
Impact of pre-operative transjugular intrahepatic portosystemic shunt on post-operative outcomes following non-transplant surgeries in patients with decompensated cirrhosis
Introduction
Patients with cirrhosis, regardless of etiology, have a high risk for morbidity and mortality in relation to abdominal surgery; mortality can be as high as 50% in emergent surgeries (1-3). Despite improvements in surgical techniques and intensive care, major abdominal surgery still remains a challenge in patients with cirrhosis. Major factors determining short- and long-term survival and perioperative complications in this patient population include severity of liver dysfunction, degree of portal hypertension (PHTN) and the presence of related complications such as ascites (4-7). In fact, PHTN is considered an independent predictor of morbidity and mortality following abdominal surgery (8). Venous congestion secondary to PHTN predisposes to an increased risk of intraabdominal hemorrhage, worsening ascites, wound dehiscence and/or peritonitis. Accordingly, it has been suggested that such complications may be prevented, or their incidence reduced by decreasing portal pressure pre-operatively by placing a transjugular intrahepatic portosystemic shunt (TIPS) (9). TIPS is a minimally invasive procedure performed to manage complications related to PHTN such as variceal bleeding, refractory ascites, hepatic hydrothorax and hepatorenal syndrome (10).
Literature on pre-operative TIPS prior to abdominal surgery is very limited and exists mostly in the form of case reports and small case series (11-16). Kim et al. described a series of 25 patients who had TIPS in place for surgery, with only 6 having prophylactic TIPS (14). They showed a 12% in-hospital mortality and 74% 1-year survival. However, there was no comparator group in this study. A retrospective study from Vinet et al. compared 18 patients with elective TIPS with 17 patients without TIPS who underwent abdominal surgeries and showed that elective TIPS placement did not improve post-operative outcome in patients with good or moderately impaired liver function (17). The survival rates were not different in the TIPS vs. no TIPS groups at 1 month (83% vs. 86%) and 1 year (54% vs. 62%) (17). The largest case control study to date by Tabchouri et al. comparing patients with cirrhosis who underwent pre-operative TIPS to controls without TIPS showed no significant differences between the two groups in post-operative complications or 90-day mortality (16).
Our aim, in this retrospective study, was to compare perioperative outcomes between patients with cirrhosis who underwent abdominal surgery after pre-operative TIPS with those that did not. We present the following article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-21-133/rc).
Methods
Patient population
All patients who underwent elective TIPS placement prior to abdominal surgeries from January 2013 to January 2018 at Houston Methodist Hospital were identified by retrospective chart review. These patients were compared with a cohort of cirrhotic patients who underwent any abdominal surgeries without TIPS placement. A matched cohort was attempted but due to the rarity of these surgeries a precisely matched cohort was not possible. We selected all the patients in our hospital who underwent abdominal or pelvic surgery where there was known cirrhosis prior to operation. Patients with non-abdominal surgeries were excluded. Patients were identified using Current Procedures Terminology (CPT) and International Classification of Disease-9th Revision (ICD-9) codes generated by their hospital stay. In this retrospective review, the decision to place the pre-operative TIPS was individualized and made by the managing physicians. The rarity of patients with decompensated hepatic cirrhosis also prevented the groups from being equally matched to the type surgery they underwent. This resulted in the majority of patients in TIPS undergoing exploratory laparotomy (85%), whereas equal numbers of patients in no TIPS group underwent laparoscopy and exploratory laparotomy (P=0.02).
Inclusion and exclusion criteria
All patients with cirrhosis aged 18 years or older who underwent abdominal and pelvic surgery with or without TIPS. Patient were excluded if the TIPS was placed more than 9 months prior to surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Houston Methodist Hospital IRB committee (IRB Number: Pro00018198). Informed consent was not required as this was a retrospective chart review using de-identified patient information.
TIPS placement
TIPS placement was performed by an interventional radiologist using the standard technique (18). Both pre- and post-TIPS portal pressures were measured with the goal being to reduce portal pressure to below 12 mmHg. Patients were managed by the treating physician’s team as clinically indicated. All patients in both groups were followed by a specialist hepatologist acting as a consultant.
Outcomes
The primary outcomes were mortality and 30 days and at 1 year following surgery. The following post-operative outcomes were also compared between the two groups: blood loss, worsening ascites, wound leak, infections, encephalopathy, liver decompensation, and length of hospitalization. Information about patient demographics (age, sex, race, etc.), body mass index (BMI), comorbidities (including history of chronic kidney disease, diabetes, hypertension, and congestive heart failure), etiology of cirrhosis, history of decompensation from cirrhosis, model for end-stage liver disease (MELD) score, and Child-Turcotte-Pugh (CTP) were obtained by reviewing patients’ electronic health records. Operative details such as estimated blood loss, indication for surgery, type of abdominal surgery, need for intra-operative blood transfusions, operative time were obtained from the operative notes and anesthesia documentation.
