Restorative pouch surgery following proctocolectomy for inflammatory bowel disease: past experience and future direction
Introduction
In the past, surgery for inflammatory bowel disease (IBD), Crohn’s disease (CD) colitis, or ulcerative colitis (UC) involved removing the entire large intestine and creating a permanent ileostomy. However, this procedure can be challenging for patients and can significantly affect their quality of life (QOL). An evolution in surgical care took place to restore intestinal continuity. A straight ileo-anal anastomosis was attempted but only found marginal success in the pediatric population, while adults suffered from frequent bowel movements (BMs) (1). Nils Kock was able to demonstrate the small bowel could be used as an intra-abdominal fluid reservoir in the 1960s, and subsequently, in the late 1970s, Parks & Nicholls described a restorative procedure for patients suffering from UC to extirpate the colorectum and restore intestinal continuity with a hand-sewn loop of ileum in the shape that resembled an “S” (2,3). The aptly named S-pouch functioned as a pelvic intestinal reservoir and was the first advancement that led to a life without a permanent ileostomy and an acceptable QOL. Concurrently, stoma appliances were undergoing major advancements by the work of Turnbull from Cleveland Clinic in patients who still required a permanent stoma (4).
Years later, with the invention of a linear stapler, the J-pouch was created in Japan by Utsunomiya, and further advancements ensued focusing on the type of anal anastomosis, hand-sewn versus stapled, in efforts to produce a repeatable operation with optimal functional outcomes.
As medical treatments advanced, especially with biologic therapy, select patients with CD were also candidates for an ileal pouch-anal anastomosis (IPAA) and the procedure evolved from a traditional open laparotomy incision to more minimally invasive techniques, like laparoscopic surgery, robotic surgery, single incision surgery, and trans-anal (taIPAA) platforms (5,6). The indication for a restorative proctocolectomy also evolved as patients presented in different stages of their disease, for example, patients in extremis with toxic colitis, with refractory disease, or with dysplasia/malignancy, each dictating the number of operations needed to restore their continuity. Hence, the steps of an IPAA were broken down into different stages, sparing the proctectomy with pouch creation until the patient was optimally conditioned, off medical therapy and mentally prepared for a complex operation.
As the IPAA became the standard of care with a high success rate, there have also been patients who have suffered from pouch dysfunction and ultimate pouch failure in a minority of patients (less than 5%) (7). This has led to a field of revisional or redo pouch surgery. Unfortunately, with the increase of minimally invasive techniques, there has been an anecdotal increase in pouch-twists (the pouch twisted along its mesenteric axis) and retained rectum (creating an ileal anal-rectal anastomosis), possibly related to the minimally invasive platform, lack of sub-specialization, or underdiagnosis of postoperative pelvic sepsis. Many patients are then labeled as having CD and treated with medical therapy instead of having their underlying mechanical issue addressed. These cases are complex and are typically performed in high-specialized referral centers.
In this article we will describe the different pouch types, their construction, the stages of the procedures, the outcomes for UC and CD, the types of operations offered, and the work-up and treatment for patients who may be suffering from pouch failure.
Pouch design
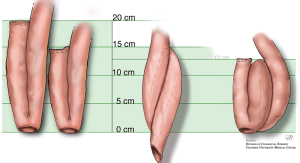
The S pouch is created by taking three loops of small intestine, each 12cm in length, created by folding the small intestine onto each other, creating enterotomies and sewing the edges to create a reservoir. It is subsequently attached it to the anus; first described in a hand-sewn manner, later with a circular stapler as it preserved the anal transitional zone and is technically easier to perform (8). Sir Alan Parks and Mr. John Nicholls are credited as the first to describe the IPAA after fashioning an S-pouch for patients with UC after proctocolectomy in 1978 (9). While the S pouch was effective in restoring intestinal continuity, it was associated with a high risk of complications, including pouchitis and other technical considerations associated with the complexity of construction (Figure 1) (9).
To address these complications, a new type of pouch called the J pouch was developed. First described in 1980 by Utsunomiya et al., the J pouch is similar to the S pouch but is made using two loops of bowel, each about 20 cm, while a linear stapler joins the adjacent limbs to create a reservoir resembling a “J” (Figure 1) (10). This shape allows for better control of bowel function, requires less bowel length to create, and is technically easier to construct than other pouches. As such, the J-pouch has quickly become the pouch of choice for most surgeons performing IPAA.
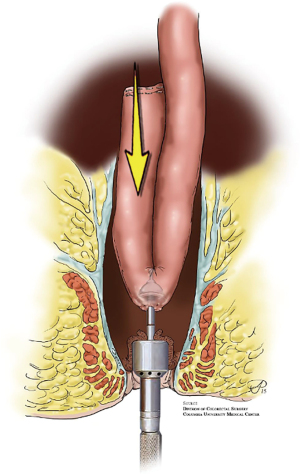
There has also been development in the way the anastomosis is created. In a hand-sewn anastomosis, mucosectomy is performed by removing the anal transition zone (ATZ) potentially extirpating the diseased rectum and any occult malignancy. A major benefit is ensuring there is no retained rectum as the pouch is anastomosed directly to the anus however a drawback is it also removes the highly-specialized nerves of the anal canal. A double-stapled IPAA, however, avoids a mucosectomy by leaving the ATZ intact and creating the anastomosis just proximal to it. Most surgeons are moving towards stapled anastomosis (Figure 2), as they are quicker to perform, and the potential benefit of mucosectomy in hand-sewn anastomoses is offset by the risk of damage to the anal sphincter and sensory nerves in the ATZ. There is also evidence that stapled IPAA results in better functional outcomes with lower incidence of incontinence and nocturnal seepage (8). Though the J-shaped-pouch, along with a double-stapled ileal-anal anastomosis has become the standard of care, a pouch-surgeon should have knowledge of S- and H-shaped pouches as well so they can effortlessly evaluate which pouch-shape will be needed, and when a hand-sewn anastomosis may be indicated (3,11).
