
Impact of immune tolerance mechanisms on the efficacy of immunotherapy in primary and secondary liver cancers
Introduction
Liver cancer is the sixth most common cancer and the fourth leading cause of cancer-related death worldwide (1). Hepatocellular carcinoma (HCC) is the most common subtype, accounting for 80–90% of primary liver cancer (PLC). Chronic inflammation and cirrhosis are the strongest risk factors for HCC. Chronic inflammation may arise from viral infections [hepatitis B (HBV) and hepatitis C (HCV) viruses], alcoholic and nonalcoholic steatohepatitis (NASH), chronic toxin exposure, or other infections (e.g., liver fluke) (1). Lifestyle factors such as dietary habits, chronic alcohol consumption, and sedentary lifestyle, have led to a continued increase in the incidence of HCC (1).
The liver is also a frequent site of metastasis. Liver metastases (LM) are 20 to 40 times more common than PLC (2). LM often originate from cancers of the gastrointestinal tract (particularly colon) but may also originate from melanoma, breast, pancreatic, bladder, and lung cancers (3). It is estimated that up to 50% of patients with various cancers will either present with, or develop, LM during their disease course (4). In a Surveillance, Epidemiology, and End Results (SEER) database query from 2010–2015, among 2.4 million patients with cancer of various types, the presence of LM was associated with reduced survival (5).
Immunotherapies offer promise in the treatment of patients with liver cancer. Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) alone or in combination with other treatments have significantly improved survival in patients with advanced malignancies including HCC (6-8). However, both preclinical and clinical data suggest that the presence of LM is associated with diminished response to ICI monotherapy (9). In this review, we provide insight into the immunosuppressive nature of the liver and reflect how this limits the response to ICI. We describe the components of the liver tumor microenvironment (TME) and highlight specific immunosuppressive mechanisms that may modulate the response to immunotherapy. Finally, we discuss the clinical efficacy of ICI in primary and secondary liver cancers and review novel therapeutic strategies that aim to improve immunotherapy efficacy by modulating the liver TME.
Liver tumor immune microenvironment
The liver is architecturally complex with distinct immune and stromal cell populations which have been documented with recent single-cell RNA (scRNAseq) studies (10-13). Recent studies exploring the interplay between myeloid and lymphoid cells in the TME have revealed the immunosuppressive nature of HCC TME (14-17). While there is substantial heterogeneity of HCC tumor cells across patients, analysis of ligand-receptor interactions between tumor and stromal cells of the TME has shown that there is consistent cross-talk between these populations—thus presenting the TME as an ideal target for immunotherapies in patients with HCC (14). Multiple groups have proposed prognostic HCC models based on gene signatures identified using scRNAseq analysis (18,19). A recent bulk RNAseq study by Gao classified HCC into different immune subclasses with different prognostic values based on TME signatures—immune desert (C1), immunogenic (C2), innate immune (C3), and mesenchymal (C4) (20). Specifically, the C1 subtype is defined by the absence of priming T cells; the C2 subtype is defined by the presence of infiltrating macrophages, CD4+, and CD8+ T cells, and B cell; the C3 subtype is associated with the presence of activated immunosuppressive macrophages, and the C4 subtype is associated with activated cancer associated fibroblasts (CAFs) which support epithelial-mesenchymal transition (EMT) (20-22). This section provides an overview of the various immune and stromal cellular components of the liver TME.
Immune components of hepatic TME
T cells are central to the surveillance and elimination of tumor cells. Tumor infiltrating lymphocytes (TILs) are primarily composed of CD3+, CD8+, CD4+, and Foxp3+ T lymphocytes (23-25). Tumor-infiltrating tumor-associated antigen (TAA) specific CD8+ T lymphocytes infiltrate the tumor bed or peritumoral region with antigen-specific anti-tumor cytotoxicity (26). Their presence has been linked to improved disease-free survival and a higher 5-year survival rate in HCC (27,28). CD4+ T lymphocytes are central to priming the CD8+ T lymphocytes; however, their subsets are heterogeneous (29). Pro-inflammatory Th1 (IFN-γ, TNF-α) and anti-inflammatory Th2 (IL-4, IL-10) are two major CD4+ T helper lymphocyte subsets. A Th1 to Th2 shift within the liver is associated with a poor prognosis (30-32). Regulatory T cells, Tregs (IL-10, TGFβ), another CD4+ T cell subset, play a role in escaping immune surveillance by suppressing the immune response and are further classified as natural Tregs (CD4+CD25+FOXP3+) or inducible Tregs (FOXP3+ or FOXP3−) (33). Type 1 regulatory T (Tr1) cells are FOXP3− and are an important source of IL-10 (34,35).
Natural killer (NK) cells are an innate lymphoid cell (ILC) population and play an important role in immune surveillance; deficiencies promote immune escape (36). Activation of NK cells lead to the release of lytic granules and cytotoxicity (37,38). Liver NK cells, particularly the CD56bright (CXCR6+CCR5+CD69+), play a critical role in local innate immune responses (36). Another subset of liver tumor-infiltrating NK cells expressing KLRC1 and KLRC2 genes, develop mitochondrial fragmentation, leading to deficiencies in NK-cell cytotoxicity and immunosurveillance (39). Meanwhile, natural killer T (NKT) cells express both NK and T-cell receptors (TCRs) (40) and exert both pro and anti-inflammatory effects (41,42).
Macrophages are a type of antigen-presenting cell (APC) typically responsible for innate immune response and can be classified as tissue-resident Kupffer cells (KCs) or monocyte-derived macrophages in the liver. KCs make up the largest macrophage population in the liver, originating from the yolk-sac-derived embryonic progenitors (43,44). Meanwhile, monocyte-derived macrophages are recruited from the bone marrow to infiltrate the liver in response to inflammation (43). Macrophages can promote inflammation and fibrogenesis through cell-cell signaling, which can contribute to the progression of chronic liver disease and subsequently the development of HCC over time (45,46). In the HCC TME, CD68+ macrophages are associated with pro-tumor effect and poor prognosis, whereas CD68+CD169+ macrophages may promote CD8+ T‐cell activation and cytotoxic function through a potential costimulatory function of CD169 (47,48). ScRNA-seq analysis of healthy human liver identified two macrophage subpopulations, CD68+MARCO+ and CD68+MARCO− macrophages (49). While CD68+ MARCO+ macrophages resembled long-lived KCs with reduced TNFα production capability, CD68+ MARCO− macrophages resembled recently recruited macrophages with a pro-inflammatory phenotype (49).
Neutrophils are short-lived innate immune cells that are often the first responders to infection. Although tumor-associated neutrophils (TANs) were originally classified into two fixed phenotypes: anti-tumorigenic (N1) and pro-tumorigenic (N2). Single-cell sequencing has demonstrated that neutrophil phenotypes are highly dynamic with underlying chromatin, transcriptional, and receptor heterogeneity (10,50). Human TANs primarily express CCL2 and CCL17 chemokines, facilitating the migration and tumor infiltration of macrophages and Tregs via the CCL2-CCR2 and CCL17-CCR4 interactions, respectively (51,52). TANs are associated with poor prognosis for HCC patients with a direct impact on tumorigenesis, tumor progression, and metastasis (51).
Dendritic cells (DCs) are professional APCs originating from CD34+ bone-marrow stem cells and are classified as either immature or mature based on their functional capability (53). Myeloid-derived type 2 conventional DCs (CD141−CD11c+CD14+) are the most abundant DCs in the human liver. Other subtypes include type 1 conventional DCs (CD141+CD11c+CD14−, lymphoid-derived plasmacytoid DCs (CD123+CD11c−CD303+CD304+), and monocyte-derived inflammatory DCs (54-56). DCs play a critical role in supporting adaptive immune response by regulating the differentiation of T cells in the liver, thus directly impacting peripheral tolerance in the liver (57,58). DCs isolated from HCC tumors express inhibitory receptor ligands including PD-1, T cell immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte-activation gene 3 (LAG-3), and CTLA-4, leading to the inhibition of adaptive immunostimulatory responses (59). Additionally, DC dysfunction and immunoinhibitory DC-T cell interactions can also promote HCC growth (60-62).
B lymphocytes mediate humoral immunity by differentiating into antibody-secreting plasma cells and can serve as APCs (63). While antigen-presentation stimulates anti-tumor adaptive immune effects, B cells also secrete immunosuppressive cytokines with pro-tumor effects, and thus the overall impact of tumor-infiltrating B cells in liver tumors remains controversial (63,64). Interestingly, recent scRNA-seq analysis revealed distinct B-cell subpopulations in the HCC TME and a significantly reduced density of B cells in the HCC TME compared to the normal liver microenvironment (65). Studies have shown that increased B cell infiltration within HCC TME is associated with improved clinical outcomes (63,64).
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells generated in the bone marrow that can be classified into two primary subsets: polymorphonuclear or granulocytic MDSC (PMN-MDSCs) and monocytic MDSCs (M-MDSCs) (66). Human PMN-MDSCs (CD33+CD11b+CD15+CD66b+) share similar features with neutrophils, while human M-MDSCs (CD33+CD11b+CD14+) express surface markers similar to monocytes, macrophages, and DCs (66). Immature MDSCs undergo differentiation into mature myeloid cells such as DCs or macrophages in response to the presence of transcription factors, growth factors, hypoxic conditions, and pro-inflammatory cytokines within the TME (67,68). MDSCs promote tumor progression through various mechanisms such as expression of immunosuppressive cytokines and proangiogenic factors, and inhibition of T cell responses (68,69). Several studies have reported a direct association between increased MDSC density and unfavorable clinical outcomes in HCC (70-72).