Statistical analysis
Demographic and clinical data were reported as frequencies and proportions for categorical variables and as median and interquartile range (IQR) for continuous variables. Differences between groups (TIPS vs. no TIPS) were compared using the Chi-square or Fischer’s exact tests for categorical variables and Wilcoxon rank-test for continuous variables as appropriate. Univariate survival analysis was performed to determine the characteristics associated with patient mortality. Kaplan Meier survival curves were used to depict patient survival. Differences between TIPS or no TIPS were analyzed using the log-rank test. All the analyses were performed on Stata version 16.1 (StataCorp LLC, College Station, TX, USA). A P value of <0.05 was considered statistically significant. The statistical methods of this study were reviewed by Edward A. Graviss and Duc T. Nguyen from Houston Methodist Hospital.
Results
Patient characteristics
Baseline characteristics and biochemical markers for patients in both groups are summarized in Table 1. A total of 38 patients with cirrhosis who underwent abdominal surgery were identified from January 2013 to January 2018. Among these, 20 patients underwent pre-operative elective TIPS placement. Demographic characteristics in the two groups were comparable in terms of age, gender ratio, and BMI. The median age was 62 (IQR, 57–69) years with a male predominance (62.5%). The majority of the patients were Caucasian (60.5%). Both groups were similar in terms of etiology of cirrhosis with hepatitis C virus (HCV) (34.2%) being most common diagnosis followed by nonalcoholic steatohepatitis (NASH) (31.6%), alcohol (23.7%), autoimmune (5.3%), Budd Chiari (2.6%) and primary biliary cholangitis (PBC) (2.6%). Presence of ascites (prior to TIPS placement), whether large (requiring paracentesis) or small (evident on imaging only), did not differ between groups. Patients with TIPS had a statistically significantly higher rate of documented esophageal varices (65% vs. 22%, P=0.01); however, rates of prior variceal bleeding were similar. Indication for TIPS included refractory ascites in 55% (11/20) patients and variceal bleeding in 10% (2/20). The other 7 patients (35%) had TIPS placed due to elevated portal pressures to facilitate surgery. Patients who underwent TIPS had lower rates of hepatic encephalopathy (HE), prior to their procedure (50% vs. 83.3%). Serum chemistries were comparable in both groups except for international normalized ratio (INR), which was higher in the TIPS group (1.4 vs. 1.3, P=0.04). Platelet count was slightly lower in the TIPS group; however, there was no statistical significance between the two groups (71.5 vs. 101.5, P=0.25).
Table 1
| Characteristics | No TIPS (n=18) | TIPS (n=20) | P value |
|---|---|---|---|
| Age (years), median [IQR] | 63 [54, 69] | 62 [60, 69] | 0.95 |
| Gender, n (%) | 0.14 | ||
| Female | 4 (22.2) | 9 (45.0) | |
| Male | 14 (77.8) | 11 (55.0) | |
| Race, n (%) | 0.13 | ||
| Caucasian | 8 (44.4) | 15 (75.0) | |
| African American | 4 (22.2) | 2 (10.0) | |
| Hispanic | 6 (33.3) | 2 (10.0) | |
| Other | 0 (0.0) | 1 (5.0) | |
| BMI (kg/m2), median [IQR] | 29.8 [26.0, 34.0] | 28.5 [24.3, 32.8] | 0.51 |
| BMI (kg/m2), n (%) | 0.81 | ||
| <18.5 | 0 (0.0) | 1 (5.0) | |
| 18.5–24.9 | 4 (22.2) | 4 (20.0) | |
| 25–29.9 | 6 (33.3) | 6 (30.0) | |
