
Endoscopic mucosal resection: tips and tricks for gastrointestinal trainees
Introduction
Endoscopic mucosal resection (EMR) is a specialized endoscopic technique designed for safe and complete resection of superficial gastrointestinal (GI) tract lesions that are not amenable to standard polypectomy. At its core, EMR utilizes techniques to separate the target lesion and surrounding mucosa from the underlying submucosa followed by complete resection of the lesion either en bloc or in a piecemeal fashion. This is compared to endoscopic submucosal dissection (ESD), which uses similar techniques of submucosal injection and lesion lifting, involves dissection of the submucosa to allow for higher chance of en bloc resection, however, ESD is more technically challenging than EMR. The development of minimally invasive, organ-saving techniques such as EMR and ESD have revolutionized the management of superficial large complex polyps and early GI malignancies. While EMR can be employed in a variety of pathologies throughout the GI tract (e.g., Barrett’s esophagus, early esophageal and gastric cancers, carcinoid tumors, duodenal adenomas), it is most frequently utilized within the colorectum (1-6). Studies indicate that there are lower costs and fewer adverse events for endoscopic resection relative to surgical resection for colorectal polyps (7,8).
However, despite its significant advantages over conventional techniques, the widespread adoption of EMR is lagging. A recent survey of 163 GI fellow physicians found that only 52.6% of the GI fellows received formal education in EMR and less than half of the fellows were able to correctly arrange the order of the steps of EMR (9). The goal of this review is to discuss when and how to perform EMR as well as to provide tips and tricks for GI trainees as they incorporate this technique into their practice.
Discussion
Identifying appropriate polyps for EMR
Polyp size and depth
The critical factor of whether a polyp is appropriate for EMR is the depth of the lesion. As the core of EMR predicates on separating the mucosa from the submucosa, EMR should typically not be used on lesions with submucosal invasion or else there would be incomplete resection of the lesion. However, it is important to note that certain reports have highlighted that EMR could at times be efficacious for select lesions with superficial submucosal invasion. Several techniques are available to predict polyp histology and depth of invasion of the lesion depending on the location. These include chromoendoscopy, narrow-band imaging (NBI), blue-light imaging, and/or endoscopic ultrasound. There is no strict polyp size limit for EMR. Generally, if the polyp is ≤2 cm in size, it can be removed en-bloc while larger lesions may need to be resected in a piecemeal fashion. Additional instances where EMR may be particularly helpful over traditional polypectomy are sessile serrated lesions, and lesions with high-grade dysplasia.
Polyp classification
Using validated classification systems to describe polyps and assess the presence of submucosal invasion is essential prior to performing EMR or deciding on if an alternative procedure such as ESD or surgery is needed. Several such classification symptoms are currently in use.
Paris classification
The Paris classification is an internationally recognized classification system that characterizes polyps based on morphology (10), and is recommended by the US Multi-Society Task Force (USMSTF) as well (11). The Paris classification classifies polyps into:
- Type 0–I: polypoid.
- 0–Ip: pedunculated.
- 0–Is: sessile.
- Type 0–II: non-polypoid and flat.
- 0–IIa: slightly elevated.
- 0–IIb: completely flat.
- 0–IIc: slightly depressed without an ulcer.
- Type 0–III: non-polypoid with an ulcer (10) (Figure 1).
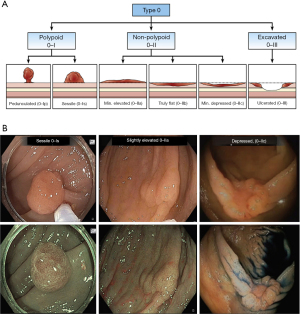
Paris Type 0–III is typically not amenable to EMR and often requires surgical resection. Additionally, a large multicenter prospective cohort study published by Burgess et al., found Paris Types 0–IIc, 0–Is, or 0–IIa + 0–Is morphologies to have a higher risk of submucosal invasion (12).