ILCs are a type of innate immune cell lacking rearranged antigen‐specific receptors (73). ILCs are classified into group 1, 2, or 3 based on transcription factors, phenotypic markers, and cytokine expression (73-75). Group 1 ILCs include NK cells and ILC1s (IFN-γ, TNFα), which are regulated by the transcription factor T-BET (74,76). Group 2 ILCs (IL-4, IL-5, and IL-13) are mainly regulated by GATA-3 and ROR-α transcription factors and promote hepatic fibrosis and HCC tumor progression (73,77-79). Group 3 ILCs (IL-17, IL-22) are regulated by the transcription factor RORγt (80).
Stromal components of TME
Hepatic stellate cells (HSCs) are liver-specific mesenchymal cells, however, their embryonic origin remains controversial since they express marker genes of all three germ layers (81-83). Inactive HSCs exist in the quiescent state in the healthy liver but undergo transition to an activated myofibroblastic state and initiate fibrosis in response to liver injury (84,85). Activated HSCs secrete immunosuppressive cytokines and angiogenic growth factors which support tumor progression (84,86,87).
Cancer-associated fibroblasts (CAFs) are mesenchymal cells derived from HSCs or tumor cells undergoing EMT (88). CAFs secrete extracellular matrix proteins and growth factors including epidermal growth factor (EGF), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), immunomodulating chemokines and cytokines, and matrix metalloproteinase (MMP) enzymes (89). The HCC CAF phenotype primarily expresses fibroblast surface markers FSP-1 and FAP (88,90). CAFs in LM can be classified into three subtypes—myofibroblastic CAFs (myCAFs), inflammatory CAFs (iCAFs), and portal fibroblasts (PF)/mesothelial CAF (PF/mesCAF) (91). A proteomic and scRNA-seq analysis categorized HCC CAFs into three subtypes which share similar features as HSC, vascular smooth muscle (VSMC), and portal fibroblast cells (92).
Liver sinusoidal endothelial cells (LSECs) are the sentinels of the liver, lining the sinusoids to form a permeable interface between parenchymal liver cells and sinusoidal vasculature (93). Hepatic LSECs are uniquely fenestrated, lack a basement membrane, and promote liver stem cell quiescence (94). They are the first to interact with and eliminate circulating pathogens and tumor cells by performing endocytosis, and thus mediate immune tolerance (93-95). In the setting of malignancy, LSECs undergo morphological and phenotypic alterations which impair their immunosurveillance ability and secrete pro-metastatic cytokines and chemokines supporting the pathogenesis of LM (96-99).
Immunosuppressive mechanisms in the hepatic TME
Liver physiologically promotes immune tolerance
The healthy liver maintains a tolerogenic environment that tempers anti-tumor immune responses (Figure 1) (100-102). This physiologically serves to prevent unwanted reactions to antigens that are filtered from the gut through the liver (103,104). In the 1960s, studies of organ transplants in animals demonstrated that porcine liver transplant recipients survived without the help of immunosuppressive agents (105), supporting the notion that the liver has a uniquely tolerogenic environment. Recent efforts have investigated the mechanisms of immune tolerance in the liver. These studies have identified T cells to be an important mediator of inappropriate responses to self-antigens and have highlighted the mechanisms by which self-reactive T cells are suppressed from propagating autoimmunity. Specifically, murine CD8+ T cells stimulated by hepatocytes presenting self-antigen undergo apoptosis after initial expansion in the liver, an important component of peripheral tolerance (106). In murine models, CD8+ T cells activated in the liver have shorter lifespans and impaired cytotoxic function compared with those activated in the lymph nodes (107). Thus, the suppression of T cell function is a key mechanism of the liver tolerogenic environment. Epithelial, stromal, and immune cell interactions with T cells contribute to hepatic immunosuppression and this will be discussed further in the context of the healthy liver and primary and secondary liver cancer.
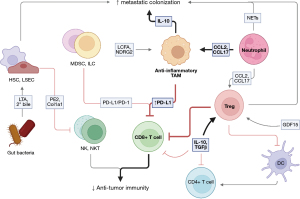
Hepatocytes make up the bulk of the liver’s structure (108) and are central to the modulation of the adaptive immune response. Hepatocytes are non-professional APCs that interact with T cells in the healthy liver (109). Low levels of co-stimulatory CD80/86 and elevated levels of PD-L1 on hepatocytes during antigen presentation to T cells lead to dysfunctional T cell activation (106,110,111). Hepatocytes also directly cause CD8+ T cell death through a process known as ‘suicidal emperipolesis’, in which autoreactive T cells actively invade hepatocytes for degradation in lysosomal compartments, leading to diminished CD8+ T cell numbers in the liver. Prevention of this process results in T cell-mediated hepatitis in preclinical models (112). Therefore, hepatocytes promote T cell tolerance in the liver.
Liver DCs also contribute to immune tolerance. Human hepatic DCs lack efficient antigen uptake, resulting in impaired CD4+ T cell proliferation and responsiveness. Furthermore, liver DCs secrete high levels of IL-10 to promote the generation of Treg and Th2 cells (113). Murine hepatic DCs have limited phagocytosis, express low levels of costimulatory molecules, and poorly activate T cells as compared to splenic DCs (114). This effect may be partly due to the low expression of TLR4 by liver DCs, resulting in impaired phagocytosis (115).
KCs limit autoimmune responses by presenting self-antigen, expanding Treg populations, and restricting CD4+ T cell priming in mice (116,117). KCs act as inefficient APCs and express low levels of MHCII to NK, NKT, and T cells, promoting tolerance by limiting the cytotoxic capabilities of these cells (118). Additionally, KCs produce immunosuppressive cytokines IL-10 and TGFβ, which suppresses T cell function and promote polarization of Tregs (119,120).
Hepatic Tregs, which are both thymically derived and peripherally induced, prevent T cell-mediated inflammation and autoimmunity by restricting immune responses to self-antigens (121). Polarization of naïve CD4+ T cells to Tregs in the liver is dependent on TGFβ signaling, which is produced by KCs or LSECs (122,123). Tregs function as an IL-2 sink, restricting the ability of other T cells to sense IL-2 and proliferate (124,125). Additionally, Treg expression of CTLA-4 restrains T cell activation (126,127). Hepatic Tregs are an important source of immunosuppressive cytokines IL-10 and TGFβ (128). Importantly, loss of hepatic Tregs and thus impaired peripheral tolerance is associated with liver injury (129-131).
Hepatic anatomy supports immune tolerance
LSECs contribute to immune tolerance in the liver. LSECs are fenestrated endothelial cells that line liver sinusoids and allow efficient antigen filtration (132). They have the ability to present antigen, which supports formation of Tregs and, causes dysfunction of OT-I T cells in vivo (123,133). These T cells are unable to regain cytotoxic function after restimulation with a clonotypic antibody (134). This may be because LSECs can express the inhibitory molecule PD-L1 (135). Lymphocytes that home to the liver must travel through the sinusoidal channels of the liver, then bind to LSECs via atypical adhesion molecules (136). Minimal shear forces in the sinusoids means this process does not require selectins (137). LSECs can sequester activated CD8+ T cells in mouse livers by binding via ICAM-1, preventing further movement through the liver, and inducing apoptosis (138). Steatohepatitis, liver fibrosis, and cirrhosis reduce tumor infiltration by T cells (139). Thus, LSECs modulate the adaptive response to self-antigens in the liver by altering the physical structure and immune signaling milieu to contribute to immune tolerance.
Immune signaling drives hepatic immunosuppression
The immune signaling milieu, including IL-10 and TGFβ, drives immunosuppression in the liver. IL-10 is central to regulating immune responses in the liver. Activating human CD4+ T cells in the presence of IL-10 induces anergy (140). IL-10+ B cells are elevated in patients with operational tolerance, which is a stable function of a transplanted organ without the use of immunosuppressives (141). IL-10 prevents infiltration of fibrosis-inducing neutrophils after liver injury in mice (142). Like IL-10, TGFβ is an essential modulator of liver-specific immune responses (143) and has been suggested to play a role in immunosuppression in the setting of cancer. Genes in the TGFβ pathway which display markers of T cell exhaustion are overexpressed in patients with HCC (144). Simultaneous targeting of TGFβ and PD-L1 increases immune infiltration and reduces tumor growth compared to anti-PD-L1 alone in murine breast and colorectal cancer (CRC) models (145). Together, these suppressive mechanisms prevent the induction of autoimmunity to antigens processed by the liver, as well as alloantigens after liver transplant. These mechanisms may also abate the immune system’s natural ability to recognize and respond to cancer.
Anti-tumor immunity is dysregulated in PLC
PLC restricts effective anti-tumor responses. CD8+ T cells express elevated levels of PD-1 and display an exhausted phenotype in HCC patients which correlates with worse survival (146,147). Exhausted NK cells present in primary tumors of HCC patients are associated with poor clinical outcomes (148). While tumor-intrinsic mechanisms may be partly to blame for the impaired cytotoxic activity of these effectors (149,150), systemic reprogramming of myeloid and lymphoid compartments by the diseased liver also contributes to immune suppression in the TME and subsequent cancer progression. Tumor-associated macrophages (TAMs) are associated with increased tumor growth and worse survival in patients with HCC (151,152). PD-L1+ KCs in the HCC stroma induce T-cell exhaustion by localizing with CD8+ T cells (153). PD-L1 expression by HCC cells promotes TAM infiltration via NF-κB and STAT3 signaling (154). Additional signaling by cancer cells and TAMs increases TAM infiltration, polarizes TAMs toward an anti-inflammatory state, and predicts poor prognosis (155-157). In a mouse model of HCC, the immunosuppressive effects of an anti-inflammatory subset of TAM, TREM-1+ TAMs, could not be reversed by PD-L1, suggesting a role in ICI resistance (158). Tregs also play a significant role in promoting PLC. Tregs correlated with carcinogenesis and worse survival of patients with HCC (159). The presence of growth differentiation factor 15 (GDF15) in patients with HCC promotes Treg infiltration into the tumor and drives cancer progression (160,161). Tumor-infiltrating Tregs lead to an immunosuppressive environment by suppressing MHCII expression on DCs and by interacting directly with CD8+ T cells through PD-L1 interactions (162,163).