| ≥30 | 8 (44.4) | 9 (45.0) | |
| Etiology of cirrhosis, n (%) | 0.79 | ||
| ETOH | 4 (22.2) | 5 (25.0) | |
| HCV | 7 (38.9) | 6 (30.0) | |
| NASH | 5 (27.8) | 7 (35.0) | |
| AIH | 1 (5.6) | 1 (5.0) | |
| Budd Chiari | 0 (0.0) | 1 (5.0) | |
| PBC | 1 (5.6) | 0 (0.0) | |
| Ascites, n (%) | 0.09 | ||
| No | 13 (72.2) | 9 (45.0) | |
| Yes | 5 (27.8) | 11 (55.0) | |
| Large ascites (requiring paracentesis), n (%) | 0.07 | ||
| Absent | 3 (16.7) | 2 (10.0) | |
| Present | 2 (11.1) | 9 (45.0) | |
| None | 13 (72.2) | 9 (45.0) | |
| Esophageal varices, n (%) | 0.01 | ||
| No | 14 (77.8) | 7 (35.0) | |
| Yes | 4 (22.2) | 13 (65.0) | |
| Esophageal variceal bleeding, n (%) | 0.59 | ||
| No | 14 (77.8) | 14 (70.0) | |
| Yes | 4 (22.2) | 6 (30.0) | |
| Previous encephalopathy, n (%) | 0.03 | ||
| No | 15 (83.3) | 10 (50.0) | |
| Yes | 3 (16.7) | 10 (50.0) | |
| Hemoglobin (mg/dL), median [IQR] | 11.3 [9.8, 12.1] | 10.9 [9.3, 11.6] | 0.26 |
| Serum albumin, median [IQR] | 3.0 [2.5, 3.5] | 3.0 [2.5, 3.2] | 0.55 |
| Serum bilirubin, median [IQR] | 1.5 [0.8, 1.7] | 1.3 [0.8, 2.3] | 1.00 |
| INR, median [IQR] | 1.3 [1.2, 1.4] | 1.4 [1.4, 1.6] | 0.04 |
| PT, median [IQR] | 16.0 [15.0, 17.5] | 17.2 [16.1, 18.9] | 0.12 |
| Platelets (×109), median [IQR] | 101.5 [73.0, 114.0] | 71.5 [52.0, 128.0] | 0.25 |
| Creatinine, median [IQR] | 0.7 [0.7, 1.0] | 0.9 [0.7, 1.1] | 0.14 |
| Diabetes, n (%) | 0.91 | ||
| No | 12 (66.7) | 13 (65.0) | |
| Yes | 6 (33.3) | 7 (35.0) | |
| Chronic kidney disease, n (%) | 0.10 | ||
| No | 17 (94.4) | 15 (75.0) | |
| Yes | 1 (5.6) | 5 (25.0) | |
| Congestive heart failure, n (%) | 0.49 | ||
| No | 16 (88.9) | 19 (95.0) | |
| Yes | 2 (11.1) | 1 (5.0) | |
| Hypertension, n (%) | 0.20 | ||
| No | 15 (83.3) | 13 (65.0) | |
| Yes | 3 (16.7) | 7 (35.0) | |
| Ejection fraction (%), median [IQR] | 60 [60, 65] | 65 [60, 65] | 0.54 |
| Pulmonary artery pressure (mmHg), median [IQR] | 33 [21, 53] | 27.5 [24, 30] | 0.35 |
| Pre-operative CTP score, median [IQR] | 7 [6, 8] | 9.0 [7.5, 10.5] | 0.003 |
| Pre-operative MELD, median [IQR] | 11 [8, 13] | 13.0 [10.5, 17.0] | 0.051 |
Values are as frequency and % for categorical variables and median [IQR] for continuous variables. IQR, interquartile range; BMI, body mass index; ETOH, alcohol consumption; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; AIH, autoimmune hepatitis; PBC, primary biliary cholangitis; INR, international normalized ratio; PT, prothrombin time; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease; TIPS, transjugular intrahepatic portosystemic shunt.
Interestingly, the average CTP score was statistically higher in patients with TIPS both before TIPS placement (9-child B vs. 7-child B, P=0.003) and on the day of surgery (Table 2; 10-child B vs. 7-child B, P<0.001). Average MELD score trended toward significance at the time of TIPS placement (13 vs. 11, P=0.051); however, MELD score was significantly higher in the TIPS group on the day of surgery (Table 2; 15.5 vs. 11, P=0.01). Both groups were comparable in regard to comorbidities such as diabetes, hypertension, chronic kidney disease and congestive heart failure. Hemodynamic values were comparable between both groups. Average left ventricular ejection fraction (60% vs. 65%) and pulmonary artery pressure (33 vs. 27.5 mmHg) measured by transthoracic echocardiogram were similar as well in both groups.