Pouch stages
Pouch surgery is typically performed in one, two or three stages, depending on the patient’s condition and the surgeon’s preference. A staged procedure is preferred in the emergency setting (sparing the proctectomy and IPAA creation at the initial operation), when patients are taking high dose steroids and when nutritional status is poor. Several studies have also demonstrated increased risk of post-operative complications in patients undergoing restorative pouch surgery while using infliximab, suggesting a staged approach for these patients as well (12).
Defining the 1 stage, 2 stage, modified 2 stage, 3 stage operations
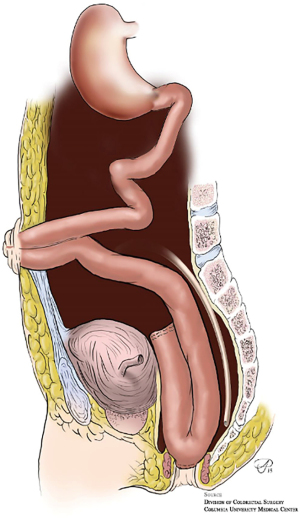
The most common method for an elective IPAA is a traditional two-staged approach. In the first stage, patients undergo restorative total proctocolectomy with IPAA (J-pouch) with a temporary diverting loop ileostomy (Figure 3). In the second stage, the ileostomy is reversed and bowel continuity is restored. There is typically a 12-week waiting period between stages to allow the anastomoses and pouch to heal before ileostomy reversal, and only with a negative water-soluble contrast enema and endoscopy ensuring no leak or stricture (13). A modified two-staged technique has also been described. In the first stage, a subtotal colectomy and end ileostomy is performed. This is followed by a completion proctectomy and definitive IPAA created 3–6 months later without a diverting ileostomy. While data remains scarce and limited to a few single-center retrospective studies, there is some evidence that the modified two-staged approach leads to lower rates of anastomotic leak following pouch creation when compared with the traditional two-stage procedure (14). In practice, the decision to perform traditional vs. modified two-staged procedure should be determined based on surgeon comfort and experience until stronger data is available.
Other surgical options include a three-staged approach, which involves subtotal colectomy and end ileostomy at the initial operation, followed by completion proctectomy and IPAA with a diverting ileostomy, and finally stoma closure a few months later. While this approach may be seen as the most conservative, several recent studies have demonstrated non-inferiority of two-staged IPAA compared with the three-staged approach. The need for an additional surgery in the three-staged approach also leads to higher healthcare costs and increased hospital stay (15,16). However, because many patients are being tried on many biologic medications before being referred for surgery, they are presenting malnourished, with minimal physiologic reserve and as a result, the 3-staged approach is becoming the most commonly needed (17).
Increasingly less common is the total proctocolectomy and IPAA creation performed in a single-staged operation, the 1-stage pouch. There are data to suggest that a single-staged operation can have similar outcomes compared to the two-staged approach in appropriately selected patients, however pouch reach issues, malnutrition, medications and other factors like body habitus limit this approach greatly (18).
Laparoscopy, robotic, and open techniques
While minimally invasive surgical (MIS) techniques have become widely accepted throughout surgery, open surgery has remained the cornerstone in the IBD population. This is likely in part because many IBD patients present malnourished with inflamed and friable tissues. Additionally, IBD surgery can be complex with high conversion rates, and open techniques has its advantages in reoperative surgery, specifically in redo pelvic pouch procedures (19). Nevertheless, recently laparoscopic and robotic surgery have been proven to positively affect patients undergoing surgery with decreased levels of pain and narcotic requirements, less intra-abdominal adhesion formation, earlier return of bowel function, shorter hospital length of stay, and improved cosmesis (19). The benefits of minimally invasive surgery are a particularly important consideration in the IBD population, given the young age of this cohort, the ability to return to their normal lifestyles quickly and improved cosmesis is an important outcome for them. Additionally, this cohort is more likely to undergo multiple operations, making the benefit of decreased adhesion formation and small bowel obstructions particularly important (19,20).
MIS techniques can be evaluated in many aspects of IBD surgery, including abdominal versus pelvic, elective versus emergent, and staged procedures like the IPAA. Several studies have demonstrated the feasibility of subtotal colectomy and proctectomy with minimally invasive techniques. It has been demonstrated that laparoscopic procedures are often associated with decreased wound infections, decreased intra-abdominal abscesses, and shorter length of stay when compared with the open technique (21). The robotic platform has also demonstrated feasibility and has been attributed to decreased conversion to open when compared with conventional laparoscopic approach (22). In terms of laparoscopic IPAA, several studies have confirmed safety and feasibility with longer operative times than open surgery, but with similar complication rates, faster postoperative recovery times, improved cosmesis, and decreased impact on fertility and sexual function (22,23). When compared to laparoscopic IPAA, robotic IPAA has demonstrated similar outcomes with longer operative times but decreased blood loss and length of stay (24).
In terms of long-term outcomes, several studies have shown similar or improved bowel function after MIS IPAA compared to open, with fewer nocturnal BMs and decreased pad usage with no differences in total daily BMs or pouch failure rates (25). It should be noted, that while the laparoscopic and robotic platforms provide improved visualization and steadiness in difficult-to-reach areas such as the deep pelvis, the limited operating space and ability to easily rotate between the abdomen and pelvis may lead to complications, such as retained rectums, ischemic strictures, and/or pouch twists that may require operative revision/correction. Overall, MIS techniques have been demonstrated to be safe and effective for IPAA procedures in the IBD population in experienced hands and appropriately selected patients.
Pouches for dysplasia or cancer
Patients with UC are at increased risk for developing colorectal cancer and most cases are thought to arise from dysplasia, making endoscopic surveillance the standard recommendation to detect dysplasia. As advancements have been made in medical therapies and endoscopic surveillance, the surgical management of dysplasia in UC has changed in parallel (26-28). Prior to advancements in endoscopy, most areas of visible dysplasia that were previously referred for surgery currently undergo endoscopic resection alone with close surveillance. Even patients with invisible dysplasia are instead first referred to an endoscopist with expertise in IBD to better inform the decision of surveillance vs. surgery (28).