Lateral spreading tumors
Endoscopists can further enhance the description of a polyp by using the granular versus non-granular surface topographic description in conjunction with the Paris 0–Is and 0–II classifications, as suggested by the USMSTF (11). Lateral spreading tumors are lesions 10 mm or larger in diameter and can be described as granular (i.e., uneven or nodular surface) or non-granular (i.e., smooth). Generally, granular lesions are less likely to be invasive and thus are more amenable to EMR (12).
Narrow-Band Imaging International Colorectal Endoscopic (NICE) classification
The NICE classification categorizes polyps based on the optical diagnosis of color, vascularity, and surface patterns using NBI. There are three main categories of polyps that are identified via the NICE classification: (I) type 1 polyps are hyperplastic or sessile polyps identified by coloring similar to surrounding mucosa and lack of specific vascular patterns; (II) type 2 polyps are adenomas that may be more brown relative to the surrounding mucosa due to the brown blood vessels surrounding white structures and can have varying surface patterns; (III) type 3 polyps often have submucosal invasion and can be identified by their dark brown color with interrupted vascularity and irregular surface pattern (13). Given the higher probability of submucosal invasion in NICE type 3 polyps, these lesions are not typically amenable to EMR.
Kudo pit pattern classification
Kudo and colleagues developed another classification system using magnifying chromoendoscopy to stratify lesions based on their pit patterns to help predict polyp histology (14). There are five distinct pit patterns:
- Type I are normal, round pit patterns (considered nontumors);
- Type II are asteroids or stellate (considered nontumors);
- Type III are tubular or round pit patterns that can be smaller (IIIs) or larger (IIIL) than normal (often considered adenomatous);
- Type IV have dendritic or gyrus-like pit patterns (often considered adenomatous);
- Type V can have an irregular arrangement (Type Vi) or amorphous structure (Type VN) (often considered cancerous).
The irregularities in the Type V pit pattern suggest submucosal invasive disease and thus are typically not appropriate for EMR.
Technical aspects of EMR
Preprocedural process
The patient’s preparation for EMR is very similar to a standard colonoscopy regarding bowel preparation and nil per oral status. EMR is associated with a higher risk of procedural adverse events as compared to screening/surveillance colonoscopy including the risk of post-EMR bleeding, post-polypectomy syndrome, and perforation. This should be clearly communicated with the patient and alternative treatments should be discussed. In addition to obtaining informed consent for patients presenting specifically for EMR, an endoscopist could consider obtaining consent for EMR at the time of index colonoscopy in case an EMR-amenable lesion is present and there is adequate time, staff, and depending on the endoscopist’s comfort and expertise. Additional factors to consider when deciding on whether to perform EMR during an index colonoscopy would be the location and size of the polyp. For instance, a 2 cm polyp might be removed via EMR on index colonoscopy but a 5 cm polyp in a challenging location might need additional time and higher risk consent considerations. Therefore, decision to perform EMR during an index colonoscopy should be obtained on a case-by-case basis. A thorough review of prior endoscopy and pathology reports should be performed with paying close attention to the location, size, and character of the target lesion and any previous interventions that may have been performed. Discuss the anticipated complexity and duration of the procedure with the anesthesia provider. Communicate your ‘game plan’ with the endoscopy nurse and technician and give them a verbal checklist of all necessary equipment. Confirm the immediate availability of equipment that may be needed if there are intraprocedural complications.
EMR techniques
There are a variety of methods to perform EMR including hot snare or conventional EMR, cold snare EMR, and underwater EMR. Regardless of which EMR method is chosen, there are general steps that should be taken in all EMR cases. After the lesion is located endoscopically, it is important to complete a careful exam with white light and narrow band imaging versus dye-based chromoendoscopy for the best characterization of the lesion using the classification systems mentioned above. Next, marking of the borders of the lesion with a snare tip or argon plasma coagulation (APC) should be performed especially for larger lesion, to ensure complete removal of the lesion. All available techniques utilize snare resection with or without additional support from an injection, banding, a cap, etc. If the lesion is not removed at the time of index colonoscopy, the USMSTF recommends marking the lesion’s location with a tattoo if outside the rectum or cecum for easier identification at future procedures. Tattoo should not be placed under the lesion and should be placed at least 3–5 cm distal to the lesion on the opposite wall of the lesion (11).
