Hepatic epithelioid hemangioendothelioma: how fast does it grow and which findings could have prevented diagnostic delay?—a case report
Introduction
Hepatic epithelioid hemangioendothelioma (HEHE) is a rare sarcoma with a varied presentation, from indolent nodules to aggressive tumors. The primary sources of the tumor tissue are pre-endothelial or vascular endothelial cells (1). Despite a similar tumor having been found in the lung and reported in 1975, the first epithelioid hemangioendothelioma description was in 1982 when the authors report an atypical vascular tumor that had been frequently misdiagnosed as carcinoma (2,3). Nearly 50% of epithelioid hemangioendothelioma are found in association with a preexisting vessel, so their microscopic appearance is of infiltrative cords of epithelioid endothelial cells implanted in a myxohyaline stroma growing within the vessel or around it (3). The tumor cells are usually positive for CD31, CD34, and cytokeratin, with a translocation that allows confirmation of the diagnosis, denoted t(1;3) WWTR1-CAMTA1 fusion, which can be detected by fluorescence in situ hybridization (FISH) (3). Less commonly, the fusion found is YAP1-TFE3, detected by TFE3 immunoreactivity (4). While HEHE has been known as a slow-growing tumor of indolent nature (5-7), this is the first case report in which the tumor growth was measured, showing nodules that enlarged and spread to other organs in a given time span. We present the following case in accordance with the CARE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-48/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The local ethics committee (named “Comitê de Ética em Pesquisa”) approved the development of this study (Protocol No. 5.235.006).
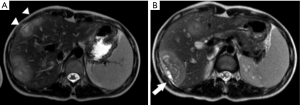
The patient is a 17-year-old female from the interior of São Paulo state, Brazil. Her first complaints were related to the mass effect caused by the liver tumor, such as abdominal pain and bloating during the last 3 years. She also reported weight loss in recent months. Painful hepatomegaly was described in her medical appointments since 2018. The first abdominal ultrasonography was performed in September 2018 and showed hypoechoic hepatic nodules of 12×31 mm. A magnetic resonance imaging (MRI) on February 8, 2019, revealed 11 hyperintense nodules in T2, filled by liquid material and peripheral enhancement by the contrast medium, thus suggesting necrotic content, which would be the first sign that the nodules deserve further investigation (Figure 1). The largest nodule measured 6.3×6.1×4.0 cm, and was located in the liver segment VI. At that time, she already had an enlarged spleen, reaching 13.5 cm. Her blood tests were unremarkable.
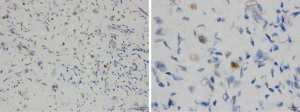
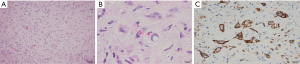
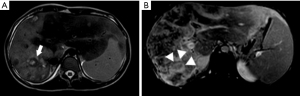
In March 2019, she was submitted to a laparoscopic liver biopsy at a local hospital. The first analysis by the local pathologists was “No signs of malignancy. The histologic specimen is formed by granulation tissue with marked fibrosis and hyalinization, embracing and compressing biliary ducts and hepatocytes. Some of these cells contain vacuolated cytoplasm and are disposed in cords”. However, the sample already had findings compatible with HEHE such as cords of epithelioid tumor cells, and intracytoplasmic vacuoles containing red blood cells (shown in Figure 2A,2B). At that moment, no malignancy had been suspected and no immunohistochemical assay was carried out. Only when the patient was forwarded to our hospital in July 2021 the immunoreaction for CD31 was observed on the same sample from the first liver biopsy (Figure 2C).
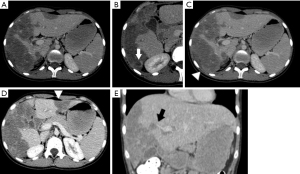
In August 2019, the patient reported fever and worsening abdominal pain. Intermittent ultrasonography assessment was maintained until reaching new signs of tumor growth. Then, a computerized tomography (CT) was done in May 2020 showing that the tumor located in the liver segment VI had dimensions of 12.9×7.3 cm (shown in Figure 3). In February 2021, the patient was submitted to a new MRI that showed delayed enhancement of the tumors by the gadolinium.
In April 2021, a second laparoscopic liver biopsy was done. Unfortunately, the histologic assessment was still deemed inconclusive by the local pathologists, who reported no malignant tissue in spite of the HEHE findings. Ascitic fluid was also assessed, but no malignant cells were found. At that time, she reported a dull pain in her knees. Due to the lack of a diagnosis at that time, the patient was forwarded to our hospital in July 2021, with painful hepatomegaly, asymptomatic splenomegaly, and pain in both knees, but no respiratory complaints. The histologic tumor samples were reassessed and the HEHE findings were finally recognized. Additional staining for CD31 was performed, thus confirming the diagnosis (shown in Figure 2C).
