
Withdrawal time in colonoscopy, past, present, and future, a narrative review
Introduction
Colorectal cancer (CRC) is the second most common cause of death from cancer worldwide. Ninety-five percent of CRC cases originate from tubular adenomas of the colon therefore high-quality colonoscopy screening with adequate adenoma detection rate (ADR) is the gold standard modality to prevent CRC (1). Back-to-back colonoscopy studies have shown that as much as 24% of tubular adenomas are missed in colonoscopies (2). The majority of interval CRCs are due to missed lesions secondary to inadequate bowel prep leading to difficulty in finding small and flat lesions and differences in endoscopists ability to find adenomas (3). Therefore, ADR, cecal intubation rate, complete polypectomy rate, quality of bowel prep, and withdrawal time (WT) have been shown to correlate with rates of interval CRC (4). ADR, defined as the number of cases with at least one adenoma detected, has been used as a primary indicator of quality of colonoscopy (5). Studies show that lower-quality colonoscopies reflected by lower ADRs are associated with increased rate of interval CRCs (6). Among the factors studied that can affect the ADR is WT. There is a knowledge gap on the ideal duration of WT since literature has been evolving in this dynamic topic. Moreover, artificial intelligence (AI) is providing new avenues for endoscopists. The objective of this review is to illustrate available evidence for WT efficacy and demonstrate the future direction and potentials of WT in colonoscopy. We present this article in accordance with the Narrative Review reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-8/rc) (7).
Methods
We conducted a comprehensive search of PubMed/MEDLINE using the search term “withdrawal time” and “colonoscopy”. The search was restricted to articles in English language only. We included all peer-reviewed observational studies and randomized controlled trials (RCTs). Society guidelines were also reviewed for historical and current recommendations on WT. All studies were cross-referenced to include relevant articles (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | Feb 28, 2023 |
| Databases and other sources searched | PubMed/MEDLINE |
| Search terms used | Colonoscopy, withdrawal time |
| Timeframe | Jan 1, 1999 to Feb 28, 2023 |
| Inclusion and exclusion criteria | Inclusion: Observational and randomized trials in English language |
| Exclusion: case reports and case series less than 10 subjects | |
| Selection process | HH and NZ conducted the search independently and consensus was obtained with discussion with MA |
WT
Definition and recommendations
WT is calculated as the duration of time from reaching the cecum or terminal ileum till the end of procedure in normal colonoscopies without any interventions. The majority of the mucosal inspection is carried out during this period. Increasing WT by spending more time for a detailed colonoscopy through inspecting behind folds and cleaning the colon can lead to increased ADR (8,9). 2021 American Gastroenterological Association (AGA) guidelines recommend endoscopists with lower ADRs, take measures including increasing their WT to achieve an ADR of 30% (5). According to these guidelines, endoscopists should achieve an average WT of at least 6 minutes for normal colonoscopies with an aspirational target of 9 minutes. These recommendations are based on the following available evidence demonstrating that setting a minimum WT ranging from 6 to 9 minutes can increase outcomes including ADR and lower interval CRC compared to WTs less than this amount.
Right colon WT
Colonoscopy has been shown to be more effective in the left colon compared to the right due to presence of serrated lesions in the right colon. Serrated lesions are more difficult to detect due to their flat morphology, fleshy appearance, and having a mucus cap (10). This requires more careful examination with maneuvers like second look in the right colon or retroflexion that can increase the ADR by 5–20% even in cases of adequate prep (11-13). The 2021 AGA guidelines consider a second look of the right colon through forward or retroflexed view best practice especially when polyps are detected on the first look increasing the chance of synchronous lesions (5). Yun et al. showed that second look maneuvers in the right colon are time consuming and proposed a need for a WT in the right colon above 3 minutes (14). KASID multicenter study also illustrated that ADR significantly improves when there is a WT of 2 minutes in the right colon compared to less duration [odds ratio (OR), 2.98; 95% confidence interval (CI), 1.72 to 5.15; P<0.001] (15). Indeed, many studies have shown increased serrated polyp detection rate (SDR) with longer WTs, which brings further evidence that increasing WT is an effective strategy to detect serrated lesions in the right colon (16-18).
