EndoFLIP assessment of pyloric sphincter in children: a single-center experience
Highlight box
Key findings
• EndoFLIP is easy to use and can predict clinical response to intra pyloric injection of Botulinum Toxin in children with symptoms of impaired gastric drainage.
• Over two third of the studied children showed clinical response to Botulinum Toxin.
• Botulinum Toxin can result in long term improvement in pylorus EndoFLIP measurements.
What is known and what is new?
• Gastrointestinal complaints are common in children with neuromuscular disabilities.
• EndoFLIP can be used to predict response to intra pyloric Botulinum Toxin injection in adults with gastroparesis.
• All EndoFLIP measurements in the study have improved after Botulinum Toxin injection.
What is the implication, and what should change now?
• EndoFLIP should be used as an added tool to assess children with suspected gastroparesis.
• EndoFLIP catheter can be inserted via existing gastrostomy tract.
Introduction
Children with neurological disabilities often have multiple associated gastrointestinal (GI) problems which can affect their nutritional status and quality of life. Ninety two per cent of children with cerebral palsy have clinically significant GI complications (1). Due to the inherent difficulties with communication, gastrointestinal symptomatology in these children can manifest as early satiety, recurrent vomiting, retching, distress with feeds and prolonged feeding times (2). The sequelae of these problems does not only compromise nutritional status, but can also cause significant distress to the affected children and their carers (3). Similar symptoms are often reported in patients with delayed gastric emptying and gastroparesis (4).
The pylorus is known to play a salient role in drainage of gastric content into the duodenum and pyloric dysfunction can contribute to the pathophysiology of delayed gastric emptying (5). The incidence of pylorospasm was reported to be higher in diabetic gastroparesis patients compared to healthy volunteers and abnormal pylorus compliance was associated with severe clinical symptoms and altered quality of life (6-8). In the absence of a single effective strategy to manage symptoms secondary to delayed gastric emptying, the consensus is to try improve symptoms and to identify the group of patient who may benefit from targeted therapy (9). Botulinum Toxin is extensively used to treat spasticity in children with neurodisabilities and in a range of gastrointestinal conditions associated with abnormal sphincter tone (10-12). It is not recommended for the routine treatment of all patients with gastroparesis but is increasingly reported to be effective in patients with abnormal pyloric motility and compliance (7,13,14).
EndoFLIP® (endolumenal functional lumen imaging probe) uses impedance planimetry to characterise the geometry of the measurement area and is able to measure lumenal distensibility, compliance and cross sectional area (CSA) (15). It consists of 16 sensors inside a distensible balloon that can be filled to characterise sphincters diameter and CSA (15). It is often used to map lower oesophageal sphincter (LOS) distensibility in achalasia, to guide gastroesophageal reflux disease (GORD) surgery and to assess oesophageal compliance in eosinophilic oesophagitis (16-18). In achalasia, the LOS distensibility, rather than pressure appears to correlate well with oesophageal emptying and predict response to pneumatic dilatation (19,20). EndoFLIP is also increasingly used to assess the pyloric muscle to identify patients with low pyloric distensibility and poor compliance who will benefit from direct intervention to the pylorus (dilatation and/or intrapyloric Botulinum Toxin) (5,7,8).
We report our experience in using EndoFLIP via existing gastrostomy tracts to assess the pyloric muscles in children with neuromuscular disabilities and the response to targeted intrapyloric Botulinum Toxin injections. To the best of our knowledge, this is the first report in paediatric use of EndoFLIP to obtain pylorus measurement and to guide therapy. We present the following article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-58/rc).
Methods
The study was registered in our hospital’s clinical governance database as a review of clinical practice, in line with Declaration of Helsinki (as revised in 2013) and a full ethical review was not required (No. 10902).
Retrospective data collection of all children who had pylorus EndoFLIP assessment at Evelina London Children’s Hospital in London, United Kingdom between March 2019 and January 2022.
All included children have neuromuscular disabilities (apart from one child), are fed via gastrostomy tubes and had significant foregut symptoms including retching, vomiting, inability to tolerate large bolus feeds and/or discomfort while feeding. None of the included children had anatomical or structural abnormalities to explain their symptoms, they had normal stomach, duodenum biopsies and their symptoms did not respond to optimum acid suppression therapy, prokinetic use or feed manipulation. Demographic data, underlying medical conditions, gastrointestinal complaints and response to treatment were collected from medical records.