Today’s guidelines reserve surgery for patients with unresectable visible dysplasia, invisible high-grade dysplasia, and multifocal low-grade dysplasia and invasive adenocarcinoma (27,28). Patients with UC found to have invasive colon or rectal cancer should undergo the same routine cancer staging followed by neoadjuvant/adjuvant therapy (if indicated) and surgery. In these patients, total proctocolectomy with or without IPAA is the standard recommendation to remove all tissue at risk for dysplasia/adenocarcinoma (26,28). Additionally, data has demonstrated the results of IPAA in these situations are satisfactory with good function and no different from those undergoing IPAA in the absence of dysplasia or cancer (29). Although not the standard oncologic care, subtotal colectomy with ileorectal anastomosis can be considered in patients with significant comorbidities or those who are not candidates for IPAA and wish to avoid a stoma, and without evidence of rectal cancer and only minimal proctitis. In this very minor subset of patients, they must be counseled extensively on the ongoing neoplasia risk and need to undergo close surveillance of the remaining rectum.
Lastly, for patients diagnosed with locally advanced rectal cancer there is little data available on short-term outcomes and long-term functional outcomes on patients with IPAA exposed to external beam radiation treatment (EBRT). The largest series to date showed that pouch function was acceptable amongst patients receiving neoadjuvant therapy, however long-term outcomes were poor for those receiving radiation with the pouch in situ (30). Therefore, patients should be counseled extensively on the risk of long-term function and pouch survival when EBRT may be a part of neoadjuvant or adjuvant therapy.
Pouches for emergencies
Emergent indications for surgery in patients with UC include acute severe ulcerative colitis (ASUC) refractory to medical management, uncontrolled sepsis, bowel perforation, toxic megacolon, and uncontrolled or severe bleeding. Typically, ASUC is diagnosed clinically using the Truelove and Witts severity criteria which combine the presence of bloody BMs with systemic signs of infection including body temperature, heart rate, hemoglobin level, and erythrocyte sedimentation rate (31). Treatment of ASUC involves immediate recognition and administration of systemic corticosteroids, followed by rescue infliximab or cyclosporine if there are no clinical signs of improvement. Cases refractory to medical management should be referred to a surgeon in a timely manner, in addition to patients who present with signs of bowel perforation, refractory bleeding, or toxic megacolon (32). In patients that require surgery, the preferred surgical approach is a total abdominal colectomy with end ileostomy. Proctectomy, along with pelvic dissection, is avoided in these scenarios as it can be associated with significant morbidity and have an impact on future operations and the ability to restore intestinal continuity. These patients can still be candidates for IPAA in the future and typically follow the three staged approach if clinically feasible.
Pouches for indeterminate colitis (IC)
Despite IPAA being the standard operative approach for patients with UC, in approximately 10–15% of colitis patients there are inadequate criteria to make a distinction between UC and CD. This group of patients are described in the literature as having IC (33,34). The outcome of IPAA in patients with IC is controversial, with some studies finding higher rates of perineal complications, development of CD, and eventual pouch loss (35,36) while other studies have suggested outcomes comparable to those with UC (37,38). The variability in the literature is most likely due to the unclear criteria and wide spread confusion over diagnosis of IC. In those with true IC, the higher pouch failure rates can be attributed to diagnostic conversion to CD, indicating that those with true IC have similar failure rates to those with UC (9,39). Overall, surgical treatment in patients with IC is often based on the likelihood of a particular phenotype. However, patients with IC are suitable candidates for IPAA, but should be counseled extensively on the risks and possible conversion to CD (9,33).
Pouches for CD
In the management of UC, IPAA has become the standard operative approach for patients requiring colectomy for dysplasia, cancer, or medically refractory disease. In contrast, historically, a proven diagnosis of CD has been exclusionary for IPAA. Creation of IPAA in the setting of CD has been associated with severe problems including impaired pouch function, need for long term medical therapy, and eventual pouch excision (40-42). Given the perceived poor long term outcomes and high risk for complications, patients with CD have typically not been offered IPAA and the role of IPAA in CD remains poorly defined. Thus, many patients with CD who require coloproctectomy undergo definitive end ileostomy (43).
This consensus has been challenged by the fact that patients with IPAA for apparent UC who are subsequently found to have CD have a variable course, suggesting that a subset of CD patients may be candidates for IPAA (41). In patients undergoing IPAA, there are three time points in which they can be diagnosed with CD. This includes preoperatively (i.e., intentional), postoperative histology (i.e., incidental), and months to years postoperatively based on clinical course (i.e., delayed) (40,42).
CD diagnosed pre-operatively (i.e., intentional)
In recent years, Panis et al., reported data on IPAA in patients with CD without anoperineal or small bowel manifestations demonstrating good results at long term follow up (44,45). More recently, the observations made in Panis et al. have been validated in a larger series from the Cleveland Clinic. Their study showed favorable results with respect to pouch retention and CD manifestations of the pouch (42). This data suggests that in patients with stable, small bowel and anoperineal free-CD, can be considered for IPAA with appropriate counseling.
CD diagnosed immediately post-operatively from IPAA (i.e., incidental) versus remotely from IPAA (i.e., delayed)
Another subset of patients with apparent UC undergoing IPAA will have their diagnosis revised to CD based on the postoperative histopathology immediately after IPAA. The last subset of patients will be diagnosed with CD months to years after IPAA based on their clinical course or histopathology. Emerging data has shown that patients with CD diagnosis after IPAA (incidental and delayed) are associated with low rates of clinically evident CD, low pouch loss rate, and good functional outcomes (41,42). In previous small studies, there have been differences noted in pouch failure rates when CD is diagnosed incidentally via surgical specimen versus remotely at a later date (46,47). This observation was also validated in the Cleveland Clinic study, citing patients with CD diagnosed remotely from IPAA had higher rates of pouch loss (50%) and greater CD manifestations (42).