Evolving evidence behind WT
Setting an average WT of more than 6 minutes by AGA is based on studies suggesting this number as a quality indicator of achieving a high-quality colonoscopy reflected in higher ADR and lower interval CRC rate. This number was pinpointed by the seminal observational study by Barclay et al. in more than seven thousand screening colonoscopies, in which WT more than 6 minutes was found to increase ADR (28.3% vs. 11.8%, P<0.001) and advanced ADR (6.4% vs. 2.6%, P=0.005) (19). This led to setting the WT of more than 6 minutes as a colonoscopy quality indicator by a 2006 American Society for Gastrointestinal Endoscopy/American College of Gastroenterology task force (20). Since these recommendations, multiple large scale observational studies have supported these findings. Another observational study on 76,810 screening colonoscopies showed improved outcomes with WT of 6 minutes. This study also showed the fewest number of interval CRCs with WT of above 8 minutes (21). Further studies also supported WT of 6 minutes for better outcomes in terms of ADR and interval CRC rates (22-24). A study by Butterly et al. on 7,996 colonoscopies found an increase in ADR and also SDR in WT 9 minutes compared to 6 minutes. Regression model showed WT of 9 minutes to be most significant for SDR, with nearly a 30% increase (16). This finding is important as serrated lesions are more prone to be missed. This increases the importance of WT in the right colon and brings up the notion of a dedicated WT for the right colon as discussed in the prior section. A retrospective US study on 31,558 colonoscopy examinations suggested a WT of 11 minutes increased both ADR (OR 1.65, 95% CI: 1.09 to 2.51) and detection rate of proximal serrated polyps (OR 1.81, 95% CI: 1.06 to 3.08) (18). In this study, ADR linearly correlated well with WT (R=0.76, P<0.001). In addition to whole colon WT, there are studies proposing WTs on segments of the colon. Multicenter studies by Jung et al. and Kashiwagi et al. have shown that setting a minimum WT in each segment of colon of more than 3 minutes also increases the PDR (15,25). The mentioned prior evidence mainly involves observational studies with their adherent bias. Recently, in a large multicenter RCT by Zhao et al., it was noted that increasing WT from 6 to 9 minutes led to significantly improved ADR especially in right colon and for colonoscopists with less experience. This is the first large multicenter RCT that proved superiority of WT of 9 minutes compared to 6 minutes (26). This brings further high-quality evidence for future guideline regarding WT for the procedures.
Interventions to improve WT
Despite recommendations to improve WT, methods to achieve this goal are understudied (4). In order to improve WT, multiple modalities are proposed. Feedback and monitoring have been shown to improve WT (27). Barclay et al. in their study on 2053 screening colonoscopies used a digital watch emitting audible signals at 2, 4, 6, and 8 minutes, therefore notifying the endoscopist when they achieved the WT of 8 minutes. This showed to improve detection of neoplasia (34.7% vs. 23.5%, P<0.0001) (23). In the era of AI, this new tool has gained interest in improving ADR through various methods including improving WT. In a study on 659 patients by Su et al., a deep convolutional neural network model showed improvement in ADR (0.289 vs. 0.165, P<0.001) and WT (7.03 vs. 5.68 minutes, P<0.001) (28). The AI had multiple functions including real-time evaluation of withdrawal speed and alerting endoscopist in case speed of withdrawal is too fast. In addition, the software was able to detect bad prep and remind the endoscopist to clean the mucosa and suction the pool of fluid. These secondary functions inadvertently increased the WT while increasing the quality of the colonoscopy. In another RCT by Gong et al., 704 patients underwent AI-assisted colonoscopy with ENDOANGEL AI system (29). ENDOANGEL was developed using deep neural networks to monitor real-time speed of withdrawal in addition to total WT. ENDOANGEL also detected the blind spots not inspected and reminded endoscopists to evaluate those areas. This secondary function increased the quality and secondarily improved the WT. This study found that ADR was significantly higher in the ENDOANGEL group in intention-to-treat analysis (OR 2·30, 95% CI: 1·40–3·77; P=0·001) (29). AI systems solely to detect polyps and not intended to increase WT, have also been shown to inadvertently increase WT. For example, in a recent large RCT on 3059 subjects by Xu et al., an AI system not intended to increase WT duration, led to an increase in WT (8.3 vs. 7.8 minutes; P=0.004) (30). This is attributed to longer time spent on endoscopist confirmation of polyps detected by AI during insertion and withdrawal. This study showed improved ADR in AI group however whether the WT prolongation spent on confirming the AI detection of polyps is beneficial or not remains unknown. We believe the recheck of polyps detected by AI is likely a positive aspect as the endoscopist will also inspect the surrounding mucosa secondarily. A recent network meta-analysis by our group showed improvement in both ADR [Relative risk (RR): 1.41, 95% CI: 1.28 to 1.54] and WT [Mean difference (MD): 0.54, 95% CI: 0.10 to 0.97] in AI compared with high-definition colonoscopy without AI (31,32).