Neutrophils, MDSCs, and ILCs all contribute to the immunosuppressive environment of HCC. Neutrophils release elevated levels of extracellular traps (NETs). NETs activate TLR4/9-COX2 signaling to induce inflammation and support the metastasis of Hepa1-6 HCC cells in mice (164). Neutrophils also recruit TAMs and Tregs, as well as inhibit T cell cytotoxicity, to promote cancer progression in patients with HCC (10,52). MDSCs are associated with poor prognosis in patients receiving anti-PD-L1 therapy. MDSC suppression improves response to anti-PD-L1 therapy by enabling CD8+ T cell function (165). HCC-associated ILC2s recruit immunosuppressive neutrophils and are associated with worse survival in patients with HCC (73). Meanwhile, ILC3s induce CD8+ T cell apoptosis through direct cell-to-cell interactions to support the growth of Hepa1-6 tumors in mice (166). Finally, non-immune factors in the tumor environment promote HCC growth. HSCs create physical barriers which reduce the infiltration of CD4+ and CD8+ T cells, Tregs, and NKT cells in fibrosis-associated HCC by depositing type I collagen (167). In the mouse gut, the secretion of lipoteichoic acid and deoxycholic acid by gram-positive bacteria induces the upregulation of COX-2 through TLR2 on HSCs, which functions to produce prostaglandin E2. Prostaglandin E2 in turn dampens the antitumor immune response by reducing IFN-γ and TNF-α production by liver immune cells (168). Furthermore, commensal gut bacteria metabolism regulates secondary bile acid concentrations in the liver, and this influences NKT-mediated tumor inhibition (169). These immune compartments contribute to immunosuppression and tumor growth in PLC.
Anti-tumor immunity is dysregulated in LM
The tolerogenic environment of the liver makes it particularly susceptible to metastasis. KCs play a key role in facilitating the colonization of circulating tumor cells in the liver. Exosomes from PAN02 pancreatic ductal adenocarcinoma (PDAC) cells induce an anti-inflammatory state in KCs that induces TGFβ and fibronectin production by HSCs. Fibronectin production leads to the recruitment of bone marrow-derived macrophages to promote metastasis in mice (170). Further, IL-10 secreted by KCs in the liver blocks the cytotoxic effect of ischemia-reperfusion injury and promotes the formation of LM by metastatic human CRC lines in nude mice (171). CXCR2 knockout and neutrophil depletion increased levels of infiltrating T cells and decreased LM, suggesting that neutrophils play a role in forming a pre-metastatic niche (172). In mice with breast cancer, these TANs facilitate the establishment of metastases by releasing NETs and chemotactically attracting cancer cells (173). Tregs also contribute to the pre-metastatic niche. Increased infiltration of Tregs in the liver of mice with PDAC after chemotherapy spurs the formation of metastases (174). Non-immune subsets, such as HSCs and LSECs, further modulate immune activity in the liver, facilitating metastasis. HSCs permit LM by inducing a quiescent state in NK cells in MDA-MB-231 metastatic breast cancer in mice (175). LSECs induce an immunosuppressive environment in B16F10 LM by binding T cells via Lyve-1, reducing the prevalence of effector T cells and leading to enhanced metastasis (176).
Anti-tumor immunity in the liver is reduced following metastatic colonization. As in PLC, the CD8+ T cell mediated anti-tumor immune response is suppressed in LM and is not rescued by ICI monotherapy (177). This suppression is antigen-specific and systemic (9). Multiple immune subsets contribute to T cell dysfunction and suppression, including macrophages, neutrophils, DCs, and MDSCs, causing metastatic growth and diminished immune response at the site of the primary tumor. NDRG2-mediated NF-κB signaling and CD36-mediated internalization of long-chain fatty acids promote anti-inflammatory macrophage polarization and metastasis in CMT93 CRC and Lewis lung carcinoma cell lines, respectively (178,179). Metastasis-associated macrophages also support peripheral immune tolerance, as selective elimination of antigen-specific CD8+ T cells via Fas-FasL interactions by LM-localized FasL+CD11b+F4/80+ monocyte-derived macrophages reduces anti-tumor immunity in B16F10 melanoma and MC38 CRC murine subcutaneous tumors, contributing to anti-PD-L1 resistance (9). TANs infiltrate LM at high levels and adopt a pro-tumoral phenotype, demonstrated by the expression of genes such as arginase-1, IL-10, and TGFβ1, that supports metastatic growth and contributes to anti-PD-1 resistance in MC38 LM models. In contrast, cross-presenting DCs are strikingly absent from CRC LM in mice, depleting activated T cells and resistance to ICI (180). Finally, MDSCs correlate with CRC LM in patients (181). Accumulation of MDSCs in MC38 LM correlates with Treg cell number (182). Finally, hepatocyte CCRK signaling mediates B16F10 and MC38 LM by promoting the infiltration of PMN-MDSCs into the liver, leading to reduced levels of effector NKT cells (183). Thus, the myriad of immunosuppressive populations in the liver which enable metastatic colonization also promote ICI resistance.
Therapeutic strategies to surmount ICI resistance in primary and secondary malignancies
The role of ICI in the management of PLCs
The PD-1 inhibitors, nivolumab and pembrolizumab, were initially studied as single arm phase II trials after sorafenib failure and showed similar overall response rates (17–20%) (184,185). Pembrolizumab was then studied in the second line setting in a placebo-controlled randomized phase III trial (KEYNOTE-224), but statistically significant improvements in overall survival (OS) and progression-free survival (PFS) were not observed. However, post-progression therapies approved during the time of the trial (e.g., nivolumab, regorafenib) may have impacted OS. Based on these data, pembrolizumab was approved as a second-line therapy after sorafenib in advanced HCC. Nivolumab was compared to sorafenib (CheckMate 459) in patients with advanced treatment-naïve HCC, and statistical significance for OS was also not met [median OS: 16.4 vs. 14.7 months; hazard ratio (HR) =0.85; P=0.075]. Nivolumab yielded a higher disease control rate (median: 7.5 vs. 5.7 months) and better safety profile (grade 3 or 4 treatment-related adverse event rate 22% vs. 49%) (186). However, due to not meeting the primary endpoint of OS, the FDA’s approval of nivolumab in the treatment of advanced HCC was overturned in 2021.
While the role of single-agent ICI in the management of HCC is uncertain, these initial studies spurred several studies evaluating various ICI combination strategies. The combination of PD-L1 inhibitor atezolizumab with the vascular endothelial growth factor (VEGF) inhibitor bevacizumab became the frontline standard of care in 2020 for HCC, based on data IMbrave150 trial, where treatment-naïve patients with advanced HCC were randomized to receive atezolizumab/bevacizumab or sorafenib. Patients who received the combination treatment had prolongation of PFS (median PFS: 6.8 vs. 4.3 months; HR =0.59; P<0.001) and OS (median OS: 19.2 vs. 13.4 months; HR =0.58; P=0.0006) (187). In addition to the reduction of tumor vascularization, VEGF blockade impacts the immune infiltration within the TME. VEGF signaling directly upregulates the proliferation of suppressive immune cells, inhibits DC maturation, increases the number of Treg cells, and promotes MDSCs. Bevacizumab was shown to reduce MDSCs in patients with lung and colon cancer (188,189), and the combination of anti-PD-1 and anti-VEGFR2 treatment is associated with decreased TAMs and increased CD8+ T cells within the liver TME in preclinical HCC models (190).
Another treatment option for HCC is dual CTLA-4 and PD-1/PD-L1 blockade. In patients with advanced HCC after progression on sorafenib, the combination of CTLA-4 inhibitor ipilimumab with nivolumab yielded an objective response rate of 32% and a median OS of 22.8 months (186). In the phase 3 HIMALAYA trial, treatment-naive patients with advanced HCC received CTLA-4 inhibitor tremelimumab plus the PD-L1 inhibitor durvalumab, durvalumab alone, or sorafenib. Patients on the combination arm had an objective response rate of 20.1% and median OS of 13.6 months. The 36-month OS rate was improved in the combination arm [30.7% vs. 24.7% (durvalumab alone) vs. 20.2%], leading to the approval of combination durvalumab and tremelimumab in the first-line setting for patients with advanced HCC in 2022.
Although there is a strong rationale to study combining anti-angiogenic tyrosine kinase inhibitors with ICI in HCC, the combination of lenvatinib and pembrolizumab compared with lenvatinib alone, as well as cabozantinib and atezolizumab compared with sorafenib alone were not shown to be superior regimens (191,192).
The role of ICI in the management of LM
The initial observation of diminished response to PD-1 blockade in patients with LM was reported by Tumeh et al. In a cohort of patients with metastatic melanoma who received pembrolizumab, presence LM was associated with a lower response rate and shorter PFS [overall response rate (ORR): 30.6% vs. 56.3%, median PFS: 5.1 vs. 20.1 months, P<0.0001] (193). A similar observation was noted in patients with advanced non-small cell lung cancer (NSCLC) treated with PD-1 blockade (193). Several studies have since reported similar findings in other solid tumors.
