA) allows a panoramic view of 330 degrees of the GI tract. It has multiple cameras placed on the tip of the scope along with LEDs (instead of xenon light source used by most other colonoscopes), which allows a wide-angle view of entire GI tract. The images taken from the FUSE are displayed on three separate monitors. Because of its broader view, FUSE is being marketed as a product to improve the visibility of GI lesions, especially flat and sessile adenomas which can be missed by conventional HD-WLE, thereby decreasing AMRs. Most studies done since the conception of this technique have been focused on checking the efficacy of this technique by comparing the AMR from FUSE vs. standard forward viewing colonoscope (FVC). As such, Gralnek et. conducted a multicenter RCT across multiple countries and results showed significantly reduced AMR in patients who underwent FUSE vs. standard FVC (7% vs. 41%; P<0.0001) (151). During this back-to-back tandem study, another notable finding was that standard FVC had missed three advanced adenomas, however, none of the adenomas missed with FUSE were advanced (151). Kudo et. al demonstrated similar results for their tandem colonoscopy trial with AMR for FUSE (11.7%) vs. standard FVC (22.9%), P<0.001. They also demonstrated efficacy of FUSE in detecting lesions <5 mm in size (AMR for <5 mm lesions with FUSE vs. standard FVC; 10.4% vs. 20.0%; P=0.0057) as well as lesions in ascending colon (AMR for ascending colon lesions with FUSE vs. standard FVC; 4.3% vs. 10.6%; P=0.0212) (152). One study compared the FUSE with colonoscopy along with scope retroflexion to better visualize the right colon. The results of this study, given as AMR which was similar to previous studies (153). They also demonstrated that FUSE and standard colonoscopy with retroflexion in right colon had similar withdrawal times (153). Hassan et al., however, demonstrated that FUSE was not better than standard FVC in detection of adenomas and sessile serrated polyps (154). A recent study conducted in Germany compared the accuracy of FUSE with HD-WLE for detection of disease activity in patients with IBD. Their results indicated that FUSE was not significantly better than HD-WLE for assessment of patients with IBD (155).
Colonoscopy “Add-on” devices
Various devices have been developed which can be attached to the distal end of colonoscope to allow better visualization of colon mucosa, thereby increasing ADR. Cap-assisted colonoscopy (CC) is a straightforward attachment on the distal end of colonoscope. It extends beyond the tip of colonoscope to various lengths and allows deflection of colon mucosa for better visualization. A recent meta-analysis of seven studies showed an overall improvement of ADR for CC compared to standard colonoscopy (156). Another large network meta-analysis including 25 RCTs demonstrated an absolute increase in ADR to 11.3% for low performing endoscopists (baseline ADR 10%) and to 45.2% for high performing endoscopists (baseline ADR 40%), albeit the evidence for CC and Endorings was very low-quality and for Endocuff was low-quality (157). The head-to-head analysis done in their study yielded very low-quality evidence, with no significant differences in ADR between three devices included in their study; CC, endocuff and Endorings. Additional devices which provide a broader view of GI tract include third-eye retroscope (TER) and third-eye panoramic (TEP). TER has been shown to increase the polyp and ADRs without causing a large increase in the withdrawal times (158,159). TEP is more recent and there is paucity of data to establish its efficacy regarding the improvement of ADR. Endocuff, Endocuff- Vision, EndoRings and Balloon assisted-colonoscopy-The G-EYE allow improved visualization of colon during withdrawal by mechanically stretching the colon folds. Multiple RCTs have demonstrated improved ADR and reduced AMR with these devices (157,160-168).
Future direction
Optivista, introduced by Pentax in 2016, uses a mechanical optical filter to enhance the characterization of colorectal polyp surfaces and blood vessels (169). A recent RCT showed that Optivista performed similar to i-SCAN with >90% NPV for rectosigmoid adenomas (170).
Another new IEE is red dichromatic imaging (RDI, Olympus). It uses red (630 nm), amber (600 nm) and green (540 nm) wavelengths to enhance the visibility of deep blood vessels (171). This helps identify potential bleeding points. RDI has three modes: mode 1 is useful for detecting bleeding points and modes 2 and 3 help in visualizing superficial and deep vessels (5). Hirai et al. in a study of 64 lesions, demonstrated improved visibility of bleeding points in acute GI bleeding compared with WLE (172).
Another new IEE technology was recently launched by Olympus called Texture and Color Enhancement Imaging (TXI), which enhances the three components of WLE (color, brightness, and texture) to better visualize subtle differences in the tissues (173). TXI divides normal white light images into a texture image and a base image, then recombines them to enhance the mucosal surface’s structure, color tone, and brightness (169). Studies have shown that TXI provides higher visibility than WLE for colorectal polyps, including SSLs, and improved visualization of colorectal lesions compared to WLE and NBI (174,175).
CAD using AI for colonoscopy is a developing technology aimed at improving polyp detection and predicting the pathology by characterization (CADx) of detected lesions (176). The first FDA-approved computer-aided polyp detection system was GI Genius (Medtronic Corp, Dublin, Ireland) (177). Several AI systems have been validated and approved for use in medical practice which include GI Genius (Medtronic Corp), Endo AID, (Olympus Corp), CAD EYE (Fujifilm Corp), Discovery, (Pentax), EndoBRAIN (Cybernet Corp, Tokyo, Japan) (178). There has been an explosion of multiple RCTs being published using AI systems from various parts of the world. which have shown significant improvement in quality metric outcomes. A recent systemic review and network meta-analysis of 50 RCTs compared CAD systems with chromoendoscopy and mucosal exposure techniques using various distal attachments. This study showed that CAD demonstrated higher ADR compared to other technologies including standard WLE, use of mucosal exposure devices and CE (179). Even though the results from these initial studies have been promising, more recent studies published from real world data have shown contradicting results with several negative studies (180-183). Several hypotheses have been postulated as possible reasons for the results not translating to the real world, some of which are possible ceiling effect of polyp detection seen with experienced endoscopists, a false sense of comfort among endoscopists assuming CAD would help with a high-quality examination, leading to decrease in the endoscopist quality of mucosal exposure and the unblinded nature of the studies leading to bias among endoscopists during examination. Moreover, most of the initial studies have been performed with strict inclusion criteria and that may certainly impact its translation to the real world when a controlled environment for patient selection does not exist. There is still a significant need for more of these real-world studies and possibly training AI algorithms with inputs from various patient groups. There is also a need for more studies comparing with, or using IEE techniques along with AI, which could certainly improve endoscopic examinations and the outcomes in the future, but as of now, it remains to be seen.