Hurdles to WT and future directions
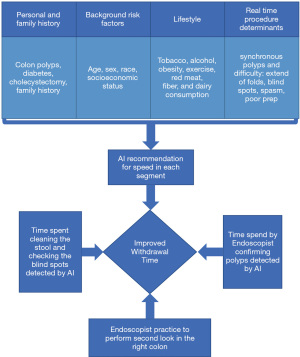
In daily life, there is more to WT than just a number. First, there are external factors that will affect the WT. If faced with external pressure of a large number of endoscopies scheduled in a limited time, for reasons including economic incentives, the endoscopists may not adhere to the minimum WT (33). Second, it is the time spent actively looking behind folds and cleaning the puddles that will improve the ADR rather than the WT, per se. Moreover, there are differences in the difficulty of colonoscopies that will require different time allocated for each individual. For example, a long colon with fair prep in a high-risk obese male with family history of CRC requires longer time than a short colon with excellent prep in an individual with no risk factors. This has led to recent proposals to change the WT of 6 minutes. Since the findings of Zhao et al., Butterly has proposed a “mean” WT of 9 minutes and a minimum WT of 6 minutes (34). Per her suggestion, the providers should strive to have WT of all colonoscopies take more than 6 minutes and the mean WT of all the procedures exceed the 9-minute threshold. We propose an individualized approach based on risk factors. There are risk factors identified for occurrence of CRC. These can be divided to background factors, lifestyle, personal and family history (Figure 1) (1). Risk factors like obesity, tobacco, alcohol, low fiber diet in addition to personal and family history of polys and CRC each increase the probability of CRC. There is a need for future studies determining a scoring system based on the patient’s preexisting risk factors of CRC and the difficulty of the procedure including the anatomy of the colon and quality of the bowel prep. In the era of AI, the AI model should be able to combine the baseline risk factors and the real-time data including the difficulty of the procedure and determine the speed of withdrawal and time spent in each segment. For example, studies have shown that finding an adenoma in a segment increases risk of synchronous or metachronous adenomas in the same segment (35-37). Therefore, finding an adenoma in a segment should lead to a more careful inspection of the same segment, a recommendation that can be reinforced with AI models similar to that used by Liu et al. (38). The AI model will recommend relook in the right colon in high-risk population. Moreover, it will not allow withdrawal from the high-risk segment until three minutes mark and reinforce evaluation of all blind spots behind folds, proper distension, and cleaning residual stool, the four components of good technique (38). There is a need for future studies to prove the efficacy of this approach and develop the appropriate AI models to guide the endoscopist to spend more time inspecting the area of interest based on baseline data prior to colonoscopy and real-time input gathered during the procedure (Figure 1).
Limitations and strengths
This review had some limitations. First, it was a narrative review and not a systematic review for literature which may not cover all the available studies. Despite this, the authors conducted a comprehensive review of literature and references of the studies and covered the main studies in this field. Due to the novelty of the subject and its evolution within the last two decades, we believe the search was able to cover the major breakthroughs in the area. Second, there has been heterogeneity in the studies regarding the definition of outcomes due to wide geographic and chronologic variety of the studies. Nevertheless, this can be considered as a strength since this narrative review was not aiming at pooling the data, rather providing the evidence, unrestricted by heterogeneity. Last, some studies were observational with their inherent biases. At the same time, some studies were high-quality RCTs without the aforementioned biases that confirmed the results seen in the observational studies. There is need for updated meta-analysis of the high-quality RCTs to evaluate the effects of the AI in improving WT and ADR.