Table 2
| Parameters/complications/outcomes | Total (n=38) | No TIPS (n=18) | TIPS (n=20) | P value |
|---|---|---|---|---|
| Operative MELD, median [IQR] | 14 [10, 16] | 11 [10, 15] | 15.5 [11.5, 17.0] | 0.01 |
| Operative CTP, median [IQR] | 8 [7, 10] | 7 [6, 8] | 10.0 [8.5, 10.5] | <0.001 |
| Operative time (min), median [IQR] | 180 [141, 234] | 197 [164, 260] | 147 [115, 202] | 0.01 |
| Laparoscopic or open, n (%) | 0.02 | |||
| Laparoscopic | 12 (31.6) | 9 (50.0) | 3 (15.0) | |
| Open | 26 (68.4) | 9 (50.0) | 17 (85.0) | |
| Operative blood loss (mL), median [IQR] | 100 [25, 350] | 125 [50, 350] | 100.0 [22.5, 350.0] | 0.61 |
| Intra- or post-operative blood transfusion, n (%) | 0.08 | |||
| No | 24 (63.2) | 14 (77.8) | 10 (50.0) | |
| Intra-operative | 8 (21.1) | 4 (22.2) | 4 (20.0) | |
| Post-operative | 3 (7.9) | 0 (0.0) | 3 (15.0) | |
| Both | 3 (7.9) | 0 (0.0) | 3 (15.0) | |
| Post-operative wound leak, n (%) | 0.91 | |||
| No | 34 (89.5) | 16 (88.9) | 18 (90.0) | |
| Yes | 4 (10.5) | 2 (11.1) | 2 (10.0) | |
| Post-operative wound infection, n (%) | 0.09 | |||
| No | 35 (92.1) | 18 (100.0) | 17 (85.0) | |
| Yes | 3 (7.9) | 0 (0.0) | 3 (15.0) | |
| Post-operative encephalopathy, n (%) | 0.84 | |||
| No | 29 (76.3) | 14 (77.8) | 15 (75.0) | |
| Yes | 9 (23.7) | 4 (22.2) | 5 (25.0) | |
| Post-operative complications, n (%) | 1.00 | |||
| No | 27 (71.1) | 13 (72.2) | 14 (70.0) | |
| Yes | 11 (28.9) | 5 (27.8) | 6 (30.0) | |
| Post-operative intra-abdominal abscess, n (%) | 0.23 | |||
| No | 35 (92.1) | 18 (100.0) | 17 (85.0) | |
| Yes | 3 (7.9) | 0 (0.0) | 3 (15.0) | |
| Anastomotic leak, n (%) | 1.00 | |||
| No | 37 (97.4) | 18 (100.0) | 19 (95.0) | |
| Yes | 1 (2.6) | 0 (0.0) | 1 (5.0) | |
| Post-operative systemic infection (sepsis, UTI, PNA), n (%) | 1.00 | |||
| No | 33 (86.8) | 16 (88.9) | 17 (85.0) | |
| Yes | 5 (13.2) | 2 (11.1) | 3 (15.0) | |
| Post-operative liver decompensation, n (%) | 0.49 | |||
| No | 36 (94.7) | 18 (100.0) | 18 (90.0) | |
| Yes | 2 (5.3) | 0 (0.0) | 2 (10.0) | |
| Post-operative ileus, n (%) | 1.00 | |||
| No | 34 (89.5) | 16 (88.9) | 18 (90.0) | |
| Yes | 4 (10.5) | 2 (11.1) | 2 (10.0) | |
| Post-operative required TIPS revision, n (%) | 1.00 | |||
| No | 37 (97.4) | 18 (100.0) | 19 (95.0) | |
| Yes | 1 (2.6) | 0 (0.0) | 1 (5.0) | |
| Post-operative ARDS, n (%) | 0.47 | |||
| No | 37 (97.4) | 17 (94.4) | 20 (100.0) | |
| Yes | 1 (2.6) | 1 (5.6) | 0 (0.0) | |
| Length of stay, median [IQR] | 8.0 [5.0, 20.0] | 8.0 [5.0, 14.0] | 8.0 [5.0, 24.5] | 0.36 |
| Overall mortality, n (%) | 0.45 | |||
| Alive | 32 (84.2) | 16 (88.9) | 16 (80.0) | |
| Dead | 6 (15.8) | 2 (11.1) | 4 (20.0) | |
| Cause of death, n (%) | 0.59 | |||
| Multiorgan failure, sepsis | 4 (10.5) | 1 (5.6) | 3 (15.0) | |
| Progression of tumor | 2 (5.3) | 1 (5.6) | 1 (5.0) | |
| Bleeding | 1 (2.6) | 0 (0.0) | 1 (5.0) | |
| Not applicable | 31 (81.6) | 16 (88.9) | 15 (75.0) | |
| One month survival, n (%) | 0.19 | |||
| No | 5 (13.2) | 1 (5.6) | 4 (20.0) | |
| Yes | 33 (86.8) | 17 (94.4) | 16 (80.0) | |
| One year survival, n (%) | 0.45 | |||
| No | 6 (15.8) | 2 (11.1) | 4 (20.0) | |
| Yes | 32 (84.2) | 16 (88.9) | 16 (80.0) |
Values are as frequency and % for categorical variables and median [IQR] for continuous variables. IQR, interquartile range; MELD, model for end-stage liver disease; CTP, Child-Turcotte-Pugh; UTI, urinary tract infection; PNA, pneumonia; ARDS, acute respiratory distress syndrome; TIPS, transjugular intrahepatic portosystemic shunt.