The wide distribution of IEE may be hindered by factors such as perceptions of technology inefficiencies and difficulties in use, high cost and low compensation, lack of standard training, and limited high-quality comparison studies (4). Further research is underway to determine the efficacy and reliability of these new IEE modalities.
Summary
Colonoscopy is an effective tool for detecting and preventing colorectal cancer, but its success depends on reliably detecting colorectal neoplasia. Incorporating advanced imaging technologies such as IEE to characterize lesions in real time diagnosis play an important role.
Since the advent of flexible GI endoscopy in the 1960s, advancements in endoscopic imaging technology have been continuous. From replacing fiber optics with charge-coupled device (CCD) (9), to introduction of chromoendoscopy in 1970s, we have come a long way (184). In recent years, “push-button” technologies such as narrowed-spectrum endoscopy (e.g., NBI, FICE, BLI, LCI, i-SCAN) and AFI has made advanced imaging more accessible, while CLE has given endoscopists the ability to view “in vivo histology” (64).
DCE is a technique in which dyes or stains are applied to the digestive tract through a spraying catheter during endoscopy, to improve the identification and assessment of abnormalities. There are two main categories of dyes used for evaluating colonic lesions: absorptive dyes (e.g., crystal violet and methylene blue) and contrast dyes (e.g., indigo carmine). DCE is falling out favor due to the longer time required for application and removal of dye and the difficulty of identifying lesions in certain situations.
NBI is a technology that filters the light used for illumination leading to improved contrast for small blood vessels close to the surface and better visibility of the structures on the mucosal surface for differentiating between abnormal and normal tissue. It has shown a consistently higher ADR compared to WLE. Classifications such as NICE and JNET were developed to standardized lesion inspection and have significantly increased accuracy of in vivo diagnosis. Both ASGE and ESGE endorse the use of NBI for diagnosis of polyps ≤5 mm. However, NBI and other electronic IEE were not found to improve neoplasia detection in patients with IBD.
CLE is a medical imaging technique used to view cells and structures within 250 micrometers of a mucosal surface. CLE has high sensitivity (93.3%) and specificity (89.9%) for polyp detection and characterization and several classifications systems, such as the Mainz and Miami classifications, have been developed for accurate identification of normal, regenerative, and dysplastic epithelium. The diagnostic yield for identification of colorectal lesions and assessment of polypoid lesions in colon has been improved through CLE and the development of CAD-based systems.
Most studies in current literature establish the efficacy of FICE for in vivo differentiation between neoplastic and non-neoplastic polyps. This differentiation is mainly done based on the classification given by Teixeira. The ADR of FICE is comparable to WLE.
BLI produces bright detailed images of microvasculature and microstructure of the colorectal tumors to allow better characterization of the tumors. BLI can accurately predict the histological diagnosis and therefore can differentiate between neoplastic and non-neoplastic polyps. The ADR with BLI was found to be comparable to WLE in multiple studies, including a large multicenter randomized control trial.
LCI has shown to improve the ADR compared to white light (58.7% vs. 46.7%; P<0.01) (90). LCI also improves detection of SSL and diminutive polyps (86,95,96). LCI keeps the color of residual as yellow, similar to white light, and therefore enhances the visibility and detection of the polyps (93).
i-SCAN has 3 modes for image enhancement: SE, CE and TE with SE being better for polyp characterization. Reports for ADR with i-SCAN have not been consistent; however, multiple studies have reported high accuracy of i-SCAN for predicting polyp histology.
AFI can differentiate between normal and abnormal mucosa based on their color differences. ADR with AFI has shown mixed results, however, the AMR seemed to improve with AFI compared with WLE. The color intensity in AFI was also found to correlate with mucosal inflammation with UC. One of the studies reported correlation of green color with number of polymorphonuclear cells (128).
EC has also been called a technique of “virtual histology”. It can efficiently differentiate between neoplastic and non-neoplastic polyps. EC can also recognize SSA/P by visualizing the oval lumens of the glands present in these lesions. The diagnostic accuracy for in vivo neoplasia recognition by EC is comparable to standard biopsy.
FUSE allows a wide-angle view of 330 degrees during colonoscopy and can better detect the lesions which are usually missed by standard HD-WLE. Many studies have demonstrated significantly lower AMR for FUSE compared to forward-viewing colonoscope.
Various add-on devices have been developed with an aim of increasing the ADR during colonoscopy. They mainly work by either improving the field of view by attaching distal devices (TER and TEP) or by mechanically flattening the colonic folds (Endocuff, Endocuff-Vision, EndoRings, The-G-EYE). Multiple RCTs and meta-analysis have supported their efficacy by demonstrating an increased ADR and reduced AMR with these devices.
New technologies are under way. Optivista, uses a mechanical optical filter to enhance the characterization of colorectal polyp surfaces and blood vessels and performs similar to i-SCAN with a high negative predictive value for rectosigmoid adenomas. Another IEE technology, RDI, uses green, amber, and red wavelengths to enhance the visibility of deep blood vessels, improving the localization of bleeding points in acute GI bleeding compared to WLE. Texture and color enhancement imaging (TXI) enhances the texture, brightness, and color of WLE to better define subtle tissue differences.
CAD using AI for colonoscopy is aimed at improving polyp detection and pathology prediction. Initial studies have shown great promise with the adaptation of AI algorithms but there is a need for more real-world data with training these algorithms using datasets from diverse population groups.
Conclusions
High-quality colonoscopy is essential to improve ADR s and decrease the rates of interval cancers. As more population-based screening programs are established around the world, the volume of colonoscopies will go up and can create a huge burden in healthcare and a drive to improve efficiency of these procedures. Several IEE technologies have been developed over time, which have assisted in improving the visualization of colonic mucosa, detection and characterization of polyps and neoplastic tissue. The role of these technologies will only continue to expand our ability to perform these procedures and strive to achieve perfection. Barriers for their standardized use in clinical practice continue to exist, given the issues with ease of access, costs, lack of standardized training, reimbursements, and constant evolution. With the advent of AI and in combination with IEE, there is more research developing in this topic, which could significantly impact the future of screening and surveillance colonoscopies, along with diagnostic and therapeutic endoscopies in general.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Gastroenterology and Hepatology for the series “Colonoscopy: Updates and Prospects”. The article has undergone external peer review.