Conclusions
To conclude, a large body of evidence suggests setting a minimum WT of 6 to 9 minutes can lead to better colonoscopy outcomes including higher ADR and lower interval CRCs. Data on methods to achieve the WT are limited, however, AI models are showing great potential. The future of the WT would be the development of AI models to guide colonoscopists to spend time on high-risk patients and the segmentation of interest based on prior and real time data. This “smart” effective withdrawal still requires a minimum WT likely between 6 to 9 minutes in majority of the procedure. Therefore, WT should be individualized based on difficulty and pretest probability of CRC rather than setting a WT number for all procedures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Gastroenterology and Hepatology for the series “Colonoscopy: Updates and Prospects”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-8/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-8/coif). The series “Colonoscopy: Updates and Prospects” was commissioned by the editorial office without any funding or sponsorship. MA served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Gastroenterology and Hepatology from September 2022 to August 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sawicki T, Ruszkowska M, Danielewicz A, et al. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers (Basel) 2021;13:2025. [Crossref] [PubMed]
- Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24-8. [Crossref] [PubMed]
- le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut 2014;63:957-63. [Crossref] [PubMed]
- May FP, Shaukat A. State of the Science on Quality Indicators for Colonoscopy and How to Achieve Them. Am J Gastroenterol 2020;115:1183-90. [Crossref] [PubMed]
- Keswani RN, Crockett SD, Calderwood AH. AGA Clinical Practice Update on Strategies to Improve Quality of Screening and Surveillance Colonoscopy: Expert Review. Gastroenterology 2021;161:701-11. [Crossref] [PubMed]
- Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298-306. [Crossref] [PubMed]
- Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med 2006;5:101-17. [Crossref] [PubMed]
- Coe SG, Crook JE, Diehl NN, et al. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol 2013;108:219-26; quiz 227. [Crossref] [PubMed]
- Wallace MB, Crook JE, Thomas CS, et al. Effect of an endoscopic quality improvement program on adenoma detection rates: a multicenter cluster-randomized controlled trial in a clinical practice setting (EQUIP-3). Gastrointest Endosc 2017;85:538-545.e4. [Crossref] [PubMed]
- Anderson JC, Srivastava A. Colorectal Cancer Screening for the Serrated Pathway. Gastrointest Endosc Clin N Am 2020;30:457-78. [Crossref] [PubMed]
- Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: an observational study. Gastrointest Endosc 2011;74:246-52. [Crossref] [PubMed]
- Tang RSY, Lee JWJ, Chang LC, et al. Two vs One Forward View Examination of Right Colon on Adenoma Detection: An International Multicenter Randomized Trial. Clin Gastroenterol Hepatol 2022;20:372-380.e2. [Crossref] [PubMed]
- Kamal F, Khan MA, Lee-Smith W, et al. Second exam of right colon improves adenoma detection rate: Systematic review and meta-analysis of randomized controlled trials. Endosc Int Open 2022;10:E1391-8. [Crossref] [PubMed]
- Yun GY, Eun HS, Kim JS, et al. Colonoscopic withdrawal time and adenoma detection in the right colon. Medicine (Baltimore) 2018;97:e12113. [Crossref] [PubMed]
- Jung Y, Joo YE, Kim HG, et al. Relationship between the endoscopic withdrawal time and adenoma/polyp detection rate in individual colonic segments: a KASID multicenter study. Gastrointest Endosc 2019;89:523-30. [Crossref] [PubMed]
- Butterly L, Robinson CM, Anderson JC, et al. Serrated and adenomatous polyp detection increases with longer withdrawal time: results from the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2014;109:417-26. [Crossref] [PubMed]
- Cavicchi M, Tharsis G, Burtin P, et al. Difference in Physician- and Patient-Dependent Factors Contributing to Adenoma Detection Rate and Serrated Polyp Detection Rate. Dig Dis Sci 2019;64:3579-88. [Crossref] [PubMed]