Based on recent data, CD should no longer be considered an absolute contraindication to offering a patient an IPAA. With effective patient selection, certain subsets of patients with CD can be considered for IPAA and have low pouch loss and favorable functional results. Additionally, patients diagnosed with CD after IPAA does not indicate a surgical disaster. Patients diagnosed with CD from operative histopathology (incidental) can be counseled to expect similar favorable results to those diagnosed pre-operatively. In contrast, outcomes in patients with delayed diagnosis of CD have been shown to be less favorable. This subset of patients can be counseled appropriately at the time of diagnosis to anticipate higher risk of pouch loss and CD manifestations and help clinicians have a higher index of suspicion when diagnosis and treating pouch complications.
Pouch function
Over 90% of patients with an IPAA are doing well and have an acceptable QOL (48). Their bowel function is predicated on the success of the index pouch, with a lesser QOL and increased seepage in patients with an initial pouch-related complication, i.e., anastomotic leak or presacral hematoma, or redo pouch surgery. Farouk et al. studied the function in patients with chronic UC who underwent hand-sewn IPAA, and found that pelvic sepsis was the primary cause of pouch failure in their cohort of 1,386 patients, with over 90 patients having subsequent pouch failure. Functional results were divided into patients under & over 45 years of age at an interval of 1 year after IPAA, and 12 years after IPAA. At 1 year, patients had an average of 5 BMs per day, and patients over 45 years old had an additional nocturnal BM (>45 years old: 2 BMs; ≤45 years old: 1 BM). At 12 years, the nocturnal BMs remained the same but there was an increase in daytime BM to 6 in both groups. The most significant finding was the usage of pads, where patients over 45 years old, after of 1 year of IPAA creation, wore pads 53% of the time (compared to 28% in <45 years old), and at 12 years, 55% continued to require pads while the under 45 group decreased to 22% (48). Starting with Parks & Nicholls, the conventional technique was a stripping of the anal canal mucosa, a mucosectomy, and performing a hand-sewn ileal-anal anastomosis. The surgeons at Cleveland Clinic advocated a technique that left the anal canal mucosa and nerves intact by stapling the rectum at the level of the ATZ (the transection at ATZ being the 1st stapling, and then connecting the pouch to the ATZ with a circular stapler (2nd stapling) to create a double-stapled IPAA). Lovegrove et al. compared the outcomes of a hand-sewn anastomosis versus a double-stapled anastomosis following IPAA in a total of 4,183 patients (8). There were no differences in postoperative complications or frequency in defecation however functional results showed that nocturnal seepage and pad use favored patients who underwent a double-stapled IPAA. There was also a non-significant finding of an increase in dysplasia in the anal transitional zone in the stapled group (3).
Pouch failure
QOL and bowel function is also paramount in those patients with failed pouches who require additional surgery. Some patients with pouch failure may choose to undergo diverting ileostomy with their pouch left in situ in their pelvis, a pouch excision with permanent ileostomy, conversion to a continent ileostomy (K-pouch) or undergo a revisional/redo pouch operation (49). While simply diverting a failing pouch may limit some sepsis and improve the patient’s QOL, it does not decrease the small, but serious risk of pouch-dysplasia or ATZ cancer. Still, most patients do well with a diverted pouch, as it resolves many symptoms, however patients with anal pain or anal seepage have been shown to do better with pouch excision and permanent ileostomy (50). Patients with pelvic pouch failure also have another option to avoid life with a permanent ileostomy. That is, converting their J-pouch to a K-pouch; named after Nils Koch. This pouch sits in the abdominal cavity and has a nipple valve that gets emptied by a Water’s tube when full, and although there is a small opening on the lower abdomen to allow intubation, it does not require pouching with a stoma appliance. A recent study by Aytac et al. analyzed patients who underwent creation of a continent ileostomy after a failed IPAA, compared to patients who had an index continent ileostomy. A total of 67 patients were included in the study, and although the need for major revisions was high at 52% vs. 61% and minor revisions of 15% vs. 19%, in the converted from J- to K-pouch group vs. index K-pouch group, respectively, there was no statistical significance. In addition, both groups intubated their pouch 5 times per day and once at night, and the number one cause of failure was enterocutaneous/enteroenteric fistula. In conclusion, patients with a failed IPAA may be offered a conversion to a continent ileostomy, in a highly motivated group of patients (51). Of note, patients with CD of the pouch, have poor outcomes if an attempt is made to convert their failed pelvic pouch to a continent ileostomy, with a failure rate of almost 50%, as this should not be attempted without a detailed informed consent (52).