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-17/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-17/coif). The series “Colonoscopy: Updates and Prospects” was commissioned by the editorial office without any funding or sponsorship. VTC served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362:1795-803. [Crossref] [PubMed]
- Sonnenberg A, Amorosi SL, Lacey MJ, et al. Patterns of endoscopy in the United States: analysis of data from the Centers for Medicare and Medicaid Services and the National Endoscopic Database. Gastrointest Endosc 2008;67:489-96. [Crossref] [PubMed]
- Stock C, Brenner H. Utilization of lower gastrointestinal endoscopy and fecal occult blood test in 11 European countries: evidence from the Survey of Health, Aging and Retirement in Europe (SHARE). Endoscopy 2010;42:546-56. [Crossref] [PubMed]
- Jang JY. The Past, Present, and Future of Image-Enhanced Endoscopy. Clin Endosc 2015;48:466-75. [Crossref] [PubMed]
- Aguila EJ, Beany A, Singh R. Advanced mucosal imaging in colonoscopy: technical details and clinical applications. Mini-invasive Surg 2022;6:55. [Crossref]
- Trivedi PJ, Braden B. Indications, stains and techniques in chromoendoscopy. QJM 2013;106:117-31. [Crossref] [PubMed]
- Osawa H, Yamamoto H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc 2014;26:105-15. [Crossref] [PubMed]
- ASGE Technology Committee. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2015;81:502.e1-502.e16. [Crossref] [PubMed]
- East JE, Vleugels JL, Roelandt P, et al. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy 2016;48:1029-45. [Crossref] [PubMed]
- Sano Y, Chiu HM, Li XB, et al. Standards of diagnostic colonoscopy for early-stage neoplasia: Recommendations by an Asian private group. Dig Endosc 2019;31:227-44. [Crossref] [PubMed]
- Dekker E, Nass KJ, Iacucci M, et al. Performance measures for colonoscopy in inflammatory bowel disease patients: European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2022;54:904-15. [Crossref] [PubMed]
- Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. [Crossref] [PubMed]
- American Society for Gastrointestinal Endoscopy Standards of Practice Committee; Shergill AK, Lightdale JR, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc 2015;81:1101-21.e1-13.
- Murthy SK, Feuerstein JD, Nguyen GC, et al. AGA Clinical Practice Update on Endoscopic Surveillance and Management of Colorectal Dysplasia in Inflammatory Bowel Diseases: Expert Review. Gastroenterology 2021;161:1043-1051.e4. [Crossref] [PubMed]
- Buchner AM. The Role of Chromoendoscopy in Evaluating Colorectal Dysplasia. Gastroenterol Hepatol (N Y) 2017;13:336-47. [PubMed]
- Singh R, Chiam KH, Leiria F, et al. Chromoendoscopy: role in modern endoscopic imaging. Transl Gastroenterol Hepatol 2020;5:39. [Crossref] [PubMed]
- Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003;124:880-8. [Crossref] [PubMed]
- Repici A, Wallace MB, East JE, et al. Efficacy of Per-oral Methylene Blue Formulation for Screening Colonoscopy. Gastroenterology 2019;156:2198-2207.e1. [Crossref] [PubMed]
- Kudo S, Tamura S, Nakajima T, et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 1996;44:8-14. [Crossref] [PubMed]
- Brown SR, Baraza W, Hurlstone P. Chromoscopy versus conventional endoscopy for the detection of polyps in the colon and rectum. Cochrane Database Syst Rev 2007;CD006439. [PubMed]
- Tamai N, Saito Y, Sakamoto T, et al. Visualization of laterally spreading colorectal tumors by using image-enhanced endoscopy. Gastroenterol Res Pract 2012;2012:638391. [Crossref] [PubMed]
- Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology 2007;132:874-82. [Crossref] [PubMed]
- Hlavaty T, Huorka M, Koller T, et al. Colorectal cancer screening in patients with ulcerative and Crohn's colitis with use of colonoscopy, chromoendoscopy and confocal endomicroscopy. Eur J Gastroenterol Hepatol 2011;23:680-9. [Crossref] [PubMed]
- Iannone A, Ruospo M, Wong G, et al. Chromoendoscopy for Surveillance in Ulcerative Colitis and Crohn's Disease: A Systematic Review of Randomized Trials. Clin Gastroenterol Hepatol 2017;15:1684-97.e11. [Crossref] [PubMed]
- Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148:639-651.e28. [Crossref] [PubMed]
- Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis 2017;11:649-70. [Crossref] [PubMed]
- Ho SH, Uedo N, Aso A, et al. Development of Image-enhanced Endoscopy of the Gastrointestinal Tract: A Review of History and Current Evidences. J Clin Gastroenterol 2018;52:295-306. [Crossref] [PubMed]
- Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 2016;28:526-33. [Crossref] [PubMed]
- Machida H, Sano Y, Hamamoto Y, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy 2004;36:1094-8. [Crossref] [PubMed]
- Hirata M, Tanaka S, Oka S, et al. Evaluation of microvessels in colorectal tumors by narrow band imaging magnification. Gastrointest Endosc 2007;66:945-52. [Crossref] [PubMed]
- Teramoto A, Hamada S, Ogino B, et al. Updates in narrow-band imaging for colorectal polyps: Narrow-band imaging generations, detection, diagnosis, and artificial intelligence. Dig Endosc 2023;35:453-70. [Crossref] [PubMed]
- Dinesen L, Chua TJ, Kaffes AJ. Meta-analysis of narrow-band imaging versus conventional colonoscopy for adenoma detection. Gastrointest Endosc 2012;75:604-11. [Crossref] [PubMed]
- Pasha SF, Leighton JA, Das A, et al. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol 2012;107:363-70; quiz 371. [Crossref] [PubMed]
- Atkinson NSS, Ket S, Bassett P, et al. Narrow-Band Imaging for Detection of Neoplasia at Colonoscopy: A Meta-analysis of Data From Individual Patients in Randomized Controlled Trials. Gastroenterology 2019;157:462-71. [Crossref] [PubMed]
- Kawasaki A, Yoshida N, Nakanishi H, et al. Usefulness of third-generation narrow band imaging and texture and color enhancement imaging in improving visibility of superficial early gastric cancer: A study using color difference. DEN Open 2022;3:e186. [Crossref] [PubMed]