In a pan-cancer analysis evaluating clinical data of a published cohort of 1,661 patients who received ICI therapy, patients with LM had significantly shorter OS than those without LM (10 vs. 20 months; HR =1.66; P<0.0001) in multivariate analysis (194). This cohort included patients with breast, colorectal, esophagogastric, head and neck, melanoma, NSCLC, renal, and non-melanoma skin cancers. A subgroup analysis showed that the presence of LM was associated with shortening of OS in the ICI monotherapy group (P<0.0001) but did not reach statistical significance in the ICI-based combination therapy group (P=0.0815) (194). Further, a meta-analysis of patients with NSCLC treated with anti-PD-1/PD-L1 ICI was conducted assessing 6,274 patients across 11 publications. The pooled results showed that anti-PD-1/PD-L1 treatments correlated with better OS (HR =0.73; 95% CI: 0.64–0.83; P<0.05) and PFS (HR =0.77; 95% CI: 0.6–0.94; P<0.05) compared with standard chemotherapy in both patients with and without LM (194). However, subgroup analysis showed that while ICI monotherapy could not prolong PFS in patients with LM, ICI-based combination therapy could. Conversely, in patients without LM, both ICI monotherapy and combination therapy prolonged both PFS and OS. Together, these findings suggest that the presence of LM diminished tumor response in patients who received ICI monotherapy, especially in NSCLC. While combination treatments may overcome hepatic resistance mechanisms to ICI, the optimal strategy remains under investigation.
Use of locoregional therapies to the liver to overcome ICI resistance
It is hypothesized that the elimination of LM by surgical resection, radiation, or other locoregional treatments can restore ICI efficacy in patients with LM (Table 1).
Table 1
| Locoregional technique | Patient population | N | Intervention | Outcome | Reference |
|---|---|---|---|---|---|
| Stereotactic body radiotherapy | Metastatic NSCLC after progression on ≥1 systemic therapy | 39 | Ipilimumab (anti-CTLA-4 antibody) in combination with radiation therapy to one metastatic site (6 Gy ×5 or 9 Gy ×3) | ORR 18%; disease control 31%; median OS 13 months | (195) |
| Unresectable HCC | 5 | SBRT followed by anti-PD-1 antibody | 2 of 5 patients with CR, 3 of 5 patients with PR, median PFS 14.9 months | (196) | |
| Unresectable or recurrent HCC | 64 | SBRT-ICI vs. TACE (propensity score matching analysis) | 12-month PFS improved in SBRT-ICI group (93.3% vs. 16.7%; P<0.001); 24-month OS improved in SBRT-ICI group (80.4% vs. 8.3%; P<0.001) | (197) | |
| Hepatic ablation | Colorectal cancer with liver metastases | 38 | RFA treatment followed by primary tumor resection | Radiofrequency ablation increased T-cell infiltration and PD-L1 expression in human colorectal tumors | (198) |
| HCC | 32 | Tremelimumab (anti-CTLA-4 antibody) in combination with ablation | 26% achieved confirmed partial response; 12-month PFS rate 33.1%; median OS 12.3 months | (199) | |
| TACE and transarterial radioembolization | Unresectable HCC | 34 | TACE plus camrelizumab (anti-PD-L1 antibody) | Objective response rate was 35.3%; median PFS 6.1 months; median OS 13.3 months | (200) |
| BCLC stage C HCC | 1 | Y-90 radioembolization in combination with nivolumab (anti-PD-1 antibody) | >50% reduction in size of primary tumor | (199) |
ICI, immune checkpoint inhibitor; PLC, primary liver cancer; LM, liver metastases; NSCLC, non-small cell lung cancer; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ORR, objective response rate; OS, overall survival; HCC, hepatocellular carcinoma; SBRT, stereotactic body radiotherapy; PD-L1, programmed death-ligand 1; CR, complete response; PR, partial response; PFS, progression-free survival; TACE, trans-arterial chemoembolization; RFA, radiofrequency ablation; BCLC, Barcelona Clinic Liver Cancer.
Stereotactic body radiation therapy (SBRT) is an effective treatment modality for non-surgical candidates in patients with primary and oligometastatic liver tumors with adequate liver function. In pre-clinical models, radiation improves ICI efficacy. For example, in mice models with melanoma, the combination of anti-CTLA-4, anti-PD-L1/PD-1, and radiation produced major tumor responses (201). While anti-CTLA-4 can suppress Treg cell number and function, thereby increasing the CD8+ T cell to Treg (CD8+/Treg) ratio, radiation enhances the diversity of the T cell receptor repertoire of intratumoral T cells. The addition of PD-L1 blockade reverses T cell exhaustion to mitigate the depression of CD8+/Treg ratio and further activates T cell expansion (201). Even in patients with advanced NSCLC, where anti-CTLA-4 antibodies have failed to demonstrate significant monotherapy activity, the addition of radiation to anti-CTLA-4 therapy achieved disease control in 31% of patients (195). Increased serum interferon-β and early dynamic changes in blood T cell clones after radiation were predictors of response. These data suggest that the combination of liver SBRT and ICI may act synergistically to improve tumor response rate and outcomes of patients with LM.
Preclinical HCC models have also shown the combination of radiation and ICI to exhibit therapeutic synergism and improved OS (202,203). Small series have shown promising signs of clinical activity in patients with HCC (196,204). A propensity score matching analysis of patients with HCC who received SBRT-ICI versus trans-arterial chemoembolization (TACE) showed a significantly improved response rate (87.5% vs. 16.7%), 24-month PFS (77.8% vs. 2.1%), and 24-month OS (80.4% vs. 8.3%) in the SBRT-ICI arm (197). Studies are ongoing evaluating the efficacy of SBRT-ICI in early and advanced stage HCC (NCT05488522, NCT04857684).
Further, in the context of LM, liver-directed radiotherapy may modulate systemic ICI response. In mice bearing subcutaneous and liver tumors treated with radiotherapy, anti-PD-L1, or the combination, it was shown that liver-directed radiotherapy did not modulate T cell number in the subcutaneous tumor on its own, but along with PD-L1 blockade significantly increased T cell infiltration into the subcutaneous tumor (9). Mice which received the combination therapy had regression of both the subcutaneous and liver tumors, suggesting that liver-directed radiotherapy improves systemic efficacy of ICI by restoring peripheral CD8+ T cells (9). Prospective clinical trials are ongoing to understand the clinical efficacy of combining hepatic SBRT with ICI in advanced malignancies with LM (NCT05169957, NCT04923776).
Local ablation increases liver immunogenicity and activation of DCs in HCC (205). In preclinical models, radiofrequency ablation increases T cell infiltration and expression of immune checkpoints (PD-L1, LAG-3) within the treatment zone and distant sites, via activation of serum and intra-tumoral cytokines (198,206,207). Thus, the addition of ICI to ablation may result in more effective antitumor immunity. This was studied in a small retrospective cohort of patients with CRC with LM, where the combination of radiofrequency ablation and PD-1 blockade significantly enhanced T cell immune responses, higher response rates and prolonged survival (198). Similarly, the combination of tremelimumab (CTLA-4 inhibitor) with tumor ablation led to 26.3% response rate with a median time to tumor progression of 7.4 months in patients with HCC (199).
TACE is a widely accepted treatment modality for patients with unresectable intermediate stage HCC. Transarterial radioembolization (TARE) using Yttrium-90 (Y90) is also an emerging and now adopted option for treating unresectable HCC (208). TACE may promote immunogenic cell death. In a cohort of patients with HCC treated with TACE, the proportion of circulating Th17 CD4+ T cells increased and was associated with improved outcomes (209,210). In another cohort of patients with HCC treated with TACE, PD-L1 and PD-1 expression on tumor cells significantly increased following treatment (200). Similarly, in patients treated with TARE, the hepatic TME after treatment suggested signs of local immune activation with higher expression of granzyme B, infiltration of CD8+ T cells, NK cells, and NKT cells. These studies indicate that the combination of TACE/TARE with ICI should be further investigated in the treatment of HCC.
Histotripsy is an investigational ultrasound ablation technique that uses short high-amplitude pulses to create inertial acoustic damage to tissues (211,212). The rapid expansion and collapse of cavitation microbubbles leads to cellular destruction of the target tissue (213) in a precise manner at the histologic level with real-time visualization by diagnostic ultrasound. In immunocompetent rat HCC models, partial histotripsy of local tumor resulted in complete response and prolonged disease-free survival compared to untreated controls (214,215). In murine tumor models, histotripsy demonstrated local and systemic anti-tumor immune responses with and without concurrent CTLA-4 blockade (211,216,217). A multi-center phase I study evaluated hepatic histotripsy in eight patients with unresectable multifocal liver tumors including HCC, CRC with LM, cholangiocarcinoma (CCA), and breast cancer with LM, in which no device-related adverse events were noted (218). A patient with CRC LM had sustained reduction of non-treated tumors in the liver following histotripsy (219). At a cellular level, histotripsy disrupts cellular structures to release tumor-specific antigens and damage-associated molecular patterns, which can stimulate innate and adaptive immune responses, and subsequently modulate the TME and diminish cancer progression (215,216). Further research is required to identify the potential role of histotripsy in clinical practice and to consider combination strategies using hepatic histotripsy and ICI to overcome immune resistance.
Use of combination systemic therapies overcome ICI resistance
The addition of VEGF blockade may restore ICI efficacy in patients with LM. Enhancement of CD8+ T cell function with anti-angiogenic agents has been demonstrated in solid malignancies including HCC (187,220-222). In patients with NSCLC and LM combination of VEGF blockade, chemotherapy, and ICI significantly prolonged PFS compared to chemotherapy and ICI alone (222). VEGF signaling has been implicated in diminished anti-tumoral immunity by several mechanisms, including reducing cytotoxic activity of peripheral CD8+ T cells (223), enhancing Treg cell activation (224-226), and inducing immunosuppressive effects of MDSCs (68). VEGF-A also directly induces FASL expression leading to apoptosis of CD8+ T cells (227). Thus, blocking the VEGF pathway in combination with PD-1/PD-L1 blockade may synergistically restore ICI efficacy in patients with LM by reducing CD8+ T cell apoptosis within the liver, and enhance T cell activity and function systemically.