Conventional (injection-assisted) or hot EMR
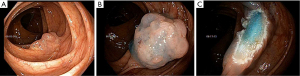
Conventional EMR involves the injection of a lifting agent into the submucosa to lift the lesion away from the underlying muscular layer for safer resection (11,15). Several lifting agents can be used and include using saline with or without the addition of a blue dye (such as Methylene blue), hetastarch as well as commercially available submucosal lifting agents. In our practice, we mix dilute Epinephrine into the lifting agent during cold EMR to limit intra-procedural oozing to help maintain better visualization during cold EMR. After identifying an appropriate polyp and the lesion is raised with the submucosal injection, it is ready for resection with a snare (Figure 2A,2B). The lesion can either be resected in one piece or in multiple pieces depending on the lesion size. For piecemeal resection, the edge of the resected area is used to pivot the snare and resect the remainder of the lesion in an overlapping fashion to mitigate the risk of residual polyp tissue. This step is repeated until the entire lesion is resected. If a lesion is resected piecemeal, then the lateral margins of the resection bed should have normal-appearing tissue and should be ablated (11,16). This can be performed either using snare tip soft coagulation (STSC) or APC (Figure 2C).
Cold EMR
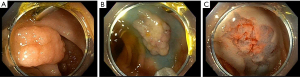
Given that the use of electrocautery in hot EMR can be associated with adverse events such as bleeding, post-polypectomy syndrome, and perforation, the cold EMR technique has been employed to help mitigate these events. Cold snare EMR follows the basic steps used in hot EMR but does not use electrocautery to resect the lesion, and thus often requires a smaller, stiffer snare with a thin cutting wire (Figure 3). A recent systematic review comparing hot and cold snare EMR suggested that cold snare EMR may be safer while maintaining excellent complete resection rates. However, larger, randomized control trial studies are needed to validate these findings (17).
Underwater EMR
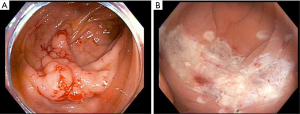
Underwater EMR is a newer technique that eliminates the need for submucosal injection by filling the lumen with water to float the lesion toward the center of the lumen, away from the underlying muscular layers (which will maintain their underlying circular structure). The lesion is then resected using electrocautery (Figure 4). It has the theoretical benefit of avoiding the seeding of malignant cells deeper into the GI tract during the injection process. Additionally, it can be useful in situations where a submucosal injection is challenging, for instance, if there is fibrosis in the resection bed of the prior EMR. A recent review and meta-analysis concluded that underwater EMR is safe and effective with comparable or lower recurrence rates and procedural adverse events compared to conventional EMR (18).
Defect closure
All EMR procedures should end with a thorough inspection of the entire treated area to evaluate for residual polyp, sites of active or potential bleeding, and perforation. Defect closure should be performed in polyps >2 cm and those located in the right colon when electrocautery was performed (19).
Adverse events
While EMR carries substantially less risk of morbidity and mortality compared to surgical intervention, adverse events can occur following EMR, and endoscopists performing EMR should be comfortable with recognizing and appropriately managing them. Adverse events related to colonic EMR include bleeding, postpolypectomy syndrome, perforation, and polyp recurrence.