- Schauer C, Chieng M, Wang M, et al. Artificial intelligence improves adenoma detection rate during colonoscopy. N Z Med J 2022;135:22-30. [PubMed]
- Wong YT, Tai TF, Wong KF, et al. The study on artificial intelligence (AI) colonoscopy in affecting the rate of polyp detection in colonoscopy: A single centre retrospective study. Surg Pract 2022;26:115-9. [Crossref]
- Gimeno-García AZ, Hernández Negrin D, Hernández A, et al. Usefulness of a novel computer-aided detection system for colorectal neoplasia: a randomized controlled trial. Gastrointest Endosc 2023;97:528-536.e1. [Crossref] [PubMed]
- Wanders LK, East JE, Uitentuis SE, et al. Diagnostic performance of narrowed spectrum endoscopy, autofluorescence imaging, and confocal laser endomicroscopy for optical diagnosis of colonic polyps: a meta-analysis. Lancet Oncol 2013;14:1337-47. [Crossref] [PubMed]
- McGill SK, Evangelou E, Ioannidis JP, et al. Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics. Gut 2013;62:1704-13. [Crossref] [PubMed]
- Chiu HM, Chang CY, Chen CC, et al. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut 2007;56:373-9. [Crossref] [PubMed]
- Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc 2013;78:625-32. [Crossref] [PubMed]
- Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012;143:599-607.e1. [Crossref] [PubMed]
- East JE, Vieth M, Rex DK. Serrated lesions in colorectal cancer screening: detection, resection, pathology and surveillance. Gut 2015;64:991-1000. [Crossref] [PubMed]
- IJspeert JE, Bastiaansen BA, van Leerdam ME, et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut 2016;65:963-70. [Crossref] [PubMed]
- Hirata D, Kashida H, Matsumoto T, et al. A Multicenter Prospective Validation Study on Selective Endoscopic Resection of Sessile Serrated Lesions Using Magnifying Colonoscopy in Clinical Practice. Digestion 2023; Epub ahead of print. [Crossref] [PubMed]
- Minegishi Y, Kudo SE, Miyata Y, et al. Comprehensive Diagnostic Performance of Real-Time Characterization of Colorectal Lesions Using an Artificial Intelligence-Assisted System: A Prospective Study. Gastroenterology 2022;163:323-325.e3. [Crossref] [PubMed]
- Kamiński MF, Hassan C, Bisschops R, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2014;46:435-49. [Crossref] [PubMed]
- von Renteln D, Kaltenbach T, Rastogi A, et al. Simplifying Resect and Discard Strategies for Real-Time Assessment of Diminutive Colorectal Polyps. Clin Gastroenterol Hepatol 2018;16:706-14. [Crossref] [PubMed]
- Efthymiou M, Allen PB, Taylor AC, et al. Chromoendoscopy versus narrow band imaging for colonic surveillance in inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2132-8. [Crossref] [PubMed]
- Dekker E, van den Broek FJ, Reitsma JB, et al. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy 2007;39:216-21. [Crossref] [PubMed]
- van den Broek FJ, Fockens P, van Eeden S, et al. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy 2011;43:108-15. [Crossref] [PubMed]
- Ignjatovic A, East JE, Subramanian V, et al. Narrow band imaging for detection of dysplasia in colitis: a randomized controlled trial. Am J Gastroenterol 2012;107:885-90. [Crossref] [PubMed]
- Leifeld L, Rogler G, Stallmach A, et al. White-Light or Narrow-Band Imaging Colonoscopy in Surveillance of Ulcerative Colitis: A Prospective Multicenter Study. Clin Gastroenterol Hepatol 2015;13:1776-1781.e1. [Crossref] [PubMed]
- Pellisé M, López-Cerón M, Rodríguez de Miguel C, et al. Narrow-band imaging as an alternative to chromoendoscopy for the detection of dysplasia in long-standing inflammatory bowel disease: a prospective, randomized, crossover study. Gastrointest Endosc 2011;74:840-8. [Crossref] [PubMed]
- Bisschops R, Bessissow T, Joseph JA, et al. Chromoendoscopy versus narrow band imaging in UC: a prospective randomised controlled trial. Gut 2018;67:1087-94. [Crossref] [PubMed]
- Parigi TL, Nardone OM, Iacucci M. Image-Enhanced Endoscopy Surveillance of Colon and Pouch Dysplasia in IBD. Dis Colon Rectum 2022;65:S119-28. [Crossref] [PubMed]
- Ignjatovic A, Thomas-Gibson S, East JE, et al. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc 2011;73:128-33. [Crossref] [PubMed]
- Rastogi A, Rao DS, Gupta N, et al. Impact of a computer-based teaching module on characterization of diminutive colon polyps by using narrow-band imaging by non-experts in academic and community practice: a video-based study. Gastrointest Endosc 2014;79:390-8. [Crossref] [PubMed]
- Takemura Y, Yoshida S, Tanaka S, et al. Computer-aided system for predicting the histology of colorectal tumors by using narrow-band imaging magnifying colonoscopy (with video). Gastrointest Endosc 2012;75:179-85. [Crossref] [PubMed]
- Tischendorf JJ, Gross S, Winograd R, et al. Computer-aided classification of colorectal polyps based on vascular patterns: a pilot study. Endoscopy 2010;42:203-7. [Crossref] [PubMed]
- Gross S, Trautwein C, Behrens A, et al. Computer-based classification of small colorectal polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc 2011;74:1354-9. [Crossref] [PubMed]
- Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 2004;127:706-13. [Crossref] [PubMed]
- Subramanian V, Ragunath K. Advanced endoscopic imaging: a review of commercially available technologies. Clin Gastroenterol Hepatol 2014;12:368-76.e1. [Crossref] [PubMed]
- Wang TD, Friedland S, Sahbaie P, et al. Functional imaging of colonic mucosa with a fibered confocal microscope for real-time in vivo pathology. Clin Gastroenterol Hepatol 2007;5:1300-5. [Crossref] [PubMed]
- Kiesslich R, Canto MI. Confocal laser endomicroscopy. Gastrointest Endosc Clin N Am 2009;19:261-72. [Crossref] [PubMed]
- Kuiper T, Kiesslich R, Ponsioen C, et al. The learning curve, accuracy, and interobserver agreement of endoscope-based confocal laser endomicroscopy for the differentiation of colorectal lesions. Gastrointest Endosc 2012;75:1211-7. [Crossref] [PubMed]
- Wallace M, Lauwers GY, Chen Y, et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 2011;43:882-91. [Crossref] [PubMed]