Adoptive cell transfer of chimeric antigen receptor-modified T cells (CAR-T) has been successful in treating hematologic malignancies but has had a modest impact on the treatment of solid tumors. In HCC, glypican-3 (GPC-3) provides a novel prognostic therapeutic target for CAR-T in HCC (228). In vitro and xenograft models of HCC have shown early signs of activity of GPC-3 targeted CAR-T cells in treating GPC3+ HCC (229). Other potential targets for CAR-T cell therapy in HCC may include AFP (NC03132792), mutant TP53, and HBV antigens (NCT03899415). The polarization of immune cells within the liver TME may be shaped by CAR-T cell therapy, as well as cell-based therapy using TILs, which may induce the release of TAAs generating an antitumor immune-response. The identification of unique antigens on aberrant cells or tumor-associated cell death pathways may emerge as a new therapeutic strategy to overcome anti-inflammatory microenvironments and reactivate tumor immunosurveillance within the liver (230).
Immune checkpoint molecules beyond PD-1, PD-L1, and CTLA-4 may be targeted to enhance an anti-tumor immune response in primary and secondary liver cancers. In HCC, increased number of PD-1+CD8+ T cells and PD-L1+ tumor cells are associated with poorer prognosis (231). TIM-3 expression on CD4+ and CD8+ TILs and TAMs inhibits T cell function in HCC, while TIM-3 expression on Treg cells further enhances T cell suppression (232). LAG-3 represents another targetable immune checkpoint, which normally is upregulated upon activation of T cells and can provide a de-activating signal to T cells. LAG-3 is preferentially expressed on tumor-specific CD4+ and CD8+ TILs as compared to other immune compartments (233). These preclinical data support further evaluation of the combination of LAG-3 and TIM-3 with PD-1 and PD-L1 blockade in the treatment of HCC.
In other solid malignancies, clinical trials are underway evaluating LAG-3 modulators along with PD-1 or CTLA-4 inhibitors. For example, LAG-3 blockade was shown to enhance TILs in preclinical models of CRC with LM (234). Relatlimab, the first commercially developed anti-LAG-3 antibody, was the first LAG-3 inhibitor to be approved along with nivolumab to treat unresectable or metastatic melanoma (235). Prospective clinical trials are needed to understand whether blockade of TIM-3, LAG-3 and/or other immune checkpoints (e.g., TIGIT, PVRIG, KIR-L, NKG2A, CD47) can enhance the efficacy of ICI in advanced malignancies with LM (236).
Conclusions
The hepatic TME of primary and secondary liver tumors is characterized by immune cells, suppressive cells, and complex pro-inflammatory and immunomodulatory signaling. The magnitude of the innate and adaptive immune response depends on the interactions between tumor cells and components of the liver TME. ICI monotherapy and along with other systemic therapies play a fundamental role in managing both primary and secondary liver cancer. However, further characterization of the underlying cellular and molecular mechanisms leading to immune evasion is needed to inform effective novel combination strategies and improve ICI efficacy in both PLC and LM.
Acknowledgments
The figure was generated using Biorender (www.biorender.com).
Funding: Funding was provided by LUNGevity, NCI (No. CA252010, PI Green), Veterans Affairs (No. I01 BX005267; PI Green), Melanoma Research Alliance (No. MRA 689853; PI Green), P01 (No. CA233452; PI Bhowmick) and NIAID Training Grant T32 to ANP.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-11/coif). ANP received Training Grant T32 from NIAID. TW has a consulting relationship with Boston Strategic Partners, Inc. ZX reports the following conflicts of interests with HistoSonics: funding; planned, issued, or pending patent; stock or stock options; receipt of equipment, materials, or other services. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Milette S, Sicklick JK, Lowy AM, et al. Molecular Pathways: Targeting the Microenvironment of Liver Metastases. Clin Cancer Res 2017;23:6390-9. [Crossref] [PubMed]
- de Ridder J, de Wilt JH, Simmer F, et al. Incidence and origin of histologically confirmed liver metastases: an explorative case-study of 23,154 patients. Oncotarget 2016;7:55368-76. [Crossref] [PubMed]
- Tsilimigras DI, Brodt P, Clavien PA, et al. Liver metastases. Nat Rev Dis Primers 2021;7:27. [Crossref] [PubMed]
- Horn SR, Stoltzfus KC, Lehrer EJ, et al. Epidemiology of liver metastases. Cancer Epidemiol 2020;67:101760. [Crossref] [PubMed]
- Kudo M. Combination Cancer Immunotherapy in Hepatocellular Carcinoma. Liver Cancer 2018;7:20-7. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019;18:197-218. [Crossref] [PubMed]
- Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27:152-64. [Crossref] [PubMed]
- Xue R, Zhang Q, Cao Q, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 2022;612:141-7. [Crossref] [PubMed]
- Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541-50. [Crossref] [PubMed]
- Thorsson V, Gibbs DL, Brown SD, et al. The Immune Landscape of Cancer. Immunity 2018;48:812-30.e14. [Crossref] [PubMed]
- Li X, Ramadori P, Pfister D, et al. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer 2021;21:541-57. [Crossref] [PubMed]
- Massalha H, Bahar Halpern K, Abu-Gazala S, et al. A single cell atlas of the human liver tumor microenvironment. Mol Syst Biol 2020;16:e9682. [Crossref] [PubMed]
- Lu Y, Yang A, Quan C, et al. A single-cell atlas of the multicellular ecosystem of primary and metastatic hepatocellular carcinoma. Nat Commun 2022;13:4594. [Crossref] [PubMed]
- Ho DW, Tsui YM, Chan LK, et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun 2021;12:3684. [Crossref] [PubMed]
- Zhang Q, He Y, Luo N, et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019;179:829-45.e20. [Crossref] [PubMed]
- Liu C, Pu M, Ma Y, et al. Intra-tumor heterogeneity and prognostic risk signature for hepatocellular carcinoma based on single-cell analysis. Exp Biol Med (Maywood) 2022;247:1741-51. [Crossref] [PubMed]
- Wang H, Yu S, Cai Q, et al. The Prognostic Model Based on Tumor Cell Evolution Trajectory Reveals a Different Risk Group of Hepatocellular Carcinoma. Front Cell Dev Biol 2021;9:737723. [Crossref] [PubMed]
- Gao X, Huang H, Wang Y, et al. Tumor Immune Microenvironment Characterization in Hepatocellular Carcinoma Identifies Four Prognostic and Immunotherapeutically Relevant Subclasses. Front Oncol 2020;10:610513. [Crossref] [PubMed]
- Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599-616. [Crossref] [PubMed]
- Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015;160:48-61. [Crossref] [PubMed]
- Yao W, He JC, Yang Y, et al. The Prognostic Value of Tumor-infiltrating Lymphocytes in Hepatocellular Carcinoma: a Systematic Review and Meta-analysis. Sci Rep 2017;7:7525. [Crossref] [PubMed]
- Zheng X, Jin W, Wang S, et al. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients With Hepatocellular Carcinoma. Front Immunol 2021;12:729705. [Crossref] [PubMed]
- Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity 2013;39:61-73. [Crossref] [PubMed]
- Fatourou EM, Koskinas JS. Adaptive immunity in hepatocellular carcinoma: prognostic and therapeutic implications. Expert Rev Anticancer Ther 2009;9:1499-510. [Crossref] [PubMed]
- Wada Y, Nakashima O, Kutami R, et al. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 1998;27:407-14. [Crossref] [PubMed]
- Cai XY, Gao Q, Qiu SJ, et al. Dendritic cell infiltration and prognosis of human hepatocellular carcinoma. J Cancer Res Clin Oncol 2006;132:293-301. [Crossref] [PubMed]
- Bian J, Lin J, Long J, et al. T lymphocytes in hepatocellular carcinoma immune microenvironment: insights into human immunology and immunotherapy. Am J Cancer Res 2020;10:4585-606. [PubMed]
- Lee HL, Jang JW, Lee SW, et al. Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci Rep 2019;9:3260. [Crossref] [PubMed]
- Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006;10:99-111. [Crossref] [PubMed]
- Momiyama K, Nagai H, Sumino Y. Changes of host immunity in relation to efficacy in liver cirrhosis patients with advanced hepatocellular carcinoma treated by intra-arterial chemotherapy. Cancer Chemother Pharmacol 2009;64:271-7. [Crossref] [PubMed]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008;8:523-32. [Crossref] [PubMed]
- Ye F, Yan S, Xu L, et al. Tr1 regulatory T cells induced by ConA pretreatment prevent mice from ConA-induced hepatitis. Immunol Lett 2009;122:198-207. [Crossref] [PubMed]
- Roncarolo MG, Bacchetta R, Bordignon C, et al. Type 1 T regulatory cells. Immunol Rev 2001;182:68-79. [Crossref] [PubMed]
- Hudspeth K, Donadon M, Cimino M, et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun 2016;66:40-50. [Crossref] [PubMed]
- Wu Y, Kuang DM, Pan WD, et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 2013;57:1107-16. [Crossref] [PubMed]
- Mikulak J, Bruni E, Oriolo F, et al. Hepatic Natural Killer Cells: Organ-Specific Sentinels of Liver Immune Homeostasis and Physiopathology. Front Immunol 2019;10:946. [Crossref] [PubMed]
- Zheng X, Qian Y, Fu B, et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol 2019;20:1656-67. [Crossref] [PubMed]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007;25:297-336. [Crossref] [PubMed]
- Seo H, Jeon I, Kim BS, et al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat Commun 2017;8:15776. [Crossref] [PubMed]
- Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol 2008;180:3627-35. [Crossref] [PubMed]
- Wen Y, Lambrecht J, Ju C, et al. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol 2021;18:45-56. [Crossref] [PubMed]
- Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015;518:547-51. [Crossref] [PubMed]
- Reid DT, Reyes JL, McDonald BA, et al. Kupffer Cells Undergo Fundamental Changes during the Development of Experimental NASH and Are Critical in Initiating Liver Damage and Inflammation. PLoS One 2016;11:e0159524. [Crossref] [PubMed]
- Miura K, Yang L, van Rooijen N, et al. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol 2012;302:G1310-21. [Crossref] [PubMed]
- Zhang Y, Li JQ, Jiang ZZ, et al. CD169 identifies an anti-tumour macrophage subpopulation in human hepatocellular carcinoma. J Pathol 2016;239:231-41. [Crossref] [PubMed]
- Ding T, Xu J, Wang F, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol 2009;40:381-9. [Crossref] [PubMed]