Bleeding
Bleeding is the most common adverse event after EMR. It can present as intraprocedural bleeding, typically amenable to endoscopic hemostatic methods like electrocautery, or can present as delayed bleeding. A large prospective study of 1,172 patients evaluating the risk factors for EMR-related bleeding of colonic lesions ≥20 mm in size found an 11.3% rate of intraprocedural bleeding requiring intervention and a 6.2% rate of clinically significant post-endoscopic bleeding (defined as any post-procedural bleeding leading to an emergency department visit, hospitalization, or required procedural intervention to treat such as endoscopy, angiography, or surgery) and typically (89%) occurred in ≤1 week (20). Risk factors for clinically significant post-polypectomy bleeding include lesions resected in the proximal colon, intraprocedural bleeding, larger polyp size, and anticoagulant use (20-22). We do have relatively recent data that would support clipping any cold resection in the right colon of lesions ≥20 mm (23). If bleeding is encountered intraprocedurally, it can be managed with standard techniques such as soft coagulation with the snare tip or coagulation grasper forceps, APC, endoscopic clips, or hemostatic powder.
Post-polypectomy syndrome
Post-polypectomy syndrome presents with generalized symptoms of fever, tachycardia, and abdominal pain. It typically occurs within hours to up to one week later. It is likely a consequence of cautery use which leads to a full-thickness injury of the bowel wall leading to inflammation and a peritonitis-like clinical picture. It generally is self-limited, treated with supportive management of fluids, bowel rest, and often antibiotics. However, a minority of patients may need surgery if they do not improve with supportive care.
Perforation
Perforation is the most serious adverse events that can occur with any endoscopic procedure, occurring about 1.5% of the time after large colonic polyps are removed, as found in a large systematic review and meta-analysis (24). Prompt recognition of perforation is key as immediate successful endoscopic management (e.g., with endoscopic closure) can be achieved in ≥87.8% of cases per a review article evaluating 466 perforations (25). Delayed or severe perforations are often treated surgically.
Inadequate resection and recurrent disease
While endoscopic management of colorectal lesions offers many benefits to surgery, it unfortunately also has a significant risk of local recurrence of disease related to initial inadequate resection. A large systematic review and meta-analysis of 33 studies showed an average local recurrence rate of 15% after EMR of nonpedunculated colorectal lesions, with piecemeal resections having a much higher risk of local recurrence compared to en bloc resections (20% versus 3%, respectively, P<0.0001). Additionally, this study found that nearly all (96%) of recurrences occurred within 6 months (26). Thus, USMSTF recommends a repeat surveillance colonoscopy six months after piecemeal EMR, then at one- and three-year intervals (11). During the surveillance colonoscopy, endoscopists can either biopsy the prior EMR scar if no residual/recurrent polypoid tissue is visible, or else a recent study has also shown the use of narrow-band imaging for scar (NBI-SCAR) classification for identifying recurrence, which has the potential to decrease the need for biopsies (27). Reassuringly, endoscopic methods can treat most local recurrences and several tools are now available for the management of recurrent polyps including hot avulsion, endoscopic full-thickness resection, ESD, and use of the powered endoscopic resection device (28,29).
Formal EMR training
There is a significant learning curve for achieving EMR competency and other advanced luminal resection skills such as ESD. However, there is a paucity of standardized training or a commonly accepted threshold number of EMR procedures completed to develop these skills and subsequently assess competency in these procedures in the United States. There are highly variable data on the number of EMR procedures that should be done in order to determine competency, as highlighted in a systematic review evaluating the learning curves for varying colorectal polyp resection methods which found that EMR competency may be achieved between 50 and 300 procedures (30). There are recent and ongoing studies to further assess this topic as well (31).
The master-apprentice model is the traditional training method for advanced endoscopic procedures in Japan. This model involves a trainee learning about the procedure through didactics and case observation, moving into practice on animal models, before finally starting to do the basic steps of the procedure under the observation of an expert with the experts completing the complicated parts, and over time the trainees complete the entire procedure.
Potential methods for providing learning curricula for endoscopic resection training are currently being developed with studies looking at the utility of simulators (32), live animal models (33), case observation, and there is even an International Pairing Program by some gastroenterology organizations that endoscopists can take part in to hone more advanced endoscopic resection skills. Additionally, there should be an emphasis on accurate polyp characterization using the common classification systems previously discussed to best discern which polyps are appropriate for EMR.
Getting started with EMR—tips and tricks for trainees
General tips
When getting started with EMR, it is important for the trainee to first have a solid foundation of endoscopic skills such as basic polypectomy and feel comfortable managing common adverse events like intraprocedural bleeding.