The following symptoms were assessed at baseline and criteria used to assess improvement were included between brackets: vomiting and regurgitation (more than 50% reduction in episodes), retching (more than 50% reduction in episodes), feed intolerance (full volume feed tolerance), pain during feeding (more than 50% reduction in episodes) and suboptimum weight gain (objective documented weight gain).
Statistical analysis
Analysis was performed using IBM SPSS® Statistics Version 25. Variables were reported as mean and standard deviation, Student’s t-test, ANOVA and Pearson’s correlation were used for analysis of variables and P<0.05 was regarded as statistically significant.
EndoFLIP measurement and technique description
EndoFLIP measurements were taken at the time of endoscopy under propofol anaesthesia. With the endoscope in the stomach, the balloon gastrostomy was removed and the EndoFLIP® catheter (EF-325N, 16 points over 80 mm measurement length along the balloon) (Medtronic©) was inserted through the gastrostomy tract into the pylorus under direct vision prior to endoscopic intubation of the pylorus. The balloon was filled with 20, 30 and 40 mL to assess minimum diameter, distensibility (defined as the resistance of the luminal wall to a distending force), compliance (defined as the change in volume in response to the change in pressure) and balloon pressure. A minimum of three measurements were obtained after a settling down period of 5 seconds (to avoid pressure during peristalsis and variation in pylorus volume) for each balloon volume. The mean value of each parameter was used and all individual measurements were used for subsequent analysis. For distensibility, there is published normality cut off of 10 mm2/mmHg for adults (8).
After EndoFLIP assessment, intrapyloric Botulinum Toxin (6 iu/kg to the maximum of 200 iu) was injected under the same anaesthesia. We used a sclerotherapy needle inserted through the biopsy channel of the endoscope to inject the Toxin in divided doses around the pylorus.
Symptoms were assessed 6 weeks after Botulinum Toxin injection.
Results
Measurements were obtained from 12 children, 5 (42%) were females, mean age ± standard deviation (SD) was 10.7±4.2 years. From each child, a minimum of three measurements were obtained with three sets of balloon volumes (20, 30 and 40 mL as detailed above). All children (apart from 1) had neuromuscular disabilities (9 had cerebral palsy, 1 had Nemaline myopathy, 1 with no unifying neuromuscular diagnosis and another had no unifying diagnosis but was neurologically able) (Table 1). EndoFLIP measurements showed the mean diameter, distensibility, compliance and pressure at balloon volumes of 20, 30 and 40 mL as summarised in Table 2. Diameter and pressure measurements at 20 mL balloon volume were at the lower limit of normal but all other measurements were outside the normal range reported in healthy adult volunteers (21). All children received intrapyloric Botulinum Toxin and all but one child showed clinical symptom improvement as detailed in Table 3. Although all EndoFLIP measurements have improved after the Botox injection during the subsequent measurement in symptomatic children (6–9 months later), the diameter, distensibility and pressure values have remained outside the normative adult values (21) (Table 2). Nine children had reassessment of pylorus 6–9 months after Botulinum Toxin injection, their EndoFLIP measurements are included in Table 2. Seven children had poor weight gain in the months prior to the procedure, 6 children showed sustained weight gain after the Botulinum Toxin injection for a minimum of 6 months duration. One child was able to stop jejunal feeding and maintain full gastric feeding.
Table 1
| Parameter | N=12 |
|---|---|
| Females, n (%) | 5 (42%) |
| Age (years), mean ± SD | 10.7±4.2 |
| Background diagnosis | |
| Cerebral palsy | 9 |
| Nemaline myopathy | 1 |
| No unifying diagnosis | 2 |
SD, standard deviation.
Table 2
| Parameter (mean ± SD) | 20 mL balloon volume | 30 mL balloon volume | 40 mL balloon volume | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | P value | Before | After | P value | Before | After | P value | |||
| Diameter (mm) | 6.5±1.3 | 6.6±1.3 | 0.013 | 7.8±2.3 | 9.4±1.8 | 0.05 | 10.1±2.7 | 11.2±2.4 | 0.03 | ||
| Balloon pressure (mmHg) | 13.6±4.4 | 9.6±2.5 | 0.046 | 20.9±8.1 | 16.2±4.4 | 0.1 | 42.3±13.1 | 35±16.3 | 0.2 | ||
| Distensibility (mm2/mmHg) | 2.6±1.1 | 3.8±1.4 | 0.011 | 2.7±1.8 | 4.4±1.4 | 0.008 | 2.1±1 | 3.0±0.6 | 0.01 | ||
| Compliance (mm3/mmHg) | 92.3±38 | 147.9±37.1 | 0.030 | 89.7±43.5 | 142.9±36.6 | 0.047 | 77±28.9 | 85.4±28.3 | 0.3 | ||
EndoFLIP, endolumenal functional lumen imaging probe; SD, standard deviation.