In order to try and elucidate if TIPS timing had effect on outcome, we performed the same analysis on patients who underwent TIPS within 30 and 90 days of surgery to try and control for TIPS placed for reasons other than acute perioperative need (Tables S1-S8, Figures S1-S4) These additional analyses revealed no significant differences.
Indications for and types of surgery
The indications for and the types of surgery performed are summarized on Table 3. The most frequent indications for surgery in the TIPS group were strangulated hernia (50%) and colon cancer (40%); whereas the most frequent indications for surgery in the non-TIPS group were acute cholecystitis (55.6%), and colon cancer (22.2%). When further comparing the groups, the type of surgery was significantly different in the two groups. The majority in the TIPS group underwent exploratory laparotomy (85%), whereas equal numbers of patients in no TIPS group underwent laparoscopy and exploratory laparotomy (P=0.02). When analysis of patients who underwent perioperative TIPS within 90 was done, the cohort decreased to 17 patients. When the analysis was limited to TIPS within 30 days, the cohort further contracted to 14 patients. Despite these changes in cohort size, the significant differences remained, with significantly higher numbers of open surgery in the TIPS group. The type of surgery also remained significantly different in the 30- and 90-day cohorts consistent with the aforementioned results.
Table 3
| Indication and type of surgery | Total (n=38) | No TIPS (n=18) | TIPS (n=20) |
|---|---|---|---|
| Indication for surgery, n (%) | |||
| Acute cholecystitis | 10 (26.3) | 10 (55.6) | 0 (0.0) |
| Colon cancer | 12 (31.6) | 4 (22.2) | 8 (40.0) |
| Strangulated hernia | 11 (28.9) | 1 (5.6) | 10 (50.0) |
| Gallstone pancreatitis | 1 (2.6) | 0 (0.0) | 1 (5.0) |
| Gallbladder polyp | 1 (2.6) | 1 (5.6) | 0 (0.0) |
| Pancreatic adenocarcinoma | 1 (2.6) | 1 (5.6) | 0 (0.0) |
| Cholangiocarcinoma | 1 (2.6) | 1 (5.6) | 0 (0.0) |
| Ovarian mass | 1 (2.6) | 0 (0.0) | 1 (5.0) |
| Type of surgery, n (%) | |||
| Cholecystectomy | 12 (31.6) | 11 (61.1) | 1 (5.0) |
| Right hemicolectomy | 11 (28.9) | 4 (22.2) | 7 (35.0) |
| Umbilical/incisional hernia repair | 9 (23.7) | 0 (0.0) | 9 (45.0) |
| Inguinal hernia repair | 2 (5.3) | 1 (5.6) | 1 (5.0) |
| Whipple | 1 (2.6) | 1 (5.6) | 0 (0.0) |
| Bile duct excision, hepaticojejunostomy | 1 (2.6) | 1 (5.6) | 0 (0.0) |
| Sigmoid colectomy | 1 (2.6) | 0 (0.0) | 1 (5.0) |
| Salphingo-opphorectomy | 1 (2.6) | 0 (0.0) | 1 (5.0) |
Values are as frequency and % for categorical variables. TIPS, transjugular intrahepatic portosystemic shunt.
TIPS placement
The interval between TIPS placement and abdominal surgery was variable and ranged from to same day to 190 days prior to surgery with a mean of 39 days. Mean pre-TIPS hepato-venous portal gradient (HVPG) was 16.5 mmHg and mean post-TIPS HVPG was 7.0 mmHg with a mean 50% improvement in HVGP. All but one patient had evidence of Clinically Significant Portal Hypertension (CSPH), defined by a HVPG ≥10 mm Hg. One patient had a HVPG of 9 mmHg who had TIPS placed for variceal bleeding. The procedure was technically successful in all patients. One patient developed hemoperitoneum and another had liver dcompensation following TIPS placement (Table 4).
Table 4
| Parameters | TIPS (n=20) |
|---|---|
| Mean duration between TIPS and abdominal surgery (days), mean ± SD | 39±52.8 |
| Pre-TIPS HVPG (mmHg), median [IQR] | 16.5 [12.0, 19.0] |
| Post-TIPS HVPG (mmHg), median [IQR] | 7.0 [6.0, 9.0] |
| Percentage change in gradient, median [IQR] | 0.5 [0.4, 0.6] |
| Complications from TIPS procedure, n (%) | |
| None | 17 (89.5) |
| Liver decompensation | 1 (5.3) |
| Hemoperitoneum | 1 (5.3) |
Values are as frequency and % for categorical variables and median [IQR]/mean ± SD for continuous variables. TIPS, transjugular intrahepatic portosystemic shunt; SD, standard deviation; HVPG, hepatovenous pressure gradient; IQR, interquartile range.