- Buchner AM, Gomez V, Heckman MG, et al. The learning curve of in vivo probe-based confocal laser endomicroscopy for prediction of colorectal neoplasia. Gastrointest Endosc 2011;73:556-60. [Crossref] [PubMed]
- André B, Vercauteren T, Buchner AM, et al. Software for automated classification of probe-based confocal laser endomicroscopy videos of colorectal polyps. World J Gastroenterol 2012;18:5560-9. [Crossref] [PubMed]
- Longcroft-Wheaton GR, Higgins B, Bhandari P. Flexible spectral imaging color enhancement and indigo carmine in neoplasia diagnosis during colonoscopy: a large prospective UK series. Eur J Gastroenterol Hepatol 2011;23:903-11. [Crossref] [PubMed]
- Pham NB, Vu KT, Nguyen NH, et al. Magnifying Chromoendoscopy with Flexible Spectral Imaging Color Enhancement, Indigo Carmine, and Crystal Violet in Predicting the Histopathology of Colorectal Polyps: Diagnostic Value in a Scare-Setting Resource. Gastroenterol Res Pract 2022;2022:6402904. [Crossref] [PubMed]
- dos Santos CE, Lima JC, Lopes CV, et al. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol 2010;22:1364-71. [Crossref] [PubMed]
- Teixeira CR, Torresini RS, Canali C, et al. Endoscopic classification of the capillary-vessel pattern of colorectal lesions by spectral estimation technology and magnifying zoom imaging. Gastrointest Endosc 2009;69:750-6. [Crossref] [PubMed]
- Aminalai A, Rösch T, Aschenbeck J, et al. Live image processing does not increase adenoma detection rate during colonoscopy: a randomized comparison between FICE and conventional imaging (Berlin Colonoscopy Project 5, BECOP-5). Am J Gastroenterol 2010;105:2383-8. [Crossref] [PubMed]
- Pohl J, Lotterer E, Balzer C, et al. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut 2009;58:73-8. [Crossref] [PubMed]
- Özdinç SA, Akpinar H, Bengi G, et al. Evaluation of colon mucosa using screening colonoscopy and flexible spectral imaging color enhancement in patients with long lasting ulcerative colitis. Croat Med J 2021;62:435-45. [Crossref] [PubMed]
- Yoshida N, Hisabe T, Inada Y, et al. The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms. J Gastroenterol 2014;49:73-80. [Crossref] [PubMed]
- Yoshida N, Yagi N, Inada Y, et al. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc 2014;26:250-8. [Crossref] [PubMed]
- Kanao H, Tanaka S, Oka S, et al. Narrow-band imaging magnification predicts the histology and invasion depth of colorectal tumors. Gastrointest Endosc 2009;69:631-6. [Crossref] [PubMed]
- Ito R, Ikematsu H, Murano T, et al. Diagnostic ability of Japan Narrow-Band Imaging Expert Team classification for colorectal lesions by magnifying endoscopy with blue laser imaging versus narrow-band imaging. Endosc Int Open 2021;9:E271-7. [Crossref] [PubMed]
- Yoshida N, Hisabe T, Hirose R, et al. Improvement in the visibility of colorectal polyps by using blue laser imaging (with video). Gastrointest Endosc 2015;82:542-9. [Crossref] [PubMed]
- Ikematsu H, Sakamoto T, Togashi K, et al. Detectability of colorectal neoplastic lesions using a novel endoscopic system with blue laser imaging: a multicenter randomized controlled trial. Gastrointest Endosc 2017;86:386-94. [Crossref] [PubMed]
- Vleugels JLA, Dekker E. Blue laser imaging: A promising new kid on the block or another tool to increase detection of low-risk adenomas? Gastrointest Endosc 2017;86:395-7. [Crossref] [PubMed]
- Shimoda R, Sakata Y, Fujise T, et al. The adenoma miss rate of blue-laser imaging vs. white-light imaging during colonoscopy: a randomized tandem trial. Endoscopy 2017;49:186-90. [PubMed]
- Koehn C, Rex DK, Allen J, et al. Optical diagnosis of colorectal polyps using novel blue light imaging classification among trainee endoscopists. Dig Endosc 2022;34:191-7. [Crossref] [PubMed]
- Yoshida N, Naito Y, Murakami T, et al. Linked color imaging improves the visibility of colorectal polyps: a video study. Endosc Int Open 2017;5:E518-25. [Crossref] [PubMed]
- Yoshida N, Hisabe T, Ikematsu H, et al. Comparison Between Linked Color Imaging and Blue Laser Imaging for Improving the Visibility of Flat Colorectal Polyps: A Multicenter Pilot Study. Dig Dis Sci 2020;65:2054-62. [Crossref] [PubMed]
- Suzuki T, Hara T, Kitagawa Y, et al. Linked-color imaging improves endoscopic visibility of colorectal nongranular flat lesions. Gastrointest Endosc 2017;86:692-7. [Crossref] [PubMed]
- Suzuki S, Aniwan S, Chiu HM, et al. Linked-Color Imaging Detects More Colorectal Adenoma and Serrated Lesions: An International Randomized Controlled Trial. Clin Gastroenterol Hepatol 2023;21:1493-1502.e4. [Crossref] [PubMed]
- Oliveira Dos Santos CE, Malaman D, Pereira-Lima JC, et al. Impact of linked-color imaging on colorectal adenoma detection. Gastrointest Endosc 2019;90:826-34. [Crossref] [PubMed]
- Min M, Deng P, Zhang W, et al. Comparison of linked color imaging and white-light colonoscopy for detection of colorectal polyps: a multicenter, randomized, crossover trial. Gastrointest Endosc 2017;86:724-30. [Crossref] [PubMed]
- Shinozaki S, Kobayashi Y, Hayashi Y, et al. Colon polyp detection using linked color imaging compared to white light imaging: Systematic review and meta-analysis. Dig Endosc 2020;32:874-81. [Crossref] [PubMed]
- Paggi S, Radaelli F, Senore C, et al. Linked-color imaging versus white-light colonoscopy in an organized colorectal cancer screening program. Gastrointest Endosc 2020;92:723-30. [Crossref] [PubMed]
- Li J, Zhang D, Wei Y, et al. Colorectal Sessile Serrated Lesion Detection Using Linked Color Imaging: A Multicenter, Parallel Randomized Controlled Trial. Clin Gastroenterol Hepatol 2023;21:328-336.e2. [Crossref] [PubMed]
- Miyaguchi K, Takabayashi K, Saito D, et al. Linked color imaging versus white light imaging colonoscopy for colorectal adenoma detection: A randomized controlled trial. J Gastroenterol Hepatol 2021;36:2778-84. [Crossref] [PubMed]
- Fujimoto D, Muguruma N, Okamoto K, et al. Linked color imaging enhances endoscopic detection of sessile serrated adenoma/polyps. Endosc Int Open 2018;6:E322-34. [Crossref] [PubMed]