Redo/revisional pouch surgery
Lastly, patients may choose to undergo revisional pouch surgery. Although many patients do well with a pelvic pouch, irrespective of CD, patients can present with a myriad of symptoms, many mimicking CD, and unfortunately many missed signs/symptoms to make a correct diagnosis of pouch failure. Typically, patients with mechanical failure develop symptoms resembling CD and are subsequently started on medical therapy for CD, rather than being appropriately worked up for a mechanical/technical reason(s) to explain their pouch dysfunction. Although 5–15% of patients develop CD of the pouch, as many as 90% of patients treated for Crohn’s of the pouch, have a potentially correctable mechanical reason for failure, despite a possible diagnosis of CD (53). Ultimately, patients with or without CD will not have a functional pouch with an acceptable QOL if inherent mechanical problems are not addressed. Mechanical problems can be acute or chronic. Many acute problems are occult as patients are most often diverted after pouch creation. Nevertheless, complications may arise, and the presence of an early complication must always raise suspicion of a mechanical problem if the patient develops signs of pouch failure early after surgery (within a year). This is a stark contrast from patients who experience years of excellent pouch function and then develop signs of pouch failure, as that raises a high index of suspicion for CD of the pouch. Early problems include bowel obstructions, portomesenteric vein thrombosis and pelvic sepsis from a pouch body, tip of the J, or anal-anastomotic leak (54). A pelvic abscess may be secondary to an anastomotic leak or presacral abscess and drain through the perineum as a perianal fistula (e), through the anastomotic defect itself, or through adjacent strictures, most often as a pouch-vaginal fistula in women. Many of these conditions resemble a Crohn’s perineum and are often confused with CD, prompting anti-inflammatory treatment to combat CD, rather than antibiotic treatment to treat an infection. This often complicates the problem and an anastomotic leak may end up leading to pouch failure. Late complications also include adhesive bowel obstructions, but variants may exist. For example, afferent limb syndrome can lead to chronic bowel obstructions that are difficult to identify with cross-sectional imaging as the proximal small bowel is trapped and kinked between the posterior wall of the pouch and the sacrum. Efferent limb syndrome may develop in patients specifically with an S-pouch, as the exit conduit may become elongated and kinked causing outlet obstruction in the bowel that is connecting the body of the pouch with the anal canal. Outlet defecation may cause subsequent pelvic floor dysfunction which may require pelvic floor physical therapy, however it is paramount to identify the underlying pathology, which may be a mechanical obstruction. Other late complications can be a pouch twist (twisted along its mesenteric axis) or retained rectum (Figure 4) (pouch not anastomosed to the anal canal, but the rectum), as both conditions are becoming more common in the era of minimally invasive surgery. Both conditions are treatable but require workup and complex revisional surgery. Chronic leaks may persist and develop into chronic draining wounds resembling CD. Pouchitis is common and is typically treated with intermittent antibiotics. Many patients will experience pouchitis, almost 50% within 2 years after IPAA creation, however only 19% will develop recurrent pouchitis, and although primary sclerosing cholangitis and previous treatment of anti-tumor necrosis factor alpha therapy are risk factors, the presence of an occult anastomotic leak must be ruled out as that too can lead to chronic pouchitis (55). Stricturing disease at the ileal-anal anastomosis may be secondary to CD, however inadequate reach to the pelvis can put tension on the pouch which subsequently causes a mild ischemia and results in a fibrotic stricture. Lastly, pouch cancer remains rare but can be very aggressive with a low incidence at around 0.01% at upwards of 30 years after IPAA creation (56).
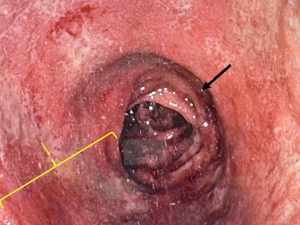
For many patients with signs of pouch failure, referral to a highly specialized IBD center is ideal to diagnose sinister conditions. The most important detail in the workup for pouch failure is the history of the patient and the operative details. Many patients will end up stating they “never felt right” after IPAA surgery, and this rather simple sentence is actually an important clue to search for a correctable mechanical complication leading to pouch failure. The work up continues with examining the pathology reports looking for signs of CD. Many patients will need all diagnostic tests, however the aim should be to start from least invasive to most invasive in search of the diagnosis, and in the proper clinical context. A thorough physical exam, and an exam under anesthesia with flexible pouchoscopy. The exam should focus on the presence of any fistulae, anastomotic stricture and retained rectum. The pouchoscopy confirms the presence of a pouch-rectal anastomosis and continues to inspect the pouch body for any signs of ulceration, pouchitis, pouch-twist, inlet stricture and inflammation of the proximal small bowel. Of note, proximal small bowel ulcerations may be caused by non-steroidal anti-inflammatory drugs (NSAIDs), however in patients without the use of NSAIDS, the presence of ileal ulcers is highly suggestive of CD (57). A gastrograffin enema (GGE) is also performed to show any presacral sinus tract or other filling abnormalities as a dynamic study. Accumulated contrast proximal to the pouch along with an empty pouch after defecation, may raise suspicion of afferent loop syndrome. A magnetic resonance imaging (MRI) is performed to check for any signs of pouch inflammation, presacral collections or fistulae. A computed tomography (CT) scan is also needed to help assess for pouch twist, chronic obstruction or narrowing/stricture (especially at a previous ileostomy closure site, with a very high index of suspicion if the closure site was performed in a stapled-side-to-side configuration), and possible cross-sectional enterography to assess for any proximal small bowel inflammation that would be consistent with CD. If the diagnosis is unclear, the patient may need a diagnostic laparoscopy to look for any subtle pathology not evident in the work up thus far. Many times, at the time of laparoscopy or laparotomy, a “thoughtful ileostomy” described by Schwartzberg et al. is created in efforts to not only diagnosis the problem but also create the ileostomy to allow the patient to regain their physical and mental strength to undergo prior to the formal corrective pouch operation. The “thoughtful ileostomy” is created 20 cm proximal to the pouch inlet in efforts to minimize any bowel loss if the subsequent operation requires pouch excision and a new pouch to be created (58).
The redo or revisional IPAA operation is typically completed after a “thoughtful ileostomy” is has been in place for 6 months, however other indications for revisional operations such as a tip of the J leak or adhesive disease like afferent limb syndrome may not require a preoperative diverting stoma (59). Tip of the J leaks may only require isolation of the leak and oversewing or re-stapling with or without diversion (60). The senior author of this article has successfully attempted and completed two laparoscopic revisional IPAA operations in highly selected patients who had their index pouch created laparoscopically, however only a limited number of minimally invasive redo pouch procedures have been attempted and the standard operation is a formal laparotomy with full abdominopelvic pouch mobilization (59,61). Robotic redo IPAA has not been published to date, however robotic pouch excision has been described (62).
The redo IPAA is a labor intensive operation and should only be performed in high-volume pouch centers. It requires a team of well-versed surgeons and operating room staff to be completed safely, as it requires an arterial line for monitoring, a Foley catheter with cystoscopy and bilateral ureteral stents, selective epidural placement, appropriate intravenous access, and an extended period of time with the patient’s arms tucked and in the modified Llyod-Davies positioned with their abdomen and perineum prepped and draped. In patients with previous laparotomies or intra-abdominal septic complications, access to the abdomen and identification of the pelvic pouch may require hours of adhesiolysis before the formal pouch operation is begun. Then the pouch is sharply mobilized to the level of the pelvic floor (63). In many circumstances, the previous pouch can be used as long as the pouch itself has not led to failure secondary to a pouch-body leak and the pouch mobilization was completed with minimal damage. Typically, a mucosectomy is needed and a subsequent hand-sewn anastomosis performed, however, in patients with a rectal cuff long enough to allow a transverse stapler at the ATZ (i.e., retained rectum), a double-stapled technique can be performed. Many patients with pouch failure can have one more complications that contribute to pouch failure, so a thorough understanding of the anatomy is paramount (i.e., retained rectum & pouch twist) (64). Patients are diverted with a loop ileostomy and a closed suction drain placed in the pelvis. Patients with a mucosectomy/hand-sewn anastomosis often require a 4–6-week course of a 22-Fr mushroom catheter placed per anus to stent the anastomosis open (63). The ileostomy is closed 3 months later after a water-soluble contrast enema demonstrates a patent pouch without signs of a leak or stricture.