- MacParland SA, Liu JC, Ma XZ, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun 2018;9:4383. [Crossref] [PubMed]
- Ballesteros I, Rubio-Ponce A, Genua M, et al. Co-option of Neutrophil Fates by Tissue Environments. Cell 2020;183:1282-97.e18. [Crossref] [PubMed]
- Geh D, Leslie J, Rumney R, et al. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2022;19:257-73. [Crossref] [PubMed]
- Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016;150:1646-58.e17. [Crossref] [PubMed]
- Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest 2006;35:459-83. [Crossref] [PubMed]
- Segura E, Touzot M, Bohineust A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013;38:336-48. [Crossref] [PubMed]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 2005;23:275-306. [Crossref] [PubMed]
- Bosma BM, Metselaar HJ, Mancham S, et al. Characterization of human liver dendritic cells in liver grafts and perfusates. Liver Transpl 2006;12:384-93. [Crossref] [PubMed]
- Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol 2018;36:247-77. [Crossref] [PubMed]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003;21:685-711. [Crossref] [PubMed]
- Zhou G, Sprengers D, Boor PPC, et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 2017;153:1107-19.e10. [Crossref] [PubMed]
- Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood 2002;100:174-7. [Crossref] [PubMed]
- Pedroza-Gonzalez A, Zhou G, Vargas-Mendez E, et al. Tumor-infiltrating plasmacytoid dendritic cells promote immunosuppression by Tr1 cells in human liver tumors. Oncoimmunology 2015;4:e1008355. [Crossref] [PubMed]
- Herber DL, Cao W, Nefedova Y, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 2010;16:880-6. [Crossref] [PubMed]
- Zhang Z, Ma L, Goswami S, et al. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. Oncoimmunology 2019;8:e1571388. [Crossref] [PubMed]
- Qin M, Wang D, Fang Y, et al. Current Perspectives on B Lymphocytes in the Immunobiology of Hepatocellular Carcinoma. Front Oncol 2021;11:647854. [Crossref] [PubMed]
- Zou J, Luo C, Xin H, et al. The role of tumor-infiltrating B cells in the tumor microenvironment of hepatocellular carcinoma and its prognostic value: a bioinformatics analysis. J Gastrointest Oncol 2022;13:1959-66. [Crossref] [PubMed]
- Ostrand-Rosenberg S, Fenselau C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol 2018;200:422-31. [Crossref] [PubMed]
- Corzo CA, Condamine T, Lu L, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010;207:2439-53. [Crossref] [PubMed]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012;12:253-68. [Crossref] [PubMed]
- Murdoch C, Muthana M, Coffelt SB, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8:618-31. [Crossref] [PubMed]
- Arihara F, Mizukoshi E, Kitahara M, et al. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 2013;62:1421-30. [Crossref] [PubMed]
- Mizukoshi E, Yamashita T, Arai K, et al. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother 2016;65:715-25. [Crossref] [PubMed]
- Gao XH, Tian L, Wu J, et al. Circulating CD14+ HLA-DR-/low myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol Res 2017;47:1061-71. [Crossref] [PubMed]
- Xu X, Ye L, Zhang Q, et al. Group-2 Innate Lymphoid Cells Promote HCC Progression Through CXCL2-Neutrophil-Induced Immunosuppression. Hepatology 2021;74:2526-43. [Crossref] [PubMed]
- Curio S, Belz GT. The unique role of innate lymphoid cells in cancer and the hepatic microenvironment. Cell Mol Immunol 2022;19:1012-29. [Crossref] [PubMed]
- Vivier E, Artis D, Colonna M, et al. Innate Lymphoid Cells: 10 Years On. Cell 2018;174:1054-66. [Crossref] [PubMed]
- O'Sullivan TE. Dazed and Confused: NK Cells. Front Immunol 2019;10:2235. [Crossref] [PubMed]
- Liang Y, Yi P, Yuan DMK, et al. IL-33 induces immunosuppressive neutrophils via a type 2 innate lymphoid cell/IL-13/STAT6 axis and protects the liver against injury in LCMV infection-induced viral hepatitis. Cell Mol Immunol 2019;16:126-37. [Crossref] [PubMed]
- McHedlidze T, Waldner M, Zopf S, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013;39:357-71. [Crossref] [PubMed]
- Gonzalez-Polo V, Pucci-Molineris M, Cervera V, et al. Group 2 innate lymphoid cells exhibit progressively higher levels of activation during worsening of liver fibrosis. Ann Hepatol 2019;18:366-72. [Crossref] [PubMed]
- Melo-Gonzalez F, Hepworth MR. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology 2017;150:265-75. [Crossref] [PubMed]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125-72. [Crossref] [PubMed]
- Geerts A. On the origin of stellate cells: mesodermal, endodermal or neuro-ectodermal? J Hepatol 2004;40:331-4. [Crossref] [PubMed]
- Yin C, Evason KJ, Asahina K, et al. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest 2013;123:1902-10. [Crossref] [PubMed]
- Coulouarn C, Corlu A, Glaise D, et al. Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res 2012;72:2533-42. [Crossref] [PubMed]
- Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol 2011;25:195-206. [Crossref] [PubMed]
- Yu MC, Chen CH, Liang X, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 2004;40:1312-21. [Crossref] [PubMed]
- Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017;14:397-411. [Crossref] [PubMed]
- Zhang J, Gu C, Song Q, et al. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci 2020;10:127. [Crossref] [PubMed]
- Khan GJ, Sun L, Khan S, et al. Versatility of Cancer Associated Fibroblasts: Commendable Targets for Anti-tumor Therapy. Curr Drug Targets 2018;19:1573-88. [Crossref] [PubMed]
- Sun L, Wang Y, Wang L, et al. Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP. J Exp Clin Cancer Res 2019;38:170. [Crossref] [PubMed]
- Affo S, Nair A, Brundu F, et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021;39:883. [Crossref] [PubMed]
- Chiavarina B, Ronca R, Otaka Y, et al. Fibroblast-derived prolargin is a tumor suppressor in hepatocellular carcinoma. Oncogene 2022;41:1410-20. [Crossref] [PubMed]
- Shetty S, Lalor PF, Adams DH. Liver sinusoidal endothelial cells - gatekeepers of hepatic immunity. Nat Rev Gastroenterol Hepatol 2018;15:555-67. [Crossref] [PubMed]
- DeLeve LD, Maretti-Mira AC. Liver Sinusoidal Endothelial Cell: An Update. Semin Liver Dis 2017;37:377-87. [Crossref] [PubMed]
- Connolly MK, Bedrosian AS, Malhotra A, et al. In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J Immunol 2010;185:2200-8. [Crossref] [PubMed]
- Géraud C, Mogler C, Runge A, et al. Endothelial transdifferentiation in hepatocellular carcinoma: loss of Stabilin-2 expression in peri-tumourous liver correlates with increased survival. Liver Int 2013;33:1428-40. [Crossref] [PubMed]
- Zhang N, Zhang WJ, Cai HQ, et al. Platelet adhesion and fusion to endothelial cell facilitate the metastasis of tumor cell in hypoxia-reoxygenation condition. Clin Exp Metastasis 2011;28:1-12. [Crossref] [PubMed]
- Benedicto A, Herrero A, Romayor I, et al. Liver sinusoidal endothelial cell ICAM-1 mediated tumor/endothelial crosstalk drives the development of liver metastasis by initiating inflammatory and angiogenic responses. Sci Rep 2019;9:13111. [Crossref] [PubMed]
- Takagi Y, Sakai N, Yoshitomi H, et al. High expression of Krüppel-like factor 5 is associated with poor prognosis in patients with colorectal cancer. Cancer Sci 2020;111:2078-92. [Crossref] [PubMed]
- Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol 2016;13:267-76. [Crossref] [PubMed]
- Giraud J, Chalopin D, Blanc JF, et al. Hepatocellular Carcinoma Immune Landscape and the Potential of Immunotherapies. Front Immunol 2021;12:655697. [Crossref] [PubMed]
- Holman NS, Church RJ, Nautiyal M, et al. Hepatocyte-Derived Exosomes Promote Liver Immune Tolerance: Possible Implications for Idiosyncratic Drug-Induced Liver Injury. Toxicol Sci 2019;170:499-508. [Crossref] [PubMed]
- Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, et al. The yin and yang of evasion and immune activation in HCC. J Hepatol 2015;62:1420-9. [Crossref] [PubMed]
- Hong SW, Krueger PD, Osum KC, et al. Immune tolerance of food is mediated by layers of CD4(+) T cell dysfunction. Nature 2022;607:762-8. [Crossref] [PubMed]
- Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature 1969;223:472-6. [Crossref] [PubMed]
- Bertolino P, Trescol-Biémont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol 1998;28:221-36. [Crossref] [PubMed]
- Bowen DG, Zen M, Holz L, et al. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest 2004;114:701-12. [Crossref] [PubMed]
- Zhou Z, Xu MJ, Gao B. Hepatocytes: a key cell type for innate immunity. Cell Mol Immunol 2016;13:301-15. [Crossref] [PubMed]
- Warren A, Le Couteur DG, Fraser R, et al. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 2006;44:1182-90. [Crossref] [PubMed]
- Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol 2003;3:51-62. [Crossref] [PubMed]
- Mühlbauer M, Fleck M, Schütz C, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol 2006;45:520-8. [Crossref] [PubMed]
- Benseler V, Warren A, Vo M, et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci U S A 2011;108:16735-40. [Crossref] [PubMed]
- Bamboat ZM, Stableford JA, Plitas G, et al. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol 2009;182:1901-11. [Crossref] [PubMed]
- Pillarisetty VG, Shah AB, Miller G, et al. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol 2004;172:1009-17. [Crossref] [PubMed]
- De Creus A, Abe M, Lau AH, et al. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol 2005;174:2037-45. [Crossref] [PubMed]
- Breous E, Somanathan S, Vandenberghe LH, et al. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 2009;50:612-21. [Crossref] [PubMed]
- Heymann F, Peusquens J, Ludwig-Portugall I, et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015;62:279-91. [Crossref] [PubMed]
- You Q, Cheng L, Kedl RM, et al. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology 2008;48:978-90. [Crossref] [PubMed]
- Knolle PA, Uhrig A, Protzer U, et al. Interleukin-10 expression is autoregulated at the transcriptional level in human and murine Kupffer cells. Hepatology 1998;27:93-9. [Crossref] [PubMed]
- Bissell DM, Wang SS, Jarnagin WR, et al. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest 1995;96:447-55. [Crossref] [PubMed]
- Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun 2010;34:1-6. [Crossref] [PubMed]
- Lüth S, Huber S, Schramm C, et al. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest 2008;118:3403-10. [Crossref] [PubMed]