Table 3
| Symptoms | Pre Botulinum (% of children) | Post Botulinum (% of children improved) |
|---|---|---|
| Vomiting and regurgitation | 100 | 83 |
| Retching | 83 | 80 |
| Feed intolerance (did not tolerate feed volume) | 100 | 78 |
| Pain during feeding | 100 | 78 |
| Suboptimum weight gain | 58 | 85 |
| Improved wellbeing (subjective reports from parents) | – | 83 |
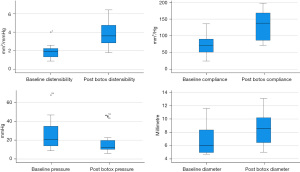
Individual EndoFLIP measurements were then categorised into three groups based on balloon volumes. There were significant changes in diameter and distensibility (P<0.05) pre and post Botulinum Toxin injection with all balloon volumes (20, 30 and 40 mL), while the pre/post difference in pressure and compliance were only statistically significant with 20 mL balloon volume as detailed in Table 2. There was a significant improvement in diameter (P<0.001) and distensibility (P<0.001) measurements in the whole group after Botulinum Toxin injection (Figure 1) and significant improvement pressure measurements (P=0.002) and increased in compliance (P=0.001) in the whole cohort (Figure 1).
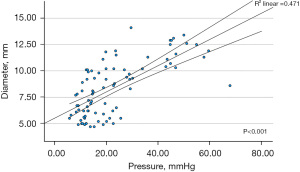
There was significant increase in balloon pressure with each incremental increase in balloon volume between 20, 30 and 40 mL (P=0.02, <0.001 and <0.001 respectively) leading to significant differences in diameter with balloon volume of 20 to 30 mL and 20 to 40 mL (P=0.026 and <0.001 respectively), and a significant change from 30 to 40 mL (P=0.004). Increasing balloon pressure appears to positively influence diameter (r=0.63, P<0.001) (Figure 2) an important factor to consider in paediatric patient as the high balloon pressure may lead to inadvertent increase in balloon diameter and subsequent pylorus dilatation. While as expected distensibility and compliance (as defined as the volume of the enclosed region between above and below the minimum diameter divided by balloon distended pressure) were strongly correlated (r=0.79, P<0.001).
Discussion
Gastrointestinal complaints are common in children with neurodisabilities and can affect up to 90% of this population (1). Regurgitation, vomiting, GORD and impaired stomach emptying can lead to feeding difficulties and malnutrition (22). In this cohort, all included children had refractory foregut symptoms including vomiting and regurgitation, retching, feed intolerance affecting their ability to maintain sufficient nutrition, pain during feeding and suboptimum weight gain, not responding to optimum medical therapy. All children appeared to have abnormal pyloric EndoFLIP measurements and had shown a clinical improvement after Botulinum Toxin injection. Although there was some improvement in EndoFLIP values in subsequent measurements, the procedures were done 6–9 months after the injection in children who have had recurrence of symptoms. Children who had a repeat Botulinum Toxin injection also showed a similar clinical improvement to the first dose.
The European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommends jejunal feeding in children intolerant to gastric feeding, in paediatric gastroparesis and GORD, as well as in the neurologically disabled (23). However, jejunal tube feeding can lead to increasing morbidity and may negatively affect quality of life (24). Transpyloric jejunal tubes require frequent replacement under a specialist care setting often under general anaesthesia. They are unpredictable and can malfunction without warning leading to unplanned hospital admissions (25,26). Feeding into the jejunum is delivered as a continuous infusion to prevent intermittent dilatation of the small bowel, this can disrupt sleep patterns if administered overnight or disturb activities and routines during daytime administration. Abnormal pyloric distensibility measured by EndoFLIP was previously reported in adults with gastroparesis and was associated with delayed gastric emptying (5). Intrapyloric Botulinum Toxin improved symptoms of poor gastric emptying in patients with abnormal pyloric distensibility (7). In children with feed intolerance and refractory foregut symptoms, assessment of pylorus by EndoFLIP may provide a useful tool to maintain gastric feeding and prevent progression to jejunal feeding. One child in our cohort was able to wean jejunal feeding and tolerated full feeds into stomach.