Additionally, it is then important to understand the basic steps of polyp recognition, optical diagnosis, and resection. Trainees should also remember that informed consent for EMR can differ from routine screening/surveillance colonoscopy since resection of larger polyps has increased risks compared to resection of polyps ≤10 mm in size.
Patient-related factors
To perform high-quality EMR, it will be critical to have adequate bowel preparation and thus visualization for the procedure. Ensure adequate patient education is provided on the bowel preparation process and preprocedural diet to try to optimize bowel preparation.
Once the patient is in the procedure, if there is difficulty in obtaining a clear view due to the specimen or any debris, try to take advantage of gravity and position the patient in a way that improves endoscopic visualization and moves debris away from the lesion.
Injection technique
The submucosal injection can be accomplished with a static injection, where the needle is kept stationary throughout the injection process, or a dynamic injection, in which a small amount of agent is initially injected to confirm correct placement, and then the needle tip is manipulated to facilitate injection that can help make subsequent resection easier.
As far as choosing an agent to use for the submucosal injection, there are many options including normal saline, sodium hyaluronic acid, sodium alginate, succinylated gelatin, hydroxyethyl starch, and fibrinogen. In a large meta-analysis assessing the efficacy and safety of these different agents in EMR and ESD procedures, there was no agent that was superior based on complete resection rate or procedural adverse events, however, the authors mention that other agents are often favored over normal saline and at least appear more effective for better mucosal lift (34). Therefore, it is likely best to try out different agents to see what works best for the endoscopist’s personal practice. Several commercially available submucosal lifting agents are also available.
Resection
After submucosal injection is performed, snare resection is undertaken. Depending on the preferred technique of resection (conventional EMR versus cold EMR), the use of cautery can be determined. The ideal technique of snare resection involves working close to the channel of the endoscope and maneuvering the snare around the lesion as the assistant slowly opens the snare. It is critical to put gentle forward pressure on the sheath of the snare as the assistant slowly closes the snare around the polyp. When using electrocautery, it is then important to lift the snare away from the mucosa to avoid deep thermal injury while resecting the polyp. On the other hand, while using cold EMR technique, lifting away from the mucosa is not necessary. If using electrocautery, either the blended current or forced coagulation current can be used based on personal preferences (35).
Intraprocedural methods to avoid perforation
As perforation is one of the most feared complications of EMR, it is important to take care in trying to prevent it. The risk of perforation can be minimized by multiple techniques throughout the procedure. First, an adequate submucosal injection is important to create the maximal distance from the deeper muscular layers of the bowel to avoid accidental perforation. Secondly, the smallest snare possible should be used to resect the lesion. After the lesion is appropriately positioned in the snare prior to resection, the endoscopist can consider gently moving the snare back and forth to verify that there is no muscularis propria trapped. If the muscularis propria is trapped in the snare, the whole wall will move rather than the lesion alone. Early perforation can be identified by the “target sign” on the resected lesion where the inadvertently resected muscularis propria appears as a gray-white central circle surrounded by the color of the dye/agent used in the submucosal injection.
Troubleshooting
Things will not always go to plan during EMR, and part of the learning process is knowing when to pivot away from the original plan. For example, if a lesion does not lift as expected after the initial submucosal injection, this could represent either incorrect injection location (i.e., either too deep or too superficial), the presence of fibrosis from a prior biopsy, or potentially could signify submucosal invasion of the lesion. In this situation, if the concern for malignancy is low then the endoscopist could consider a transition to underwater EMR. If there is a higher concern for submucosal invasion and thus malignancy, the endoscopist may need to abandon EMR altogether in favor of other endoscopic methods like ESD, endoscopic full thickness resection (EFTR) or surgery. In this case, the location of the lesion should be marked, such as with a tattoo, for easy localization during the repeat procedure.