- Carambia A, Freund B, Schwinge D, et al. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J Hepatol 2014;61:594-9. [Crossref] [PubMed]
- Pandiyan P, Zheng L, Ishihara S, et al. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 2007;8:1353-62. [Crossref] [PubMed]
- Chinen T, Kannan AK, Levine AG, et al. An essential role for the IL-2 receptor in T(reg) cell function. Nat Immunol 2016;17:1322-33. [Crossref] [PubMed]
- Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 2000;192:295-302. [Crossref] [PubMed]
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med 2000;192:303-10. [Crossref] [PubMed]
- Chang KM. Regulatory T cells and the liver: a new piece of the puzzle. Hepatology 2005;41:700-2. [Crossref] [PubMed]
- Liu Z, Gu J, Qin Z, et al. Decreased Foxp3 and function of Tregs caused immune imbalance and liver injury in patients with autoimmune liver diseases post-liver transplantation. Ann Transl Med 2020;8:534. [Crossref] [PubMed]
- Longhi MS, Hussain MJ, Mitry RR, et al. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol 2006;176:4484-91. [Crossref] [PubMed]
- Longhi MS, Ma Y, Bogdanos DP, et al. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol 2004;41:31-7. [Crossref] [PubMed]
- Poisson J, Lemoinne S, Boulanger C, et al. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol 2017;66:212-27. [Crossref] [PubMed]
- von Oppen N, Schurich A, Hegenbarth S, et al. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology 2009;49:1664-72. [Crossref] [PubMed]
- Limmer A, Ohl J, Kurts C, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med 2000;6:1348-54. [Crossref] [PubMed]
- Diehl L, Schurich A, Grochtmann R, et al. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 2008;47:296-305. [Crossref] [PubMed]
- Guidotti LG, Iannacone M. Effector CD8 T cell trafficking within the liver. Mol Immunol 2013;55:94-9. [Crossref] [PubMed]
- Wong J, Johnston B, Lee SS, et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest 1997;99:2782-90. [Crossref] [PubMed]
- Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J Immunol 1999;163:3202-10. [Crossref] [PubMed]
- Heinrich B, Brown ZJ, Diggs LP, et al. Steatohepatitis Impairs T-cell-Directed Immunotherapies Against Liver Tumors in Mice. Gastroenterology 2021;160:331-45.e6. [Crossref] [PubMed]
- Groux H, Bigler M, de Vries JE, et al. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med 1996;184:19-29. [Crossref] [PubMed]
- Glass MC, Glass DR, Oliveria JP, et al. Human IL-10-producing B cells have diverse states that are induced from multiple B cell subsets. Cell Rep 2022;39:110728. [Crossref] [PubMed]
- Louis H, Van Laethem JL, Wu W, et al. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology 1998;28:1607-15. [Crossref] [PubMed]
- Schon HT, Weiskirchen R. Immunomodulatory effects of transforming growth factor-β in the liver. Hepatobiliary Surg Nutr 2014;3:386-406. [PubMed]
- Sia D, Jiao Y, Martinez-Quetglas I, et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017;153:812-26. [Crossref] [PubMed]
- Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med 2018;10:eaan5488. [Crossref] [PubMed]
- Kim HD, Song GW, Park S, et al. Association Between Expression Level of PD1 by Tumor-Infiltrating CD8(+) T Cells and Features of Hepatocellular Carcinoma. Gastroenterology 2018;155:1936-50.e17. [Crossref] [PubMed]
- Ma J, Zheng B, Goswami S, et al. PD1(Hi) CD8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer 2019;7:331. [Crossref] [PubMed]
- Sun H, Huang Q, Huang M, et al. Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma. Hepatology 2019;70:168-83. [Crossref] [PubMed]
- Zhang PF, Gao C, Huang XY, et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer 2020;19:110. [Crossref] [PubMed]
- Chui NN, Cheu JW, Yuen VW, et al. Inhibition of CMTM4 Sensitizes Cholangiocarcinoma and Hepatocellular Carcinoma to T Cell-Mediated Antitumor Immunity Through PD-L1. Hepatol Commun 2022;6:178-93. [Crossref] [PubMed]
- Yeung OW, Lo CM, Ling CC, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol 2015;62:607-16. [Crossref] [PubMed]
- Bao D, Zhao J, Zhou X, et al. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene 2019;38:5007-20. [Crossref] [PubMed]
- Wu K, Kryczek I, Chen L, et al. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res 2009;69:8067-75. [Crossref] [PubMed]
- Chen J, Li G, Meng H, et al. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother 2012;61:101-8. [Crossref] [PubMed]
- Ke M, Zhang Z, Cong L, et al. MicroRNA-148b-colony-stimulating factor-1 signaling-induced tumor-associated macrophage infiltration promotes hepatocellular carcinoma metastasis. Biomed Pharmacother 2019;120:109523. [Crossref] [PubMed]
- Li Z, Li H, Zhao ZB, et al. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARδ signalling-mediated alternative activation of macrophages. J Exp Clin Cancer Res 2019;38:469. [Crossref] [PubMed]
- Wu L, Zhang X, Zheng L, et al. RIPK3 Orchestrates Fatty Acid Metabolism in Tumor-Associated Macrophages and Hepatocarcinogenesis. Cancer Immunol Res 2020;8:710-21. [Crossref] [PubMed]
- Wu Q, Zhou W, Yin S, et al. Blocking Triggering Receptor Expressed on Myeloid Cells-1-Positive Tumor-Associated Macrophages Induced by Hypoxia Reverses Immunosuppression and Anti-Programmed Cell Death Ligand 1 Resistance in Liver Cancer. Hepatology 2019;70:198-214. [Crossref] [PubMed]
- Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 2007;13:902-11. [Crossref] [PubMed]
- Zhou YF, Song SS, Tian MX, et al. Cystathionine β-synthase mediated PRRX2/IL-6/STAT3 inactivation suppresses Tregs infiltration and induces apoptosis to inhibit HCC carcinogenesis. J Immunother Cancer 2021;9:e003031. [Crossref] [PubMed]
- Wang Z, He L, Li W, et al. GDF15 induces immunosuppression via CD48 on regulatory T cells in hepatocellular carcinoma. J Immunother Cancer 2021;9:e002787. [Crossref] [PubMed]
- Suthen S, Lim CJ, Nguyen PHD, et al. Hypoxia-driven immunosuppression by Treg and type-2 conventional dendritic cells in HCC. Hepatology 2022;76:1329-44. [Crossref] [PubMed]
- Langhans B, Nischalke HD, Krämer B, et al. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. Cancer Immunol Immunother 2019;68:2055-66. [Crossref] [PubMed]
- Yang LY, Luo Q, Lu L, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol 2020;13:3. [Crossref] [PubMed]
- Zhou J, Liu M, Sun H, et al. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut 2018;67:931-44. [Crossref] [PubMed]
- Liu Y, Song Y, Lin D, et al. NCR(-) group 3 innate lymphoid cells orchestrate IL-23/IL-17 axis to promote hepatocellular carcinoma development. EBioMedicine 2019;41:333-44. [Crossref] [PubMed]
- Filliol A, Saito Y, Nair A, et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature 2022;610:356-65. [Crossref] [PubMed]
- Loo TM, Kamachi F, Watanabe Y, et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE(2)-Mediated Suppression of Antitumor Immunity. Cancer Discov 2017;7:522-38. [Crossref] [PubMed]
- Ma C, Han M, Heinrich B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018;360:eaan5931. [Crossref] [PubMed]
- Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015;17:816-26. [Crossref] [PubMed]
- Jessup JM, Samara R, Battle P, et al. Carcinoembryonic antigen promotes tumor cell survival in liver through an IL-10-dependent pathway. Clin Exp Metastasis 2004;21:709-17. [Crossref] [PubMed]
- Steele CW, Karim SA, Leach JDG, et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016;29:832-45. [Crossref] [PubMed]
- Yang L, Liu Q, Zhang X, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020;583:133-8. [Crossref] [PubMed]
- D'Costa Z, Jones K, Azad A, et al. Gemcitabine-Induced TIMP1 Attenuates Therapy Response and Promotes Tumor Growth and Liver Metastasis in Pancreatic Cancer. Cancer Res 2017;77:5952-62. [Crossref] [PubMed]
- Correia AL, Guimaraes JC, Auf der Maur P, et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature 2021;594:566-71. [Crossref] [PubMed]
- Jauch AS, Wohlfeil SA, Weller C, et al. Lyve-1 deficiency enhances the hepatic immune microenvironment entailing altered susceptibility to melanoma liver metastasis. Cancer Cell Int 2022;22:398. [Crossref] [PubMed]
- Lee JC, Mehdizadeh S, Smith J, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol 2020;5:eaba0759. [Crossref] [PubMed]
- Li M, Lai X, Zhao Y, et al. Loss of NDRG2 in liver microenvironment inhibits cancer liver metastasis by regulating tumor associate macrophages polarization. Cell Death Dis 2018;9:248. [Crossref] [PubMed]
- Yang P, Qin H, Li Y, et al. CD36-mediated metabolic crosstalk between tumor cells and macrophages affects liver metastasis. Nat Commun 2022;13:5782. [Crossref] [PubMed]
- Ho WW, Gomes-Santos IL, Aoki S, et al. Dendritic cell paucity in mismatch repair-proficient colorectal cancer liver metastases limits immune checkpoint blockade efficacy. Proc Natl Acad Sci U S A 2021;118:e2105323118. [Crossref] [PubMed]
- Lin Q, Ren L, Jian M, et al. The mechanism of the premetastatic niche facilitating colorectal cancer liver metastasis generated from myeloid-derived suppressor cells induced by the S1PR1-STAT3 signaling pathway. Cell Death Dis 2019;10:693. [Crossref] [PubMed]
- Ham B, Wang N, D'Costa Z, et al. TNF Receptor-2 Facilitates an Immunosuppressive Microenvironment in the Liver to Promote the Colonization and Growth of Hepatic Metastases. Cancer Res 2015;75:5235-47. [Crossref] [PubMed]
- Zeng X, Zhou J, Xiong Z, et al. Cell cycle-related kinase reprograms the liver immune microenvironment to promote cancer metastasis. Cell Mol Immunol 2021;18:1005-15. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-52. [Crossref] [PubMed]
- Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022;23:77-90. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Limagne E, Euvrard R, Thibaudin M, et al. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res 2016;76:5241-52. [Crossref] [PubMed]
- Feng PH, Chen KY, Huang YC, et al. Bevacizumab Reduces S100A9-Positive MDSCs Linked to Intracranial Control in Patients with EGFR-Mutant Lung Adenocarcinoma. J Thorac Oncol 2018;13:958-67. [Crossref] [PubMed]
- Rahma OE, Hodi FS. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res 2019;25:5449-57. [Crossref] [PubMed]
- Finn RS, Kudo M, Merle P, et al. LBA34 Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol 2022;33:abstr S1401.
- Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:995-1008. [Crossref] [PubMed]
- Tumeh PC, Hellmann MD, Hamid O, et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res 2017;5:417-24. [Crossref] [PubMed]
- Chen XJ, Ren A, Zheng L, et al. Pan-Cancer Analysis Identifies Liver Metastases as Negative Predictive Factor for Immune Checkpoint Inhibitors Treatment Outcome. Front Immunol 2021;12:651086. [Crossref] [PubMed]
- Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845-51. [Crossref] [PubMed]
- Chiang CL, Chan ACY, Chiu KWH, et al. Combined Stereotactic Body Radiotherapy and Checkpoint Inhibition in Unresectable Hepatocellular Carcinoma: A Potential Synergistic Treatment Strategy. Front Oncol 2019;9:1157. [Crossref] [PubMed]
- Chiang CL, Chiu KW, Lee FA, et al. Combined Stereotactic Body Radiotherapy and Immunotherapy Versus Transarterial Chemoembolization in Locally Advanced Hepatocellular Carcinoma: A Propensity Score Matching Analysis. Front Oncol 2021;11:798832. [Crossref] [PubMed]
- Shi L, Chen L, Wu C, et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin Cancer Res 2016;22:1173-84. [Crossref] [PubMed]
- Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:545-51. [Crossref] [PubMed]
- Montasser A, Beaufrère A, Cauchy F, et al. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology 2021;79:36-46. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Young KH, Baird JR, Savage T, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS One 2016;11:e0157164. [Crossref] [PubMed]
- Wehrenberg-Klee E, Goyal L, Dugan M, et al. Y-90 Radioembolization Combined with a PD-1 Inhibitor for Advanced Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2018;41:1799-802. [Crossref] [PubMed]
- Ali MY, Grimm CF, Ritter M, et al. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J Hepatol 2005;43:817-22. [Crossref] [PubMed]
- Fei Q, Pan Y, Lin W, et al. High-dimensional single-cell analysis delineates radiofrequency ablation induced immune microenvironmental remodeling in pancreatic cancer. Cell Death Dis 2020;11:589. [Crossref] [PubMed]
- Wu H, Li SS, Zhou M, et al. Palliative Radiofrequency Ablation Accelerates the Residual Tumor Progression Through Increasing Tumor-Infiltrating MDSCs and Reducing T-Cell-Mediated Anti-Tumor Immune Responses in Animal Model. Front Oncol 2020;10:1308. [Crossref] [PubMed]
- Han G, Berhane S, Toyoda H, et al. Prediction of Survival Among Patients Receiving Transarterial Chemoembolization for Hepatocellular Carcinoma: A Response-Based Approach. Hepatology 2020;72:198-212. [Crossref] [PubMed]
- Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51-72. [Crossref] [PubMed]
- Liao Y, Wang B, Huang ZL, et al. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma: a prospective study. PLoS One 2013;8:e60444. [Crossref] [PubMed]
- Worlikar T, Vlaisavljevich E, Gerhardson T, et al. Histotripsy for Non-Invasive Ablation of Hepatocellular Carcinoma (HCC) Tumor in a Subcutaneous Xenograft Murine Model. Annu Int Conf IEEE Eng Med Biol Soc 2018;2018:6064-7. [Crossref] [PubMed]
- Xu Z, Hall TL, Vlaisavljevich E, et al. Histotripsy: the first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int J Hyperthermia 2021;38:561-75. [Crossref] [PubMed]
- Smolock AR, Cristescu MM, Vlaisavljevich E, et al. Robotically Assisted Sonic Therapy as a Noninvasive Nonthermal Ablation Modality: Proof of Concept in a Porcine Liver Model. Radiology 2018;287:485-93. [Crossref] [PubMed]
- Worlikar T, Mendiratta-Lala M, Vlaisavljevich E, et al. Effects of Histotripsy on Local Tumor Progression in an in vivo Orthotopic Rodent Liver Tumor Model. BME Front 2020;2020:9830304. [Crossref] [PubMed]
- Worlikar T, Zhang M, Ganguly A, et al. Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model. Cancers (Basel) 2022;14:1612. [Crossref] [PubMed]
- Qu S, Worlikar T, Felsted AE, et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 2020;8:e000200. [Crossref] [PubMed]
- Pepple AL, Guy JL, McGinnis R, et al. Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation. Front Immunol 2023;14:1012799. [Crossref] [PubMed]
- Vidal-Jove J, Serres X, Vlaisavljevich E, et al. First-in-man histotripsy of hepatic tumors: the THERESA trial, a feasibility study. Int J Hyperthermia 2022;39:1115-23. [Crossref] [PubMed]
- Vidal-Jove J, Serres-Creixams X, Ziemlewicz TJ, et al. Liver Histotripsy Mediated Abscopal Effect-Case Report. IEEE Trans Ultrason Ferroelectr Freq Control 2021;68:3001-5. [Crossref] [PubMed]
- Shigeta K, Datta M, Hato T, et al. Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma. Hepatology 2020;71:1247-61. [Crossref] [PubMed]
- Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun 2016;7:12624. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Ziogas AC, Gavalas NG, Tsiatas M, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer 2012;130:857-64. [Crossref] [PubMed]
- Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol 2011;11:702-11. [Crossref] [PubMed]
- Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol 2018;9:978. [Crossref] [PubMed]
- Kandalaft LE, Motz GT, Busch J, et al. Angiogenesis and the tumor vasculature as antitumor immune modulators: the role of vascular endothelial growth factor and endothelin. Curr Top Microbiol Immunol 2011;344:129-48. [Crossref] [PubMed]
- Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 2014;20:607-15. [Crossref] [PubMed]
- Szoor A, Vaidya A, Velasquez MP, et al. T Cell-Activating Mesenchymal Stem Cells as a Biotherapeutic for HCC. Mol Ther Oncolytics 2017;6:69-79. [Crossref] [PubMed]
- Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 2014;20:6418-28. [Crossref] [PubMed]
- Zhang Z, Zhang Y, Xia S, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020;579:415-20. [Crossref] [PubMed]
- Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011;128:887-96. [Crossref] [PubMed]
- Yan W, Liu X, Ma H, et al. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut 2015;64:1593-604. [Crossref] [PubMed]
- Andrews LP, Marciscano AE, Drake CG, et al. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 2017;276:80-96. [Crossref] [PubMed]
- Zhou G, Noordam L, Sprengers D, et al. Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology 2018;7:e1448332. [Crossref] [PubMed]
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med 2022;386:24-34. [Crossref] [PubMed]
- Archilla-Ortega A, Domuro C, Martin-Liberal J, et al. Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J Exp Clin Cancer Res 2022;41:62. [Crossref] [PubMed]
Cite this article as: Sankar K, Pearson AN, Worlikar T, Perricone MD, Holcomb EA, Mendiratta-Lala M, Xu Z, Bhowmick N, Green MD. Impact of immune tolerance mechanisms on the efficacy of immunotherapy in primary and secondary liver cancers. Transl Gastroenterol Hepatol 2023;8:29.