Outcomes
Post-operative mortality was compared over the first month following surgery and for up to 12 months in the two groups. Mortality at 1 month was not statistically different between the TIPS (20%) and non-TIPS groups (5.6%, P=0.19). The 1-year mortality was also not statistically different between the two groups: 20% vs. 11.1%, P=0.36. For patients undergoing TIPS within 30 or 90 days of surgery this also was not statistically significant. Cumulative survival rates, as shown in Figure 1, were similar in both groups (80% vs. 88.9%, P=0.45) at 1 year. The same lack of significant difference was also noted in the patients who underwent TIPS within 30 or 90 days, respectively.
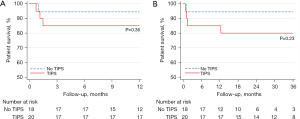
In total, 6 patients died (2 in the no TIPS group and 4 in the TIPS group). At 12-month follow-up, 1 in the no TIPS group died (with 5 other patients having follow-up time <12 months) vs. 3 in the TIPS group (P=0.36). The TIPS group had a numerically higher overall mortality (4 vs. 2, P=0.36). The four patients in the TIPS group who died had evidence of decompensated liver disease with 2 patients being CTP B and 2 being CTP C at the time of their surgery. Three patients died during hospitalization (multi-organ failure from sepsis, bleeding); all had undergone exploratory laparotomy and right hemi-colectomy for colon adenocarcinoma. The first of these patients had a pre-operative MELD score of 16 and underwent surgery 8 days following TIPS placement. Operative time was 180 minutes and blood loss 600 mL. The post-operative course was complicated by HE, anastomotic leak, progressive liver decompensation and sepsis and the patient died 35 days after surgery. The second patient had a pre-operative MELD score of 13 and underwent surgery 13 days after TIPS placement. Operative time was 143 minutes and blood loss 200 mL. Post-operative course was complicated by HE, intra-abdominal abscesses from a suspected anastomotic leak or bowel injury, and multi-organ failure from sepsis. The patient died 45 days after surgery. The third patient had pre-operative MELD score of 11 and underwent surgery 6 days after TIPS. Operative time was 385 minutes and significant intra-operative bleeding led to a blood loss of 1,500 mL. Post-operative course was complicated by pneumonia, sepsis and progressive liver decompensation. The patient died 22 days after surgery. The 4th patient who died following discharge had a laparoscopic umbilical hernia repair 3 days after TIPS placement. His hospital course was uneventful and was discharged after 1 week. He died from unrelated cardiogenic shock 11 months after surgery.
Two patients (1 CTP A, 1 CTP B) in the non-TIPS group died and both underwent exploratory laparotomy and right hemi-colectomy for colon cancer. The first patient’s pre-operative MELD score was 11. Operative time was 180 minutes and intra-operative course was uneventful. Post-operatively he developed HE with subsequent aspiration pneumonia, sepsis and multi-organ failure. He was discharged to hospice after 30 days from surgery and died the next day. The second patient had a pre-operative MELD score of 9. Operative time was 276 minutes and intra- and post-operative courses were uneventful. He was discharged 8 days after surgery but died 1 month later due to tumor progression.
The median operative time was statistically less in patients in the TIPS group (147 vs. 197 minutes, P=0.01) despite more undergoing laparotomy. Overall median operative blood loss was similar in both group (100 vs. 125 mL). Surprisingly, more patients in the TIPS group required either intra- or post-operative blood transfusion (10 vs. 4); however, this difference was not statistically significant (P=0.08). Overall, patients who underwent TIPS had numerically higher rates of post-operative wound infections (3 vs. 0), HE (5 vs. 4), confirmed anastomotic leaks (1 vs. 0), intra-abdominal abscesses (3 vs. 0), and post-operative liver decompensation (2 vs. 0); however, none of these differences were statistically significant (Table 2). One patient in the TIPS group had refractory encephalopathy and required TIPS revision. Rates for other miscellaneous complications such as ileus, systemic infections [pneumonia, urinary tract infection (UTI), sepsis] and respiratory failure were also similar in both groups. The median length of hospital stay was also similar in both groups (8 vs. 8 days).
Core tip
Patients with cirrhosis have a high risk for morbidity and mortality in relation to abdominal surgery; mortality can be as high as 50% in emergent surgeries. Despite improvements in surgical techniques and intensive care, major abdominal surgery still remains a challenge. We performed a retrospective chart review of patients who underwent elective TIPS at Houston Methodist Hospital. These patients were compared with a cohort of cirrhotic patients who underwent any abdominal surgeries without TIPS placement. We did not find any statistically significant difference in mortality or rate of post-operative complications between those who received pre-operative TIPS and those who did not in our age-matched cohort; however, TIPS may have allowed patients to proceed with surgery who may have not been surgical candidates.