A chest CT showed 7-mm nodules diffusely distributed in both lungs, associated with longitudinal fissure thickening (shown in Figure 4). A new MRI showed an enlarged liver, containing infiltrative tumors occupying most parts of the right lobe and part of the liver segment III, causing retraction of the liver capsule on segment VI, similar to the findings observed in the last CT (shown in Figure 5). The tumor was heterogeneous and led to hepatic vessels distortion. It was filled by nodules with a target aspect, reaching up to 17 mm, with progressive enhancement during the contrast medium injection.
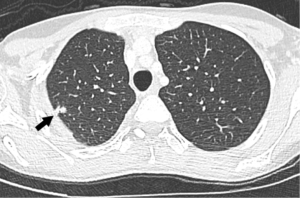
In the same month, bone scintigraphy was carried out, revealing bone metastasis. In spite of good outcomes reported in a large cohort of patients with metastatic HEHE submitted to liver transplantation and liver resection (8), multi-organ disease prevents liver transplantation in Brazil, and liver resection could not be performed due to the hepatic spreading of the tumors. Therefore, no surgical treatment was done. Ten sessions of radiotherapy was done and sorafenib was started due to the benefits reported in another case (9). Despite prior reports considering thalidomide as another option (9,10), the drug was not used because the effect on vascular endothelial growth factor (VEGF) is not as powerful as that observed with sorafenib (9). The treatment was well tolerated by the patient, who adhered properly to the medical recommendations.
Aiming to estimate the tumor growth between the two MRIs carried out in 2019 and 2021, a 2.5D volumetry of the liver was made using 3D Slicer® 4.11 on the MRI T2 weighted arterial images (11). The segmentation employs a supervised semiautomatic nodule segmentation algorithm based on open-source software. First, seed points were placed within the liver region to initialize the segmentation using a region-growing algorithm. When the image contained multiple nodules, a seed point was placed inside each nodule in axial, sagittal, and coronal directions. The contour propagation was governed by a smoothing process, which minimizes the contour curvature by a Gaussian filter of size 5 pixels. A final analysis was carried out and, if a segmented region mismatches the liver (or tumor) area, it was manually cropped or added to the segmentation. The results are shown in Table 1 and in Figures 6,7.
Table 1
| Analysis | 2019 | 2021 | Ratio 2021/2019 |
|---|---|---|---|
| LV (cm³) | 949.581 | 1,207.15 | 127.12% |
| TV (cm³) | 87.96 | 848.225 | 964.33% |
| Ratio TV/LV | 9.26% | 70.27% |
LV, liver volume; TV, tumor volume.
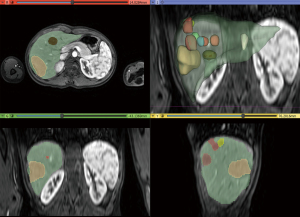
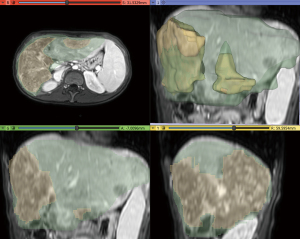
As explicit in Table 1, the liver volume measured in February 2019 increased by 27.12% in July 2021. In the same period, the tumor volume grew from 9.26% to 70.27% of the liver. Figures 6,7 show that many nodules coalesced, thus changing the nodules to giant tumors and causing hepatomegaly in this time span. The tumor cells presented TFE3 immunoreactivity (shown in Figure 8), suggesting the presence of the YAP1-TFE3 fusion, a distinct histological HEHE pattern in which mature lumina and epithelioid eosinophilic cells are observed (4).
Discussion
Three HEHE clinical findings could be used to suggest it as a possible diagnosis. First, the liver is the most commonly affected organ. Second, the tumor is more common among women. Third, abdominal pain is the main symptom. Lau et al. and Mehrabi et al. reported the largest reviews, containing 206 and 434 cases, respectively (12,13). In the study by Lau et al., the liver was involved in 99 cases (32%), the lung in 89, and the bones in 48. The patients’ age at the diagnosis ranged from 7 to 81, and the female proportion was 61%. Single-organ involvement occurred in 131 patients, of which 21% had only liver tumors. Pain was the most common symptom, occurring in 74% of the symptomatic patients (12). Right upper quadrant pain, hepatomegaly, and weight loss are the most common symptoms, and the male-to-female ratio is 2:3 (13). Despite the estimated prevalence of less than one in 1 million (8), dealing with hepatic nodules requires knowledge on HEHE as a possible diagnosis, especially when the three most common symptoms are present.