The success of the redo IPAA operation is largely dependent on repetition and skill set. In the largest series published, Remzi et al. analyzed the outcomes in over 500 patients who underwent a redo IPAA at a single institution (63). Five hundred and two patients, with a median age of 38 underwent revisional surgery. Fifty-nine percent of patients kept their index pouch, and 82% required mucosectomy with a hand-sewn anastomosis. The primary cause of revisional pouch surgery was a leak/fistula (52%, pouch-vaginal fistula, 17%), obstruction (23%), dysfunction (10%), pelvic perianal abscess (9%), pouchitis (3%), and prolapse or neoplasia in 2%. The mortality was zero, but the morbidity exceeded 50%. The anastomotic leak rate after redo surgery was 8%, with over a 98% pouch survival at 1 year, 90.2% at 5 years and 82.4% at 10 years (63). The main cause of failure was pelvic sepsis. Sadly, in the patient distribution, the number of patients with pouch failure requiring redo pouch surgery increased across each time period; 23 patients between 1983–1993, 141 patients between 1994–2003, and 338 patients between 2004–2014. And though not explicitly stated, but difficult to ignore, is the fact that the main difference between the patients suffering pouch failure from the start of the data collection period to the end date, was the introduction and widespread adoption of minimally invasive techniques. However, there may be other correlations as well, such as the evolving residency training program requirements but this has not been explicitly studied (63).
The QOL and functional results of the Remzi study demonstrated positive functional outcome of the redo pouch patients Though limited by survey response, the daytime and nighttime stool frequency was 6 [1–15] and 2 [0–9], respectively (63). Seepage using the day was 48%, while seepage at night was 54%, and pad use during the day & night was 49% and 57%, respectively. Although dietary restrictions occurred in over 30% of patients, sexual, social and work restrictions were found in 22%, 18% and 18% of patients, respectively. Overall, 92% of patients stated they would undergo the surgery again, and 93% would recommend a redo pouch operation to others, which clearly speaks to the importance of revision surgery (63).
Conclusions
A multidisciplinary approach is critical for the successful management of patients undergoing IPAA surgery. Patients with IBD often have complex medical needs, and their care requires a team of healthcare professionals, including gastroenterologists, nutritionists, and specialized stoma nurses. High volume specialized centers offer patients access to surgeons who perform IPAA regularly, as well as access to these multidisciplinary services who can help with pre-operative planning and optimization and offer ongoing support in the post-operative period.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Keith Sultan) for the special series “Controversies and Updates in Inflammatory Bowel Disease” published in Translational Gastroenterology and Hepatology for the series. The article has undergone external peer review.
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-28/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-28/coif). The series “Controversies and Updates in Inflammatory Bowel Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taylor BM, Beart RW Jr, Dozois RR, et al. Straight ileoanal anastomosis v ileal pouch--anal anastomosis after colectomy and mucosal proctectomy. Arch Surg 1983;118:696-701. [Crossref] [PubMed]
- Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J 1978;2:85-8. [Crossref] [PubMed]
- Miller-Ocuin JL, Dietz DW. The Evolution of Pelvic Pouch Surgery: Optimal Pouch Design for an Ileal Pouch Anal Anastomosis. Clin Colon Rectal Surg 2022;35:453-7. [Crossref] [PubMed]
- Dietz K. Skin problems after ostomy surgery. Can Fam Physician 1978;24:252-4. [PubMed]
- de Buck van Overstraeten A, Mark-Christensen A, Wasmann KA, et al. Transanal Versus Transabdominal Minimally Invasive (Completion) Proctectomy With Ileal Pouch-anal Anastomosis in Ulcerative Colitis: A Comparative Study. Ann Surg 2017;266:878-83. [Crossref] [PubMed]
- Baek SJ, Dozois EJ, Mathis KL, et al. Safety, feasibility, and short-term outcomes in 588 patients undergoing minimally invasive ileal pouch-anal anastomosis: a single-institution experience. Tech Coloproctol 2016;20:369-74. [Crossref] [PubMed]
- Pappou EP, Kiran RP. The Failed J Pouch. Clin Colon Rectal Surg 2016;29:123-9. [Crossref] [PubMed]
- Lovegrove RE, Constantinides VA, Heriot AG, et al. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg 2006;244:18-26. [Crossref] [PubMed]
- Ng KS, Gonsalves SJ, Sagar PM. Ileal-anal pouches: A review of its history, indications, and complications. World J Gastroenterol 2019;25:4320-42. [Crossref] [PubMed]
- Utsunomiya J, Iwama T, Imajo M, et al. Total colectomy, mucosal proctectomy, and ileoanal anastomosis. Dis Colon Rectum 1980;23:459-66. [Crossref] [PubMed]
- Kirat HT, Esen E, Schwartzberg DM, et al. The usefulness of S- and H-pouch configurations in ileal pouch salvage surgery - a video vignette. Colorectal Dis 2020;22:112. [Crossref] [PubMed]