In this study, all included children had impaired pyloric distensibility which was consistent with three different balloon volume (20, 30 and 40 mL). Pyloric distensibility improved after Botulinum Toxin injection (Figure 1). Although there are no reported paediatric values, these readings are far below the accepted adult cut off value of 10 mm2/mmHg (6,8), age or the size of the child did not appear to affect distensibility measurements. All children received intrapyloric Botulinum Toxin 6 iu/kg up to a maximum of 200 iu in divided doses, all (apart from one child) showed clinical symptom improvement after 6 weeks and sustained weight gain.
The incremental increase in balloon volume led to significant increase in balloon pressure which showed strong positive correlation with the diameter of the pylorus. This raises an important question about the optimum balloon volume in children. Although the EndoFLIP balloon is complained and does not cause hollow organ dilatation, it appears to cause unplanned dilatation of the pylorus with increasing intra balloon pressure, with no effect on distensibility or compliance. We did not observe any adverse effect from this and the intra balloon pressure remained well below the default safety pressure (60 mmHg), it is an area where further research is warranted and clinicians will need to be aware of the association between pressure and diameter as it may have clinical implications in small children. Although this is a small sample size, inflating the balloon volume to 40 mL did not produce added measurement value but did produce undue pyloric stretch.
Conclusions
In conclusion, we report our experience in the assessment of pyloric distensibility, compliance and diameter in children with neurodisabilities by EndoFLIP via existing gastrostomy tract. This can be easily performed under the same general anaesthetic as endoscopy, does not add much time to the total procedure duration (added an average of extra 10 minutes to the endoscopy time) and can guide the administration of intrapyloric Botulinum Toxin. The injections are an effective therapy to improve foregut symptoms in a subset of children, showing good clinical response and a trend of improvement in EndoFLIP measurements. Paediatricians can use EndoFLIP to select the cohort of children who may respond to intra pyloric Botulinum Toxin injection, this can potentially allow continuation of gastric feeding, avoidance of jejunal feeding and overall improvement in foregut symptoms, although more data from larger studies will be required to study the pathophysiology of pylorus muscle in children.
This study has a number of limitations. It is a retrospective design in a small number of children but to the best of our knowledge this is the first report in paediatric pylorus assessment by using EndoFLIP documented measurement parameters and guiding clinical management. We did not use breath test or scintigraphy to assess gastric emptying as our study’s population of children with neurodisabilities are unlikely to comply with such tests.
Acknowledgments
We would like to acknowledge the contribution of Ms. Emily Kelly for her help in language editing in the early stages of manuscript preparation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-58/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-58/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-58/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-58/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Del Giudice E, Staiano A, Capano G, et al. Gastrointestinal manifestations in children with cerebral palsy. Brain Dev 1999;21:307-11. [Crossref] [PubMed]
- Quitadamo P, Thapar N, Staiano A, et al. Gastrointestinal and nutritional problems in neurologically impaired children. Eur J Paediatr Neurol 2016;20:810-5. [Crossref] [PubMed]
- Sullivan PB, Juszczak E, Bachlet AM, et al. Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy. Dev Med Child Neurol 2004;46:796-800. [Crossref] [PubMed]
- Parkman HP. Idiopathic gastroparesis. Gastroenterol Clin North Am 2015;44:59-68. [Crossref] [PubMed]
- Gourcerol G, Tissier F, Melchior C, et al. Impaired fasting pyloric compliance in gastroparesis and the therapeutic response to pyloric dilatation. Aliment Pharmacol Ther 2015;41:360-7. [Crossref] [PubMed]