Discussion
In our study, we did not find any statistically significant differences in mortality or rate of post-operative complications between those who received pre-operative TIPS and those who did not in our comparison cohort. These findings correlate with observations made by Vinet and colleagues in their retrospective study (17). We did, however, record a higher absolute mortality in the TIPS group. This lack of benefit may reflect differences between the two cohorts. Despite our attempt to match the groups, we still ended up with higher MELD/CTP scores in the TIPS group than in with those who did not have a TIPS. Theoretically, placing TIPS prior to surgery is thought to be viable option to improve surgical outcome in a subset of patients with decompensated cirrhosis. A recently published prospective study evaluating risk factors for patients with cirrhosis undergoing surgery showed that an HVPG >16 mmHg was associated with a high risk of post-surgical mortality (19). The evidence for TIPS efficacy and safety has been sparse, with few studies to date (15,16). In a recent meta-analysis involving 2 case reports, 5 case series (2–7 patients) and 1 retrospective comparative study yielding a total of 43 patients, those who underwent prophylactic TIPS were found to experience zero mortality in the perioperative period following abdominal surgery. No major abdominal bleeding attributable to PHTN was reported for this cohort. One patient had poor wound healing post-surgery (4.2%), one had right heart failure (4.2%), and five developed HE (20.8%) post-surgery (20). The meta-analysis did not have a comparator arm and only used outcomes in the perioperative periods to define success. In our study, rates of mortality were higher in the perioperative period, but only one of the deaths in the TIPS groups was related to liver failure using the criteria applied by Jain et al. (20). In contrast to previously published series, patients in our series receiving TIPS had higher CTP and MELD scores at the time of abdominal surgery. This may help to explain the numerically higher number of deaths recorded in this patient group. It may have been that TIPS was used to allow decompensated cirrhotic patients to undergo an operation. The 3 patients who died in the hospital after TIPS all underwent operations for colonic tumors. In contrast, laparoscopic cholecystectomy was the most common operation in the non-TIPS group. It is possible that the pre-operative TIPS may have permitted patients who otherwise would have been considered too high risk for surgery to have an operation and thus contributed to the rate of complications.
We feel that a major strength of our study was the use of a comparator group to try and better determine if TIPS is beneficial in reducing post-operative mortality. The comparison of these groups does add important findings to the literature as only one other series has compared these types of patients. We acknowledge that matching between the groups was not perfect. We attempted to match the group in terms of demographic characteristic and etiology of cirrhosis. However, we encountered difficulty in matching all the attributes due to the rarity of these types of surgeries, which resulted in significant differences in the type of surgery (laparoscopy vs. laparotomy), CTP scores, as well as the indications for surgery in the two groups. The study is somewhat limited in that it is unclear if portal pressures were different at the time of surgery as gradients were not available from the majority of the patients in non-TIPS group. However, indirect indicators of a clinically significant portosystemic gradient, such as ascites, thrombocytopenia, encephalopathy and history of variceal bleeding were equally frequent in the two groups. It is possible that patients in the TIPS group had significantly higher portosystemic gradients compared to the non-TIPS and placing a TIPS might have helped to decrease portal pressure to the extent that post-operative outcomes were comparable. To overcome this limitation, we performed multivariate analysis and found no differences in outcome after adjusting for CTP score.
The timing of TIPS placement was variable in our group with a range of zero to 190 days. We had concerns that true perioperative TIPS may have different results than a TIPS placed for another indication. However, limiting our analysis to those who underwent TIPS within 30 or 90 days of surgery did not affect the comparison of outcomes (Tables S1-S8, Figures S1-S4). Similar significant difference were also see in the smaller TIPS cohorts as compared to the groups as a whole. While TIPS placement may immediately reduce the risk of bleeding due to a reduction in portosystemic gradient, the benefit of a TIPS may be seen more distantly. The resolution of ascites (largely dependent on natriuresis), improvement of metabolic function and nutritional status, that may take up to several weeks or longer, may play critical roles in outcomes following surgery.