- Sampietro GM, Colombo F, Casiraghi S, et al. Ileal Pouch-Anal Anastomosis Surgery: Surgical Techniques. In: Tonolini M. editor. Imaging of Ulcerative Colitis. Milano: Springer; 2014:113-24. doi:
10.1007/978-88-470-5409-7_14 .10.1007/978-88-470-5409-7_14 - Mège D, Figueiredo MN, Manceau G, et al. Three-stage Laparoscopic Ileal Pouch-anal Anastomosis Is the Best Approach for High-risk Patients with Inflammatory Bowel Disease: An Analysis of 185 Consecutive Patients. J Crohns Colitis 2016;10:898-904. [Crossref] [PubMed]
- Zittan E, Wong-Chong N, Ma GW, et al. Modified Two-stage Ileal Pouch-Anal Anastomosis Results in Lower Rate of Anastomotic Leak Compared with Traditional Two-stage Surgery for Ulcerative Colitis. J Crohns Colitis 2016;10:766-72. [Crossref] [PubMed]
- Hicks CW, Hodin RA, Bordeianou L. Possible overuse of 3-stage procedures for active ulcerative colitis. JAMA Surg 2013;148:658-64. [Crossref] [PubMed]
- Swenson BR, Hollenbeak CS, Poritz LS, et al. Modified two-stage ileal pouch-anal anastomosis: equivalent outcomes with less resource utilization. Dis Colon Rectum 2005;48:256-61. [Crossref] [PubMed]
- Abelson JS, Michelassi F, Mao J, et al. Higher Surgical Morbidity for Ulcerative Colitis Patients in the Era of Biologics. Ann Surg 2018;268:311-7. [Crossref] [PubMed]
- Heuschen UA, Hinz U, Allemeyer EH, et al. One- or two-stage procedure for restorative proctocolectomy: rationale for a surgical strategy in ulcerative colitis. Ann Surg 2001;234:788-94. [Crossref] [PubMed]
- Schwartzberg DM, Remzi FH. The Role of Laparoscopic, Robotic, and Open Surgery in Uncomplicated and Complicated Inflammatory Bowel Disease. Gastrointest Endosc Clin N Am 2019;29:563-76. [Crossref] [PubMed]
- Lee GC, Bhama AR. Minimally Invasive and Robotic Surgery for Ulcerative Colitis. Clin Colon Rectal Surg 2022;35:463-8. [Crossref] [PubMed]
- Bartels SA, Gardenbroek TJ, Ubbink DT, et al. Systematic review and meta-analysis of laparoscopic versus open colectomy with end ileostomy for non-toxic colitis. Br J Surg 2013;100:726-33. [Crossref] [PubMed]
- Holder-Murray J, Marsicovetere P, Holubar SD. Minimally invasive surgery for inflammatory bowel disease. Inflamm Bowel Dis 2015;21:1443-58. [Crossref] [PubMed]
- Larson DW, Cima RR, Dozois EJ, et al. Safety, feasibility, and short-term outcomes of laparoscopic ileal-pouch-anal anastomosis: a single institutional case-matched experience. Ann Surg 2006;243:667-70; discussion 670-2. [Crossref] [PubMed]
- Flynn J, Larach JT, Kong JCH, et al. Robotic versus laparoscopic ileal pouch-anal anastomosis (IPAA): a systematic review and meta-analysis. Int J Colorectal Dis 2021;36:1345-56. [Crossref] [PubMed]
- Singh P, Bhangu A, Nicholls RJ, et al. A systematic review and meta-analysis of laparoscopic vs open restorative proctocolectomy. Colorectal Dis 2013;15:e340-51. [Crossref] [PubMed]
- DeLeon MF, Stocchi L. Elective and Emergent Surgery in the Ulcerative Colitis Patient. Clin Colon Rectal Surg 2022;35:437-44. [Crossref] [PubMed]
- Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148:639-651.e28. [Crossref] [PubMed]
- Ullman T, Odze R, Farraye FA. Diagnosis and management of dysplasia in patients with ulcerative colitis and Crohn's disease of the colon. Inflamm Bowel Dis 2009;15:630-8. [Crossref] [PubMed]
- Tonelli F, Di Martino C, Amorosi A, et al. Surgery for ulcerative colitis complicated with colorectal cancer: when ileal pouch-anal anastomosis is the right choice. Updates Surg 2022;74:637-47. [Crossref] [PubMed]
- Lightner AL, Spinelli A, McKenna NP, et al. Does external beam radiation therapy to the pelvis portend worse ileal pouch outcomes? An international multi-institution collaborative study. Colorectal Dis 2019;21:219-25. [Crossref] [PubMed]
- Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955;2:1041-8. [Crossref] [PubMed]
- Holvoet T, Lobaton T, Hindryckx P. Optimal Management of Acute Severe Ulcerative Colitis (ASUC): Challenges and Solutions. Clin Exp Gastroenterol 2021;14:71-81. [Crossref] [PubMed]
- M'Koma AE. Inflammatory Bowel Disease: Clinical Diagnosis and Surgical Treatment-Overview. Medicina (Kaunas) 2022;58:567. [Crossref] [PubMed]
- Martland GT, Shepherd NA. Indeterminate colitis: definition, diagnosis, implications and a plea for nosological sanity. Histopathology 2007;50:83-96. [Crossref] [PubMed]
- Delaney CP, Remzi FH, Gramlich T, et al. Equivalent function, quality of life and pouch survival rates after ileal pouch-anal anastomosis for indeterminate and ulcerative colitis. Ann Surg 2002;236:43-8. [Crossref] [PubMed]
- Shen B, Fazio VW, Remzi FH, et al. Risk factors for clinical phenotypes of Crohn's disease of the ileal pouch. Am J Gastroenterol 2006;101:2760-8. [Crossref] [PubMed]
- Dayton MT, Larsen KR, Christiansen DD. Similar functional results and complications after ileal pouch-anal anastomosis in patients with indeterminate vs ulcerative colitis. Arch Surg 2002;137:690-4; discussion 694-5. [Crossref] [PubMed]