Additionally, the endoscopist should only resect what they can finish. Incomplete resections can lead to submucosal fibrosis and scarring, potentially leading to recurrent disease and possibly an inability to safely resect the lesion in the future.
Conclusions
EMR is an endoscopic method that allows minimally invasive resection and cure of both pre-malignant and malignant lesions in the GI tract. Thus, providing many benefits to the patient and healthcare system at large by avoiding surgical resection, which was previously the standard of care. Learning to proficiently perform EMR requires knowledge of the indications for EMR, learning which polyps are amenable to EMR, the various techniques that may be used depending on the lesion, and evaluating closely for and managing procedural adverse events as they arise. While EMR is relatively technically easy to learn, more structured curricula are needed in the United States to help trainees enhance their endoscopic resection skills.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Muhammad Aziz, Zubair Khan and Viveksandeep Thoguluva Chandrasekar) for the series “Colonoscopy: Updates and Prospects” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-23/coif). The series “Colonoscopy: Updates and Prospects” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Konda VJ, Gonzalez Haba Ruiz M, Koons A, et al. Complete endoscopic mucosal resection is effective and durable treatment for Barrett's-associated neoplasia. Clin Gastroenterol Hepatol 2014;12:2002-10.e1-2.
- Guo HM, Zhang XQ, Chen M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol 2014;20:5540-7. [Crossref] [PubMed]
- Kim GH, Jung HY. Endoscopic Resection of Gastric Cancer. Gastrointest Endosc Clin N Am 2021;31:563-79. [Crossref] [PubMed]
- Ichikawa J, Tanabe S, Koizumi W, et al. Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy 2003;35:203-6. [Crossref] [PubMed]
- Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc 2010;71:754-9. [Crossref] [PubMed]
- De Ceglie A, Hassan C, Mangiavillano B, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol 2016;104:138-55. [Crossref] [PubMed]
- Jayanna M, Burgess NG, Singh R, et al. Cost Analysis of Endoscopic Mucosal Resection vs Surgery for Large Laterally Spreading Colorectal Lesions. Clin Gastroenterol Hepatol 2016;14:271-8.e1-2.
- Keswani RN, Law R, Ciolino JD, et al. Adverse events after surgery for nonmalignant colon polyps are common and associated with increased length of stay and costs. Gastrointest Endosc 2016;84:296-303.e1. [Crossref] [PubMed]
- Garg S, Inamdar S, Tharian B, et al. Education and gastroenterology fellow knowledge about endoscopic mucosal resection of colon adenomas: a survey-based study. Endosc Int Open 2021;9:E1227-33. [Crossref] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic Removal of Colorectal Lesions-Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;158:1095-129. [Crossref] [PubMed]
- Burgess NG, Hourigan LF, Zanati SA, et al. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology 2017;153:732-742.e1. [Crossref] [PubMed]
- Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 2016;28:526-33. [Crossref] [PubMed]
- Kudo S, Tamura S, Nakajima T, et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 1996;44:8-14. [Crossref] [PubMed]
- Klein A, Bourke MJ. How to Perform High-Quality Endoscopic Mucosal Resection During Colonoscopy. Gastroenterology 2017;152:466-71. [Crossref] [PubMed]
- Abu Arisha M, Scapa E, Wishahi E, et al. Impact of margin ablation after EMR of large nonpedunculated colonic polyps in routine clinical practice. Gastrointest Endosc 2023;97:559-67. [Crossref] [PubMed]
- Thoguluva Chandrasekar V, Spadaccini M, Aziz M, et al. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm: a systematic review and pooled-analysis. Gastrointest Endosc 2019;89:929-936.e3. [Crossref] [PubMed]
- Choi AY, Moosvi Z, Shah S, et al. Underwater versus conventional EMR for colorectal polyps: systematic review and meta-analysis. Gastrointest Endosc 2021;93:378-89. [Crossref] [PubMed]
- Buddingh KT, Herngreen T, Haringsma J, et al. Location in the right hemi-colon is an independent risk factor for delayed post-polypectomy hemorrhage: a multi-center case-control study. Am J Gastroenterol 2011;106:1119-24. [Crossref] [PubMed]
- Burgess NG, Metz AJ, Williams SJ, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol 2014;12:651-61.e1-3.