In such cases, a multidisciplinary team is needed to evaluate the imaging exams. In 2019 the patient already had tumors that could be easily distinguished from hemangiomas or other non-threatening lesions, as shown in Figure 1. Peripheral nodules with central hypodensity, calcifications, and capsular retraction are common signs of HEHE in CT and MRI images (14). Capsular retraction was already observed, suggesting malignant compromise. Of note, it occurs in less than 25% of patients, and signs of portal hypertension may also be observable due to the tumors’ growth (14). Since the patient had painful hepatomegaly and the radiological images were not recognized as HEHE, the decision to perform a biopsy at that time was correct.
As shown in Figure 2, the liver biopsies had typical findings to make the diagnosis, with cords of epithelioid tumor cells, intracytoplasmic vacuoles containing red blood cells and endothelial markers in immunohistochemistry (3). However, since the images in hematoxylin-eosin staining (HE) were not properly identified in the first evaluations, immunohistochemistry was not carried out, and the pathology conclusions were mistaken. Only when skilled pathologists assessed the samples was the disease diagnosed and further confirmed by immunohistochemistry. Regrettably, diagnostic mistakes are not rare in EHE, and this case shows how the lack of an experienced pathologist can be deleterious, especially assessing liver nodules. The two initial histological analyzes overlooked the tumor cell cords, mistakenly assuming that they were hepatocytes. As a result, no further staining or immunohistochemistry assay was carried out, and the presence of a malignant disease was wrongly denied. After two laparoscopic-guided biopsies suggesting no malignancy, the pediatricians withheld the diagnostic investigation. Certainly, a consultation with a more experienced pathologist could have avoided this terrible mistake. CD34 is expressed in more than 90% of the vascular tumors, showing a good sensitivity for initial evaluation, but is not specific to diagnose HEHE (1). CD31 is more specific and could be enough to confirm the diagnosis if the other signs of HEHE had been identified (1). In some cells, intracytoplasmatic vacuoles can displace the nucleus, the so-called blister cells, leading to a signet ring-like structure (14,15). A common finding in liver HEHE is the fibrotic stromal background (15). Unfortunately, none of these signs were recognized or described after two laparoscopic-guided biopsies.
The same problem occurred with the radiological findings. HEHE can appear as multiple nodules in the initial stages, but they tend to coalesce to form large lesions that grow diffusely into the liver (14,16). Despite the signs of malignancy observed in 2019, the disease was not diagnosed, allowing spreading to other organs. Bone involvement can cause pain, neurological symptoms, and even fractures, whereas pulmonary disease is usually manifested as coughing (16). Most patients with the single-organ disease receive surgical treatment and have no recurrence in 5 years (15). On the other hand, multi-organ disease and tumors with poor or moderate cell differentiation are findings of worse prognosis (15,17). Since the first symptom of our patient was painful hepatomegaly, maybe she could have been submitted to a curative treatment. However, the metastasis led us to offer only palliative (radiotherapy) and off-label (sorafenib) options, which were well-tolerated and provided relief from the bone pain, but did not change the liver and lung disease.
While the nodular type can be non-specific, the diffuse HEHE type is considered a slow-growing tumor, occupying the liver periphery, and not leading to capsular bulging (13). However, the time elapsed between the first symptoms and the diagnosis is usually long, leading to massive tumor growth, usually occupying both liver lobes when the disease is finally recognized (13). For the first time, the tumor growth was precisely estimated, showing that in a 29-month period the liver commitment was markedly changed, increasing 964.33% and leading to significant liver enlargement (127.12%). Fan et al. reported a fast-growing HEHE in which one of the tumors increased 9.45% within 2 months (18). However, only the growing of the largest lesion was documented in a short time span, thus reducing the measurement accuracy. As a result, the term “slow-growing tumor” seems inadequate for HEHE in patients as the one reported herein. This case is a clear example that a rapid growth can be observed in multinodular disease when its first signs are not recognized.
In spite of the limitations of a case report, this is the first time that HEHE growth was accurately measured in a given period. The results show that each sign of the disease must be recognized as soon as possible, thus avoiding diagnostic delay in similar cases.