- Yamasaki Y, Harada K, Yamamoto S, et al. Blue laser imaging and linked color imaging improve the color difference value and visibility of colorectal polyps in underwater conditions. Dig Endosc 2020;32:791-800. [Crossref] [PubMed]
- Sivanathan V, Tontini GE, Möhler M, et al. Advanced endoscopic imaging for diagnosis of inflammatory bowel diseases: Present and future perspectives. Dig Endosc 2018;30:441-8. [Crossref] [PubMed]
- Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with i-scan technology. World J Gastroenterol 2010;16:1043-9. [Crossref] [PubMed]
- Aziz M, Ahmed Z, Haghbin H, et al. Does i-scan improve adenoma detection rate compared to high-definition colonoscopy? A systematic review and meta-analysis. Endosc Int Open 2022;10:E824-31. [Crossref] [PubMed]
- Hoffman A, Sar F, Goetz M, et al. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy 2010;42:827-33. [Crossref] [PubMed]
- Kidambi TD, Terdiman JP, El-Nachef N, et al. Effect of I-scan Electronic Chromoendoscopy on Detection of Adenomas During Colonoscopy. Clin Gastroenterol Hepatol 2019;17:701-708.e1. [Crossref] [PubMed]
- Hong SN, Choe WH, Lee JH, et al. Prospective, randomized, back-to-back trial evaluating the usefulness of i-SCAN in screening colonoscopy. Gastrointest Endosc 2012;75:1011-1021.e2. [Crossref] [PubMed]
- Bouwens MW, de Ridder R, Masclee AA, et al. Optical diagnosis of colorectal polyps using high-definition i-scan: an educational experience. World J Gastroenterol 2013;19:4334-43. [Crossref] [PubMed]
- Pigò F, Bertani H, Manno M, et al. i-Scan high-definition white light endoscopy and colorectal polyps: prediction of histology, interobserver and intraobserver agreement. Int J Colorectal Dis 2013;28:399-406. [Crossref] [PubMed]
- Picot J, Rose M, Cooper K, et al. Virtual chromoendoscopy for the real-time assessment of colorectal polyps in vivo: a systematic review and economic evaluation. Health Technol Assess 2017;21:1-308. [Crossref] [PubMed]
- Lee CK, Lee SH, Hwangbo Y. Narrow-band imaging versus I-Scan for the real-time histological prediction of diminutive colonic polyps: a prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc 2011;74:603-9. [Crossref] [PubMed]
- Glover B, Patel N, Ashrafian H, et al. Diagnostic accuracy of i-scan image enhancement for real-time endoscopic diagnosis of small colorectal polyps: a meta-analysis. Therap Adv Gastroenterol 2018;11:1756284818814948. [Crossref] [PubMed]
- Lee JS, Jeon SW, Kwon YH. Comparative Study of Narrow-Band Imaging and i-scan for Predicting the Histology of Intermediate-to-Large Colorectal Polyps: A Prospective, Randomized Pilot Study. Clin Endosc 2021;54:881-7. [Crossref] [PubMed]
- Neumann H, Vieth M, Günther C, et al. Virtual chromoendoscopy for prediction of severity and disease extent in patients with inflammatory bowel disease: a randomized controlled study. Inflamm Bowel Dis 2013;19:1935-42. [Crossref] [PubMed]
- Iacucci M, Kiesslich R, Gui X, et al. Beyond white light: optical enhancement in conjunction with magnification colonoscopy for the assessment of mucosal healing in ulcerative colitis. Endoscopy 2017;49:553-9. [Crossref] [PubMed]
- González-Bernardo O, Riestra S, Vivas S, et al. Chromoendoscopy With Indigo Carmine vs Virtual Chromoendoscopy (iSCAN 1) for Neoplasia Screening in Patients With Inflammatory Bowel Disease: A Prospective Randomized Study. Inflamm Bowel Dis 2021;27:1256-62. [Crossref] [PubMed]
- López-Serrano A, Suárez MJ, Besó P, et al. Virtual chromoendoscopy with iSCAN as an alternative method to dye-spray chromoendoscopy for dysplasia detection in long-standing colonic inflammatory bowel disease: a case-control study. Scand J Gastroenterol 2021;56:820-8. [Crossref] [PubMed]
- Iacucci M, Fort Gasia M, Hassan C, et al. Complete mucosal healing defined by endoscopic Mayo subscore still demonstrates abnormalities by novel high definition colonoscopy and refined histological gradings. Endoscopy 2015;47:726-34. [Crossref] [PubMed]
- Boparai KS, van den Broek FJ, van Eeden S, et al. Hyperplastic polyposis syndrome: a pilot study for the differentiation of polyps by using high-resolution endoscopy, autofluorescence imaging, and narrow-band imaging. Gastrointest Endosc 2009;70:947-55. [Crossref] [PubMed]
- Moriichi K, Fujiya M, Okumura T. The efficacy of autofluorescence imaging in the diagnosis of colorectal diseases. Clin J Gastroenterol 2016;9:175-83. [Crossref] [PubMed]
- Zhao ZY, Guan YG, Li BR, et al. Detection and miss rates of autofluorescence imaging of adenomatous and polypoid lesions during colonoscopy: a systematic review and meta-analysis. Endosc Int Open 2015;3:E226-35. [Crossref] [PubMed]
- Moriichi K, Fujiya M, Sato R, et al. Back-to-back comparison of auto-fluorescence imaging (AFI) versus high resolution white light colonoscopy for adenoma detection. BMC Gastroenterol 2012;12:75. [Crossref] [PubMed]
- Takeuchi Y, Sawaya M, Oka S, et al. Efficacy of autofluorescence imaging for flat neoplasm detection: a multicenter randomized controlled trial (A-FLAT trial). Gastrointest Endosc 2019;89:460-9. [Crossref] [PubMed]
- Matsuda T, Saito Y, Fu KI, et al. Does autofluorescence imaging videoendoscopy system improve the colonoscopic polyp detection rate?--a pilot study. Am J Gastroenterol 2008;103:1926-32. [Crossref] [PubMed]
- van den Broek FJ, Fockens P, Van Eeden S, et al. Clinical evaluation of endoscopic trimodal imaging for the detection and differentiation of colonic polyps. Clin Gastroenterol Hepatol 2009;7:288-95. [Crossref] [PubMed]
- Rotondano G, Bianco MA, Sansone S, et al. Trimodal endoscopic imaging for the detection and differentiation of colorectal adenomas: a prospective single-centre clinical evaluation. Int J Colorectal Dis 2012;27:331-6. [Crossref] [PubMed]
- Kuiper T, van den Broek FJ, Naber AH, et al. Endoscopic trimodal imaging detects colonic neoplasia as well as standard video endoscopy. Gastroenterology 2011;140:1887-94. [Crossref] [PubMed]