- Patel VD, Thompson WK, Lapin BR, et al. Screening Colonoscopy Withdrawal Time Threshold for Adequate Proximal Serrated Polyp Detection Rate. Dig Dis Sci 2018;63:3084-90. [Crossref] [PubMed]
- Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533-41. [Crossref] [PubMed]
- Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2006;63:S16-28. [Crossref] [PubMed]
- Shaukat A, Rector TS, Church TR, et al. Longer Withdrawal Time Is Associated With a Reduced Incidence of Interval Cancer After Screening Colonoscopy. Gastroenterology 2015;149:952-7. [Crossref] [PubMed]
- Lee TJ, Blanks RG, Rees CJ, et al. Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: evidence from the Bowel Cancer Screening Programme in England. Endoscopy 2013;45:20-6. [PubMed]
- Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol 2008;6:1091-8. [Crossref] [PubMed]
- Overholt BF, Brooks-Belli L, Grace M, et al. Withdrawal times and associated factors in colonoscopy: a quality assurance multicenter assessment. J Clin Gastroenterol 2010;44:e80-6. [Crossref] [PubMed]
- Kashiwagi K, Inoue N, Yoshida T, et al. Polyp detection rate in transverse and sigmoid colon significantly increases with longer withdrawal time during screening colonoscopy. PLoS One 2017;12:e0174155. [Crossref] [PubMed]
- Zhao S, Yang X, Wang S, et al. Impact of 9-Minute Withdrawal Time on the Adenoma Detection Rate: A Multicenter Randomized Controlled Trial. Clin Gastroenterol Hepatol 2022;20:e168-81. [Crossref] [PubMed]
- Nielsen AB, Nielsen OH, Hendel J. Impact of feedback and monitoring on colonoscopy withdrawal times and polyp detection rates. BMJ Open Gastroenterol 2017;4:e000142. [Crossref] [PubMed]
- Su JR, Li Z, Shao XJ, et al. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: a prospective randomized controlled study (with videos). Gastrointest Endosc 2020;91:415-424.e4. [Crossref] [PubMed]
- Gong D, Wu L, Zhang J, et al. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol Hepatol 2020;5:352-61. [Crossref] [PubMed]
- Xu H, Tang RSY, Lam TYT, et al. Artificial Intelligence-Assisted Colonoscopy for Colorectal Cancer Screening: A Multicenter Randomized Controlled Trial. Clin Gastroenterol Hepatol 2023;21:337-346.e3. [Crossref] [PubMed]
- Aziz M, Fatima R, Dong C, et al. The impact of deep convolutional neural network-based artificial intelligence on colonoscopy outcomes: A systematic review with meta-analysis. J Gastroenterol Hepatol 2020;35:1676-83. [Crossref] [PubMed]
- Aziz M, Haghbin H, Sayeh W, et al. Comparison of Artificial Intelligence With Other Interventions to Improve Adenoma Detection Rate for Colonoscopy: A Network Meta-analysis. J Clin Gastroenterol 2022; Epub ahead of print. [Crossref] [PubMed]
- Whitson MJ, Bodian CA, Aisenberg J, et al. Is production pressure jeopardizing the quality of colonoscopy? A survey of U.S. endoscopists' practices and perceptions. Gastrointest Endosc 2012;75:641-8. [Crossref] [PubMed]
- Butterly LF. Withdrawal Time: Is Nine the New Six? Clin Gastroenterol Hepatol 2022;20:e22-4. [Crossref] [PubMed]
- Pohl H, Robertson DJ, Mott LA, et al. Association between adenoma location and risk of recurrence. Gastrointest Endosc 2016;84:709-16. [Crossref] [PubMed]
- Rosser R, Corfe BM, Chapple KS. Metachronous Colorectal Adenomas Occur Close to the Index Lesion. J Clin Gastroenterol 2022; Epub ahead of print. [Crossref] [PubMed]
- El Rahyel A, Lahr RE, Rex DK. Prevalence of synchronous neoplasia in patients with large pedunculated colorectal polyps. Endoscopy 2023; Epub ahead of print. [Crossref] [PubMed]
- Liu W, Wu Y, Yuan X, et al. Artificial intelligence-based assessments of colonoscopic withdrawal technique: a new method for measuring and enhancing the quality of fold examination. Endoscopy 2022;54:972-9. [Crossref] [PubMed]
Cite this article as: Haghbin H, Zakirkhodjaev N, Aziz M. Withdrawal time in colonoscopy, past, present, and future, a narrative review. Transl Gastroenterol Hepatol 2023;8:19.