- Murrell ZA, Melmed GY, Ippoliti A, et al. A prospective evaluation of the long-term outcome of ileal pouch-anal anastomosis in patients with inflammatory bowel disease-unclassified and indeterminate colitis. Dis Colon Rectum 2009;52:872-8. [Crossref] [PubMed]
- Trigui A, Frikha F, Rejab H, et al. Ileal pouch-anal anastomosis: Points of controversy. J Visc Surg 2014;151:281-8. [Crossref] [PubMed]
- Fleshner P. Pouches for Indeterminate Colitis and Crohn's Disease: Act now, Pay Later? In: Irving PM, Siegel CA, Rampton DS, et al. editors. Clinical Dilemmas in Inflammatory Bowel Disease: New Challenges, Second Edition. 2011. Available online:
10.1002/9781444342574.ch42 10.1002/9781444342574.ch42 - Hartley JE, Fazio VW, Remzi FH, et al. Analysis of the outcome of ileal pouch-anal anastomosis in patients with Crohn's disease. Dis Colon Rectum 2004;47:1808-15. [Crossref] [PubMed]
- Melton GB, Fazio VW, Kiran RP, et al. Long-term outcomes with ileal pouch-anal anastomosis and Crohn's disease: pouch retention and implications of delayed diagnosis. Ann Surg 2008;248:608-16. [Crossref] [PubMed]
- Connelly TM, Lincango E, Holubar SD. Crohn's of the Pouch: Now What? Clin Colon Rectal Surg 2022;35:475-86. [Crossref] [PubMed]
- Panis Y, Poupard B, Nemeth J, et al. Ileal pouch/anal anastomosis for Crohn's disease. Lancet 1996;347:854-7. [Crossref] [PubMed]
- Regimbeau JM, Panis Y, Pocard M, et al. Long-term results of ileal pouch-anal anastomosis for colorectal Crohn's disease. Dis Colon Rectum 2001;44:769-78. [Crossref] [PubMed]
- Braveman JM, Schoetz DJ Jr, Marcello PW, et al. The fate of the ileal pouch in patients developing Crohn's disease. Dis Colon Rectum 2004;47:1613-9. [Crossref] [PubMed]
- Brown CJ, MacLean AR, Cohen Z, et al. Crohn’s disease and indeterminate colitis and the ileal pouch-anal anastomosis: outcomes and patterns of failure. Dis Colon Rectum 2005;48:1542-9. [Crossref] [PubMed]
- Farouk R, Pemberton JH, Wolff BG, et al. Functional outcomes after ileal pouch-anal anastomosis for chronic ulcerative colitis. Ann Surg 2000;231:919-26. [Crossref] [PubMed]
- Shuford R, Ashburn JH. Don't Forget about the K-Pouch!. Clin Colon Rectal Surg 2022;35:499-504. [Crossref] [PubMed]
- Kiran RP, Kirat HT, Rottoli M, et al. Permanent ostomy after ileoanal pouch failure: pouch in situ or pouch excision? Dis Colon Rectum 2012;55:4-9. [Crossref] [PubMed]
- Aytac E, Dietz DW, Ashburn J, et al. Is Conversion of a Failed IPAA to a Continent Ileostomy a Risk Factor for Long-term Failure? Dis Colon Rectum 2019;62:217-22. [Crossref] [PubMed]
- Aytac E, Dietz DW, Ashburn J, et al. Long-term Outcomes After Continent Ileostomy Creation in Patients With Crohn's Disease. Dis Colon Rectum 2017;60:508-13. [Crossref] [PubMed]
- Barnes EL, Kochar B, Jessup HR, et al. The Incidence and Definition of Crohn's Disease of the Pouch: A Systematic Review and Meta-analysis. Inflamm Bowel Dis 2019;25:1474-80. [Crossref] [PubMed]
- Holubar SD. Prevention, Diagnosis, and Treatment of Complications of the IPAA for Ulcerative Colitis. Dis Colon Rectum 2018;61:532-6. [Crossref] [PubMed]
- Barnes EL, Herfarth HH, Kappelman MD, et al. Incidence, Risk Factors, and Outcomes of Pouchitis and Pouch-Related Complications in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol 2021;19:1583-1591.e4. [Crossref] [PubMed]
- Mark-Christensen A, Erichsen R, Brandsborg S, et al. Long-term Risk of Cancer Following Ileal Pouch-anal Anastomosis for Ulcerative Colitis. J Crohns Colitis 2018;12:57-62. [Crossref] [PubMed]
- Wolf JM, Achkar JP, Lashner BA, et al. Afferent limb ulcers predict Crohn's disease in patients with ileal pouch-anal anastomosis. Gastroenterology 2004;126:1686-91. [Crossref] [PubMed]
- Schwartzberg DM, Esen E, Remzi FH. Thoughtful Ileostomy Creation in Patients Undergoing Redo IPAA. Dis Colon Rectum 2020;63:117-20. [Crossref] [PubMed]
- Lynn PB, Brandstetter S, Schwartzberg DM. Pelvic Pouch Failure: Treatment Options. Clin Colon Rectal Surg 2022;35:487-94. [Crossref] [PubMed]
- Kirat HT, Kiran RP, Oncel M, et al. Management of leak from the tip of the "J" in ileal pouch-anal anastomosis. Dis Colon Rectum 2011;54:454-9. [Crossref] [PubMed]
- Addison P, Abouzeid M, Schwartzberg D. Minimally Invasive Revisional-IPAA Surgery: The Future of Redo Pouches? In: American Society of Colon & Rectal Surgeons, National Meeting. Orlando, FL; 2022.
- Toffaha A, Yousif M, Ahmed A, et al. Robotic excision of ileal pouch anal anastomosis with abdominoperineal resection - A video vignette. Colorectal Dis 2023;25:1311-2. [Crossref] [PubMed]
- Remzi FH, Aytac E, Ashburn J, et al. Transabdominal redo ileal pouch surgery for failed restorative proctocolectomy: lessons learned over 500 patients. Ann Surg 2015;262:675-82. [Crossref] [PubMed]
- Eren E, Schwartzberg D, Kirat H, et al. The dilemma in complication ileal pouch anal anastomosis: Rethink before blaming Crohn’s. In: Digestive Disease Week. San Diego; 2019.
Cite this article as: Cohen D, Silvestri C, Schwartzberg DM. Restorative pouch surgery following proctocolectomy for inflammatory bowel disease: past experience and future direction. Transl Gastroenterol Hepatol 2023;8:27.