Conclusions
With the increasing prevalence of chronic liver disease, improved long term survival, and ever lengthening liver transplantation waiting times, a growing number of patients with chronic liver disease are likely to need abdominal surgical procedures. The challenge, therefore, is to implement effective and safe perioperative measures to improve morbidity and mortality. The present study suggests that pre-operative TIPS placement does not improve mortality after non-hepatic abdominal surgery in patients with decompensated cirrhosis. However, TIPS may allow those with more advanced liver disease (according to CTP score) to become surgical candidates. More evidence is needed from prospective randomized controlled trials to determine optimal criteria for patient selection, timing of TIPS placement, and selection of appropriate stent size to allow its safe use with concomitant improvement in perioperative morbidity and mortality.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-21-133/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-21-133/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-21-133/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-21-133/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Houston Methodist Hospital IRB committee (IRB Number: Pro00018198). Informed consent was not required as this was a retrospective chart review using de-identified patient information.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zarski JP, Bichard P, Rachail M. Extrahepatic digestive surgery in the cirrhotic patient. J Chir (Paris) 1988;125:597-600. [PubMed]
- Aranha GV, Greenlee HB. Intra-abdominal surgery in patients with advanced cirrhosis. Arch Surg 1986;121:275-7. [Crossref] [PubMed]
- Mansour A, Watson W, Shayani V, et al. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery 1997;122:730-5; discussion 735-6. [Crossref] [PubMed]
- Metcalf AM, Dozois RR, Wolff BG, et al. The surgical risk of colectomy in patients with cirrhosis. Dis Colon Rectum 1987;30:529-31. [Crossref] [PubMed]
- Jakab F, Ráth Z, Sugár I, et al. Complications following major abdominal surgery in cirrhotic patients. Hepatogastroenterology 1993;40:176-9. [PubMed]
- Sirinek KR, Burk RR, Brown M, et al. Improving survival in patients with cirrhosis undergoing major abdominal operations. Arch Surg 1987;122:271-3. [Crossref] [PubMed]
- del Olmo JA, Flor-Lorente B, Flor-Civera B, et al. Risk factors for nonhepatic surgery in patients with cirrhosis. World J Surg 2003;27:647-52. [Crossref] [PubMed]
- Kadry Z, Schaefer EW, Shah RA, et al. Portal Hypertension: An Underestimated Entity? Ann Surg 2016;263:986-91. [Crossref] [PubMed]
- Azoulay D, Buabse F, Damiano I, et al. Neoadjuvant transjugular intrahepatic portosystemic shunt: a solution for extrahepatic abdominal operation in cirrhotic patients with severe portal hypertension. J Am Coll Surg 2001;193:46-51. [Crossref] [PubMed]
- Boyer TD, Haskal ZJAmerican Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005;41:386-400. [Crossref] [PubMed]
- Gil A, Martínez-Regueira F, Hernández-Lizoain JL, et al. The role of transjugular intrahepatic portosystemic shunt prior to abdominal tumoral surgery in cirrhotic patients with portal hypertension. Eur J Surg Oncol 2004;30:46-52. [Crossref] [PubMed]
- Norton SA, Vickers J, Callaway MP, et al. The role of preoperative TIPSS to facilitate curative gastric surgery. Cardiovasc Intervent Radiol 2003;26:398-9. [Crossref] [PubMed]
- Liverani A, Solinas L, Di Cesare T, et al. Preoperative trans-jugular porto-systemic shunt for oncological gastric surgery in a cirrhotic patient. World J Gastroenterol 2015;21:997-1000. [Crossref] [PubMed]
- Kim JJ, Dasika NL, Yu E, et al. Cirrhotic patients with a transjugular intrahepatic portosystemic shunt undergoing major extrahepatic surgery. J Clin Gastroenterol 2009;43:574-9. [Crossref] [PubMed]
- Schlenker C, Johnson S, Trotter JF. Preoperative transjugular intrahepatic portosystemic shunt (TIPS) for cirrhotic patients undergoing abdominal and pelvic surgeries. Surg Endosc 2009;23:1594-8. [Crossref] [PubMed]
- Tabchouri N, Barbier L, Menahem B, et al. Original Study: Transjugular Intrahepatic Portosystemic Shunt as a Bridge to Abdominal Surgery in Cirrhotic Patients. J Gastrointest Surg 2019;23:2383-90. [Crossref] [PubMed]
- Vinet E, Perreault P, Bouchard L, et al. Transjugular intrahepatic portosystemic shunt before abdominal surgery in cirrhotic patients: a retrospective, comparative study. Can J Gastroenterol 2006;20:401-4. [Crossref] [PubMed]
- Rössle M. TIPS: 25 years later. J Hepatol 2013;59:1081-93. [Crossref] [PubMed]
- Reverter E, Cirera I, Albillos A, et al. The prognostic role of hepatic venous pressure gradient in cirrhotic patients undergoing elective extrahepatic surgery. J Hepatol 2019;71:942-50. [Crossref] [PubMed]
- Jain D, Mahmood E. Preoperative elective transjugular intrahepatic portosystemic shunt for cirrhotic patients undergoing abdominal surgery. Ann Gastroenterol 2018;31:330-7. [Crossref] [PubMed]
Cite this article as: Patel P, Irani M, Graviss EA, Nguyen DT, Quigley EMM, Victor DW 3rd. Impact of pre-operative transjugular intrahepatic portosystemic shunt on post-operative outcomes following non-transplant surgeries in patients with decompensated cirrhosis. Transl Gastroenterol Hepatol 2023;8:9.