- Albéniz E, Fraile M, Ibáñez B, et al. A Scoring System to Determine Risk of Delayed Bleeding After Endoscopic Mucosal Resection of Large Colorectal Lesions. Clin Gastroenterol Hepatol 2016;14:1140-7. [Crossref] [PubMed]
- Boumitri C, Mir FA, Ashraf I, et al. Prophylactic clipping and post-polypectomy bleeding: a meta-analysis and systematic review. Ann Gastroenterol 2016;29:502-8. [PubMed]
- Gupta S, Sidhu M, Shahidi N, et al. Effect of prophylactic endoscopic clip placement on clinically significant post-endoscopic mucosal resection bleeding in the right colon: a single-centre, randomised controlled trial. Lancet Gastroenterol Hepatol 2022;7:152-60. [Crossref] [PubMed]
- Hassan C, Repici A, Sharma P, et al. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut 2016;65:806-20. [Crossref] [PubMed]
- Verlaan T, Voermans RP, van Berge Henegouwen MI, et al. Endoscopic closure of acute perforations of the GI tract: a systematic review of the literature. Gastrointest Endosc 2015;82:618-28.e5. [Crossref] [PubMed]
- Belderbos TD, Leenders M, Moons LM, et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388-402. [Crossref] [PubMed]
- Zorron Cheng Tao Pu L, Chiam KH, Yamamura T, et al. Narrow-band imaging for scar (NBI-SCAR) classification: from conception to multicenter validation. Gastrointest Endosc 2020;91:1146-1154.e5. [Crossref] [PubMed]
- Hayat M, Azeem N, Bilal M. Colon Polypectomy with Endoscopic Submucosal Dissection and Endoscopic Full-Thickness Resection. Gastrointest Endosc Clin N Am 2022;32:277-98. [Crossref] [PubMed]
- Wilson N, Are VS, Osorio Cintron R, et al. Use of the endoscopic powered resection device for the management of scarred polyps. VideoGIE 2023;8:211-6. [Crossref] [PubMed]
- Rajendran A, Pannick S, Thomas-Gibson S, et al. Systematic literature review of learning curves for colorectal polyp resection techniques in lower gastrointestinal endoscopy. Colorectal Dis 2020;22:1085-100. [Crossref] [PubMed]
- Yang D, Perbtani YB, Wang Y, et al. Evaluating learning curves and competence in colorectal EMR among advanced endoscopy fellows: a pilot multicenter prospective trial using cumulative sum analysis. Gastrointest Endosc 2021;93:682-690.e4. [Crossref] [PubMed]
- Chen MJ, Wang HY, Chang CW, et al. A novel artificial tissue simulator for endoscopic submucosal resection training - a pilot study. BMC Gastroenterol 2016;16:112. [Crossref] [PubMed]
- Küttner-Magalhães R, Dinis-Ribeiro M, Bruno MJ, et al. Training in endoscopic mucosal resection and endoscopic submucosal dissection: Face, content and expert validity of the live porcine model. United European Gastroenterol J 2018;6:547-57. [Crossref] [PubMed]
- Ferreira AO, Moleiro J, Torres J, et al. Solutions for submucosal injection in endoscopic resection: a systematic review and meta-analysis. Endosc Int Open 2016;4:E1-E16. [PubMed]
- Pohl H, Grimm IS, Moyer MT, et al. Effects of Blended (Yellow) vs Forced Coagulation (Blue) Currents on Adverse Events, Complete Resection, or Polyp Recurrence After Polypectomy in a Large Randomized Trial. Gastroenterology 2020;159:119-128.e2. [Crossref] [PubMed]
Cite this article as: Herman T, Megna B, Pallav K, Bilal M. Endoscopic mucosal resection: tips and tricks for gastrointestinal trainees. Transl Gastroenterol Hepatol 2023;8:25.