It was also demonstrated that HEHE nodules are able to occupy the liver and spread to other organs in a short time span, a finding that is not compatible with a slow-growing tumor.
Acknowledgments
The authors wish to acknowledge the patient and her family for giving their consent to make this study available, thus contributing for a better understanding of the disease. The authors are grateful to James Richard Welsh for the English language editing of the manuscript.
Funding: This study was funded by São Paulo Research Foundation (FAPESP; Grant No. 2017/25592-9). FGR received funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-48/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-48/coif). FGR reports that the study was funded by São Paulo Research Cientific Foundation (FAPESP; Grant No. 2017/25592-9), but only the Institution received the payments. None of them was done for the researcher. The researcher has received funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The funding is a scholarship for researchers according to their scientific production. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The local ethics committee (named “Comitê de Ética em Pesquisa”) approved the development of this study (Protocol No. 5.235.006).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosenberg A, Agulnik M. Epithelioid Hemangioendothelioma: Update on Diagnosis and Treatment. Curr Treat Options Oncol 2018;19:19. [Crossref] [PubMed]
- Dail DH, Liebow AA. Intravascular bronchioalveolar tumor. Am J Pathol 1975;78:6a-7a.
- Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer 1982;50:970-81. [Crossref] [PubMed]
- Antonescu CR, Le Loarer F, Mosquera JM, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 2013;52:775-84. [Crossref] [PubMed]
- Krasnodębski M, Grąt M, Morawski M, et al. Hepatic Epithelioid Hemangioendothelioma: A Rare Disease With Favorable Outcomes After Liver Transplantation. Transplant Proc 2020;52:2447-9. [Crossref] [PubMed]
- Cao L, Hong J, Zhou L, et al. Selection of treatment for hepatic epithelioid hemangioendothelioma: a single-center experience. World J Surg Oncol 2019;17:183. [Crossref] [PubMed]
- Kyriazoglou A, Koutsoukos K, Zagouri F, et al. Metastatic Hepatic Epithelioid Hemangioendothelioma Treated with Olaratumab: A Falling Star Rising? Ther Clin Risk Manag 2020;16:141-6. [Crossref] [PubMed]
- Lai Q, Feys E, Karam V, et al. Hepatic Epithelioid Hemangioendothelioma and Adult Liver Transplantation: Proposal for a Prognostic Score Based on the Analysis of the ELTR-ELITA Registry. Transplantation 2017;101:555-64. [Crossref] [PubMed]
- Sangro B, Iñarrairaegui M, Fernández-Ros N. Malignant epithelioid hemangioendothelioma of the liver successfully treated with Sorafenib. Rare Tumors 2012;4:e34. [Crossref] [PubMed]
- Salech F, Valderrama S, Nervi B, et al. Thalidomide for the treatment of metastatic hepatic epithelioid hemangioendothelioma: a case report with a long term follow-up. Ann Hepatol 2011;10:99-102. [Crossref] [PubMed]
- Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 2012;30:1323-41. [Crossref] [PubMed]
- Lau K, Massad M, Pollak C, et al. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest 2011;140:1312-8. [Crossref] [PubMed]
- Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 2006;107:2108-21. [Crossref] [PubMed]
- Lyburn ID, Torreggiani WC, Harris AC, et al. Hepatic epithelioid hemangioendothelioma: sonographic, CT, and MR imaging appearances. AJR Am J Roentgenol 2003;180:1359-64. [Crossref] [PubMed]
- Rosenbaum E, Jadeja B, Xu B, et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol 2020;33:591-602. [Crossref] [PubMed]
- Radin DR, Craig JR, Colletti PM, et al. Hepatic epithelioid hemangioendothelioma. Radiology 1988;169:145-8. [Crossref] [PubMed]
- Dogeas E, Mokdad AA, Bhattatiry M, et al. Tumor Biology Impacts Survival in Surgically Managed Primary Hepatic Vascular Malignancies. J Surg Res 2021;264:481-9. [Crossref] [PubMed]
- Fan YH, Tang HN, Zhou JP, et al. Fast-growing epithelioid hemangioendothelioma of the liver: A case report. Medicine (Baltimore) 2020;99:e22077. [Crossref] [PubMed]
Cite this article as: Ribeiro MCDO, Lemos JVB, de Toledo Moraes MP, Oliver FA, Alvarez M, Silva GF, Qi X, Romeiro FG. Hepatic epithelioid hemangioendothelioma: how fast does it grow and which findings could have prevented diagnostic delay?—a case report. Transl Gastroenterol Hepatol 2023;8:12.