- Sato R, Fujiya M, Watari J, et al. The diagnostic accuracy of high-resolution endoscopy, autofluorescence imaging and narrow-band imaging for differentially diagnosing colon adenoma. Endoscopy 2011;43:862-8. [Crossref] [PubMed]
- Moriichi K, Fujiya M, Sato R, et al. Autofluorescence imaging and the quantitative intensity of fluorescence for evaluating the dysplastic grade of colonic neoplasms. Int J Colorectal Dis 2012;27:325-30. [Crossref] [PubMed]
- Moriichi K, Fujiya M, Ijiri M, et al. Quantification of autofluorescence imaging can accurately and objectively assess the severity of ulcerative colitis. Int J Colorectal Dis 2015;30:1639-43. [Crossref] [PubMed]
- Osada T, Arakawa A, Sakamoto N, et al. Autofluorescence imaging endoscopy for identification and assessment of inflammatory ulcerative colitis. World J Gastroenterol 2011;17:5110-6. [Crossref] [PubMed]
- van den Broek FJ, Fockens P, van Eeden S, et al. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut 2008;57:1083-9. [Crossref] [PubMed]
- Ueno N, Fujiya M, Moriichi K, et al. Endosopic autofluorescence imaging is useful for the differential diagnosis of intestinal lymphomas resembling lymphoid hyperplasia. J Clin Gastroenterol 2011;45:507-13. [Crossref] [PubMed]
- Sasajima K, Kudo SE, Inoue H, et al. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc 2006;63:1010-7. [Crossref] [PubMed]
- Pirogov SS, Sokolov VV, Kaprin AD, et al. Endocytoscopy--novel endoscopic diagnostics approach: principles and procedure. Eksp Klin Gastroenterol 2015;12-21. [PubMed]
- Takamaru H, Wu SYS, Saito Y. Endocytoscopy: technology and clinical application in the lower GI tract. Transl Gastroenterol Hepatol 2020;5:40. [Crossref] [PubMed]
- Kudo SE, Wakamura K, Ikehara N, et al. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy 2011;43:869-75. [Crossref] [PubMed]
- Rotondano G, Bianco MA, Salerno R, et al. Endocytoscopic classification of preneoplastic lesions in the colorectum. Int J Colorectal Dis 2010;25:1111-6. [Crossref] [PubMed]
- Utsumi T, Sano Y, Iwatate M, et al. Prospective real-time evaluation of diagnostic performance using endocytoscopy in differentiating neoplasia from non-neoplasia for colorectal diminutive polyps (≤ 5 mm). World J Gastrointest Oncol 2018;10:96-102. [Crossref] [PubMed]
- Mori Y, Kudo SE, Ogawa Y, et al. Diagnosis of sessile serrated adenomas/polyps using endocytoscopy (with videos). Dig Endosc 2016;28:43-8. [Crossref] [PubMed]
- Kutsukawa M, Kudo SE, Ikehara N, et al. Efficiency of endocytoscopy in differentiating types of serrated polyps. Gastrointest Endosc 2014;79:648-56. [Crossref] [PubMed]
- Ogawa Y, Kudo SE, Mori Y, et al. Use of endocytoscopy for identification of sessile serrated adenoma/polyps and hyperplastic polyps by quantitative image analysis of the luminal areas. Endosc Int Open 2017;5:E769-74. [Crossref] [PubMed]
- Mori Y, Kudo S, Ikehara N, et al. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy 2013;45:98-105. [Crossref] [PubMed]
- Kudo T, Kudo SE, Wakamura K, et al. Diagnostic performance of endocytoscopy for evaluating the invasion depth of different morphological types of colorectal tumors. Dig Endosc 2015;27:754-61. [Crossref] [PubMed]
- Nakamura H, Kudo SE, Misawa M, et al. Evaluation of microvascular findings of deeply invasive colorectal cancer by endocytoscopy with narrow-band imaging. Endosc Int Open 2016;4:E1280-5. [Crossref] [PubMed]
- Sugihara Y, Kudo SE, Miyachi H, et al. In vivo detection of desmoplastic reaction using endocytoscopy: A new diagnostic marker of submucosal or more extensive invasion in colorectal carcinoma. Mol Clin Oncol 2017;6:291-5. [Crossref] [PubMed]
- Mori Y, Kudo SE, Chiu PW, et al. Impact of an automated system for endocytoscopic diagnosis of small colorectal lesions: an international web-based study. Endoscopy 2016;48:1110-8. [Crossref] [PubMed]
- Takeda K, Kudo SE, Mori Y, et al. Accuracy of diagnosing invasive colorectal cancer using computer-aided endocytoscopy. Endoscopy 2017;49:798-802. [Crossref] [PubMed]
- Misawa M, Kudo SE, Mori Y, et al. Accuracy of computer-aided diagnosis based on narrow-band imaging endocytoscopy for diagnosing colorectal lesions: comparison with experts. Int J Comput Assist Radiol Surg 2017;12:757-66. [Crossref] [PubMed]
- Mori Y, Kudo SE, Wakamura K, et al. Novel computer-aided diagnostic system for colorectal lesions by using endocytoscopy (with videos). Gastrointest Endosc 2015;81:621-9. [Crossref] [PubMed]
- Bessho R, Kanai T, Hosoe N, et al. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J Gastroenterol 2011;46:1197-202. [Crossref] [PubMed]
- Gheorghe C, Becheanu G, Iacob R, et al. The role of confocal laser endomicroscopy in assessing mucosal healing in patients with ulcerative proctitis. Endoscopy 2017;49:1285. [Crossref] [PubMed]
- Neumann H, Vieth M, Neurath MF, et al. Endocytoscopy allows accurate in vivo differentiation of mucosal inflammatory cells in IBD: a pilot study. Inflamm Bowel Dis 2013;19:356-62. [Crossref] [PubMed]
- Gralnek IM, Siersema PD, Halpern Z, et al. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol 2014;15:353-60. [Crossref] [PubMed]
- Kudo T, Saito Y, Ikematsu H, et al. New-generation full-spectrum endoscopy versus standard forward-viewing colonoscopy: a multicenter, randomized, tandem colonoscopy trial (J-FUSE Study). Gastrointest Endosc 2018;88:854-64. [Crossref] [PubMed]
- Papanikolaou IS, Apostolopoulos P, Tziatzios G, et al. Lower adenoma miss rate with FUSE vs. conventional colonoscopy with proximal retroflexion: a randomized back-to-back trial. Endoscopy 2017;49:468-75. [Crossref] [PubMed]
- Hassan C, Senore C, Radaelli F, et al. Full-spectrum (FUSE) versus standard forward-viewing colonoscopy in an organised colorectal cancer screening programme. Gut 2017;66:1949-55. [PubMed]
- Albrecht H, Vieth M, Tontini GE, et al. Accuracy of the Full Spectrum Endoscopy (FUSE) system for assessment of disease activity in Inflammatory Bowel Diseases (IBD) compared to high-definition endoscopy. Arch Clin Gastroenterol 2019;5:034-8.