- Saadi M, Yu D, Malik Z, et al. Pyloric sphincter characteristics using EndoFLIP® in gastroparesis. Características del esfínter pilórico utilizando EndoFLIP® en gastroparesia. Rev Gastroenterol Mex 2018;83:375-84. (Engl Ed). [Crossref]
- Desprez C, Melchior C, Wuestenberghs F, et al. Pyloric distensibility measurement predicts symptomatic response to intrapyloric botulinum toxin injection. Gastrointest Endosc 2019;90:754-760.e1. [Crossref] [PubMed]
- Malik Z, Sankineni A, Parkman HP. Assessing pyloric sphincter pathophysiology using EndoFLIP in patients with gastroparesis. Neurogastroenterol Motil 2015;27:524-31. [Crossref] [PubMed]
- Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers 2018;4:41. [Crossref] [PubMed]
- Multani I, Manji J, Hastings-Ison T, et al. Botulinum Toxin in the Management of Children with Cerebral Palsy. Paediatr Drugs 2019;21:261-81. [Crossref] [PubMed]
- Vanuytsel T, Bisschops R, Farré R, et al. Botulinum toxin reduces Dysphagia in patients with nonachalasia primary esophageal motility disorders. Clin Gastroenterol Hepatol 2013;11:1115-1121.e2. [Crossref] [PubMed]
- Lacy BE, Weiser K, Kennedy A. Botulinum toxin and gastrointestinal tract disorders: panacea, placebo, or pathway to the future? Gastroenterol Hepatol (N Y) 2008;4:283-95. [PubMed]
- Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013;108:18-37; quiz 38. [Crossref] [PubMed]
- Thomas A, de Souza Ribeiro B, Malespin M, et al. Botulinum Toxin as a Treatment for Refractory Gastroparesis: a Literature Review. Curr Treat Options Gastroenterol 2018;16:479-88. [Crossref] [PubMed]
- McMahon BP, Frøkjaer JB, Liao D, et al. A new technique for evaluating sphincter function in visceral organs: application of the functional lumen imaging probe (FLIP) for the evaluation of the oesophago-gastric junction. Physiol Meas 2005;26:823-36. [Crossref] [PubMed]
- Donnan EN, Pandolfino JE. Applying the Functional Luminal Imaging Probe to Esophageal Disorders. Curr Gastroenterol Rep 2020;22:10. [Crossref] [PubMed]
- Su B, Callahan ZM, Novak S, et al. Using Impedance Planimetry (EndoFLIP) to Evaluate Myotomy and Predict Outcomes After Surgery for Achalasia. J Gastrointest Surg 2020;24:964-71. [Crossref] [PubMed]
- Su B, Callahan ZM, Kuchta K, et al. Use of Impedance Planimetry (Endoflip) in Foregut Surgery Practice: Experience of More than 400 Cases. J Am Coll Surg 2020;231:160-71. [Crossref] [PubMed]
- Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 2012;143:328-35. [Crossref] [PubMed]
- Benitez AJ, Budhu S, Burger C, et al. Use of the functional luminal imaging probe in pediatrics: A comparison study of patients with achalasia before and after endoscopic dilation and non-achalasia controls. Neurogastroenterol Motil 2021;33:e14133. [Crossref] [PubMed]
- Jagtap N, Kalapala R, Reddy DN. Assessment of Pyloric Sphincter Physiology Using Functional Luminal Imaging Probe in Healthy Volunteers. J Neurogastroenterol Motil 2020;26:391-6. [Crossref] [PubMed]
- Kuperminc MN, Stevenson RD. Growth and nutrition disorders in children with cerebral palsy. Dev Disabil Res Rev 2008;14:137-46. [Crossref] [PubMed]
- Broekaert IJ, Falconer J, Bronsky J, et al. The Use of Jejunal Tube Feeding in Children: A Position Paper by the Gastroenterology and Nutrition Committees of the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition 2019. J Pediatr Gastroenterol Nutr 2019;69:239-58. [Crossref] [PubMed]
- Fortunato JE, Darbari A, Mitchell SE, et al. The limitations of gastro-jejunal (G-J) feeding tubes in children: a 9-year pediatric hospital database analysis. Am J Gastroenterol 2005;100:186-9. [Crossref] [PubMed]
- Blumenstein I, Shastri YM, Stein J. Gastroenteric tube feeding: techniques, problems and solutions. World J Gastroenterol 2014;20:8505-24. [Crossref] [PubMed]
- Al-Zubeidi D, Demir H, Bishop WP, et al. Gastrojejunal feeding tube use by gastroenterologists in a pediatric academic center. J Pediatr Gastroenterol Nutr 2013;56:523-7. [Crossref] [PubMed]
Cite this article as: Popescu M, White E, Mutalib M. EndoFLIP assessment of pyloric sphincter in children: a single-center experience. Transl Gastroenterol Hepatol 2023;8:17.