- Nutalapati V, Kanakadandi V, Desai M, et al. Cap-assisted colonoscopy: a meta-analysis of high-quality randomized controlled trials. Endosc Int Open 2018;6:E1214-23. [Crossref] [PubMed]
- Facciorusso A, Del Prete V, Buccino RV, et al. Comparative Efficacy of Colonoscope Distal Attachment Devices in Increasing Rates of Adenoma Detection: A Network Meta-analysis. Clin Gastroenterol Hepatol 2018;16:1209-1219.e9. [Crossref] [PubMed]
- DeMarco DC, Odstrcil E, Lara LF, et al. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the Third Eye Retroscope study group. Gastrointest Endosc 2010;71:542-50. [Crossref] [PubMed]
- Leufkens AM, DeMarco DC, Rastogi A, et al. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest Endosc 2011;73:480-9. [Crossref] [PubMed]
- Floer M, Biecker E, Fitzlaff R, et al. Higher adenoma detection rates with endocuff-assisted colonoscopy - a randomized controlled multicenter trial. PLoS One 2014;9:e114267. [Crossref] [PubMed]
- Biecker E, Floer M, Heinecke A, et al. Novel endocuff-assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol 2015;49:413-8. [Crossref] [PubMed]
- Ngu WS, Bevan R, Tsiamoulos ZP, et al. Improved adenoma detection with Endocuff Vision: the ADENOMA randomised controlled trial. Gut 2019;68:280-8. [Crossref] [PubMed]
- Tsiamoulos ZP, Misra R, Bourikas LA, et al. Sa1423 Endocuff-Vision: Impact on Colonoscopist Performance During Screening. Gastrointest Endosc 2015;81:AB209. [Crossref]
- Facciorusso A, Mohan BP, Crinò SF, et al. Impact of EndoRings on colon adenoma detection rate: A meta-analysis of randomized trials. J Gastroenterol Hepatol 2021;36:337-43. [Crossref] [PubMed]
- Dik VK, Gralnek IM, Segol O, et al. Multicenter, randomized, tandem evaluation of EndoRings colonoscopy--results of the CLEVER study. Endoscopy 2015;47:1151-8. [Crossref] [PubMed]
- Hendel J, Mizrahi M, Hoffman A, et al. 435 Prospective Randomized Multicenter Trial to Compare Adenoma Detection Rate of HD Colonoscopy With Standard HD Colonoscopy - Intermediate Results. Gastrointest Endosc 2015;81:AB145-6. [Crossref]
- Halpern Z, Gross SA, Gralnek IM, et al. Comparison of adenoma detection and miss rates between a novel balloon colonoscope and standard colonoscopy: a randomized tandem study. Endoscopy 2015;47:238-44. [Crossref] [PubMed]
- Shirin H, Shpak B, Epshtein J, et al. 1006 Comparison of Adenoma Detection Rate by a High Definition Colonoscopy versus Standard High Definition Colonoscopy- A Prospective Randomized Multicenter Trial. Gastrointest Endosc 2016;83:AB192. [Crossref]
- Chang WY, Chiu HM. Can image-enhanced endoscopy improve adenoma detection rate? Dig Endosc 2022;34:284-96. [Crossref] [PubMed]
- Djinbachian R, Pohl H, Marques P, et al. Sa2010 Optical diagnosis of small colorectal polyps using Optivista and iSCAN image enhanced endoscopy: a randomized controlled trial. Gastrointest Endosc 2020;91:AB240. [Crossref]
- Al-Sabban AHM, Al-Kawas FH. Red dichromatic imaging in acute GI bleeding: Does it make a difference? Gastrointest Endosc 2022;95:701-2. [Crossref] [PubMed]
- Hirai Y, Fujimoto A, Matsutani N, et al. Evaluation of the visibility of bleeding points using red dichromatic imaging in endoscopic hemostasis for acute GI bleeding (with video). Gastrointest Endosc 2022;95:692-700.e3. [Crossref] [PubMed]
- Sato T. TXI: Texture and Color Enhancement Imaging for Endoscopic Image Enhancement. J Healthc Eng 2021;2021:5518948. [Crossref] [PubMed]
- Nishizawa T, Toyoshima O, Yoshida S, et al. TXI (Texture and Color Enhancement Imaging) for Serrated Colorectal Lesions. J Clin Med 2021;11:119. [Crossref] [PubMed]
- Tamai N, Horiuchi H, Matsui H, et al. Visibility evaluation of colorectal lesion using texture and color enhancement imaging with video. DEN Open 2022;2:e90. [Crossref] [PubMed]
- Kudo SE, Mori Y, Abdel-Aal UM, et al. Artificial intelligence and computer-aided diagnosis for colonoscopy: where do we stand now? Transl Gastroenterol Hepatol 2021;6:64. [Crossref] [PubMed]
- Spadaccini M, Marco A, Franchellucci G, et al. Discovering the first US FDA-approved computer-aided polyp detection system. Future Oncol 2022;18:1405-12. [Crossref] [PubMed]
- Taghiakbari M, Mori Y, von Renteln D. Artificial intelligence-assisted colonoscopy: A review of current state of practice and research. World J Gastroenterol 2021;27:8103-22. [Crossref] [PubMed]
- Spadaccini M, Iannone A, Maselli R, et al. Computer-aided detection versus advanced imaging for detection of colorectal neoplasia: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2021;6:793-802. [Crossref] [PubMed]
- Ladabaum U, Shepard J, Weng Y, et al. Computer-aided Detection of Polyps Does Not Improve Colonoscopist Performance in a Pragmatic Implementation Trial. Gastroenterology 2023;164:481-483.e6. [Crossref] [PubMed]
- Levy I, Bruckmayer L, Klang E, et al. Artificial Intelligence-Aided Colonoscopy Does Not Increase Adenoma Detection Rate in Routine Clinical Practice. Am J Gastroenterol 2022;117:1871-3. [Crossref] [PubMed]
- Ahmad A, Wilson A, Haycock A, et al. Evaluation of a real-time computer-aided polyp detection system during screening colonoscopy: AI-DETECT study. Endoscopy 2023;55:313-9. [Crossref] [PubMed]
- Wei MT, Shankar U, Parvin R, et al. Evaluation of Computer-Aided Detection During Colonoscopy in the Community (AI-SEE): A Multicenter Randomized Clinical Trial. Am J Gastroenterol 2023; Epub ahead of print. [Crossref] [PubMed]
- Tada M, Katoh S, Kohli Y, et al. On the dye spraying method in colonofiberscopy. Endoscopy 1977;8:70-4. [Crossref] [PubMed]
Cite this article as: Shahsavari D, Waqar M, Thoguluva Chandrasekar V. Image enhanced colonoscopy: updates and prospects—a review. Transl Gastroenterol Hepatol 2023;8:26.


