
Giant cell hepatitis associated with autoimmune hemolytic anemia: an update
Introduction
Giant hepatitis associated with autoimmune hemolytic anemia (GCH-AHA) is a rare disease characterized by autoimmune hemolysis associated with acute liver injury histologically defined by widespread giant cell transformation (1,2). It is a severe and progressive disease exclusively affecting infants and young children. Mechanism of liver injury is hypothesized to be secondary to an autoimmune process, however, GCH-AIH has a less favorable response to immunosuppressive treatment compared to classical juvenile autoimmune hepatitis (JAIH) and it is burdened by high mortality despite aggressive immunosuppression and recurrence after liver transplant (Table 1).
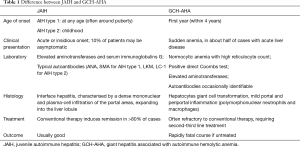
Full table
It was first described in 1981 by Bernard et al., who reported four young children with giant cell hepatitis and Coombs-positive hemolytic anemia of the immunoglobulin G-positive (IgG+) C type (1). Although three of the patients died of liver failure, in one child immunosuppressive treatment with prednisone and azathioprine was beneficial. Since its first description, 54 cases have been reported in the English medical literature from Europe, North America, Asia, Africa and Oceania mainly in the forms of single case reports and more recently as small case series.
Insight into GCH-AHA pathogenesis
In the neonatal period the transformation of hepatocytes into giant multinucleated cells is considered a non-specific reaction of immature hepatocytes. Outside of the neonatal era, this condition occurs more rarely and it can be idiopathic or secondary to different forms of aggression such as viral (Paramixovirus, HCV, HEV, HHV-6, HIV), autoimmune and toxic (herbal remedies) insults and in some genetic cholestasis (PFIC 2). Regardless of the etiology it is considered a poor prognostic factor (1,2).
Although autoantibodies classically seen in autoimmune hepatitis are usually absent, GCH-AHA is thought to be immune-mediated due to (I) the constant association with an autoimmune disease (hemolytic anemia) and occasionally with other immune-mediated disorders; (II) the strong family history of immune-mediated disorders in fist degree relatives; (III) the response to immunosuppressive treatment and relapse on its withdrawal (3).
However, GCH-AGA liver disease does not share the same histologic characteristics as JAIH. Specifically, classical interface hepatitis is rarely present and the portal infiltrate of GCH-AHA consists of macrophages and neutrophils rather than of lymphocytes and monocytes suggesting a different pathogenesis (1,2,4-7).
In 2014, Whitington et al. significantly advanced the understanding of this rare disease (4). Given that gestational alloimmune liver disease (GALD), the cause of liver injury in neonatal hemochromatosis is also characterized by a widespread giant cell transformation, these authors hypothesized that a similar humoral immune mechanism is responsible for the similarity in liver histopathology in GCH-AHA and GALD. In the latter, maternal IgG bind to fetal liver target antigens resulting in pathological amounts of C5b-9 complex on the hepatocytes and driving the progressive hepatocyte injury. In order to test their hypothesis, the authors compared liver biopsies of 6 patients with GCH-AHA and 6 patients with JAIH.
Interestingly, C5b-9 staining showed high-grade complement- mediated pan-lobular hepatocyte injury in GCH-AHA patients, which was not seen in the JAIH ones. Such finding suggested that systemic B-cell autoimmunity has a fundamental role in GCH-AIH. Further studies will be helpful to give more insight into GCH-AHA pathogenesis.
Clinical and biochemical manifestations
GCH-AHA occurs after the neonatal period, most commonly in the first year of life and within 4 years of age and it affects both sexes indistinctively. Onset is usually sudden with signs and symptoms of anemia (pallor, asthenia, occasionally fever) that, in about half of patients, are associated with findings suggestive of acute liver disease. In less than 10% of patient a seizure disorder is present early on or during follow up. Regardless of type of onset, nearly all patients at diagnosis have jaundice and hepatomegaly, and about half of them have fever, pallor and splenomegaly. When the diagnosis of anemia precedes that of hepatitis, liver disease is diagnosed within 1 month to 4 years (median 2 months); however, interestingly, about 90% of these patients already have evidence of increased aminotransferase levels at diagnosis.
An infectious process such as acute otitis media, urinary infection, Mycoplasma, EBV, VZV, and Parvovirus B19 infection, was found to precede GCH-AHA diagnosis in about a quarter of patients.
Blood work is consistent with normocytic anemia with high reticulocyte count (although a transient reticulocytopenia may be observed in some patients) and positive direct Coombs test (IgG + C’). Mild leukocytosis and thrombocytosis are common, but thrombocytopenia can also occur. Aminotransferases levels can be markedly elevated to almost 200 times the upper limit of normal with GGT within reference range or only mildly elevated. Total bilirubin and its direct fraction are usually elevated and in case of severe liver damage decreased PT and albuminemia occur. Total gamma globulin levels can be high, usually moderately, or even normal. Typical JAIH autoantibodies such as ANA, SMA, LKM are only identifiable occasionally (1,2,4-7).
Standard of diagnosis: role of currently performed liver tests in assessing GCH-AHA
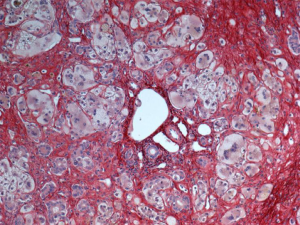
Unfortunately, no laboratory test specific for the diagnosis of GCH-AHA exists. However, GCH-AHA must always be suspected when evaluating a patient in the first year of life presenting with AHA and/or with acute liver disease of unknown etiology (1,2). A direct Coombs test should be ordered even if overt anemia or reticulocytosis are lacking and any increase in aminotransferase level should be taken into account even if mild when in presence of hemolytic autoimmune anemia. Liver biopsy helps making the diagnosis due to the presence of the characteristic widespread giant cell transformation of the hepatocytes (Figure 1), and the mild portal and periportal inflammation with an infiltrate consisting predominantly of polymorphonuclear neutrophils and macrophages. Fibrosis can be severe, with distortion of the lobular architecture. The association with other immune-mediated disorders, which can be observed at any stages of the disease, can also point toward GCH-AHA.
Existing and emerging therapies
GCH-AHA is a serious, potentially lethal disease, even with prompt diagnosis and treatment. Main causes of mortality are liver failure, complications of liver transplantation, bacterial sepsis, and epileptic encephalopathy.
Conventional first line therapy for GCH-AHA, as reported since the first description by Bernard et al. in 1981, consisted of prednisone/prednisolone (in some case IV methylprednisolone) at 2–3 mg/kg/day, combined with azathioprine (1 to 2 mg/kg/day) (1,2).
Prednisone has been used as first line treatment with varying degrees of success and with high risk of relapses during tapering, as well as severe side effects related to its prolonged use.
Azathioprine has been used in association with steroids as a steroid-sparing agent leading, in some cases, to complete remission (1,2,5,6,8). However, the clinical course of GCH-AHA is generally aggressive. In most patients there is an initial response to first line therapy, but relapses occur commonly, and second line therapy is required. Several immunosuppressive drugs, commonly used in the treatment of JAIH, have been tried as second line therapy for GCH-AHA, such as cyclosporine (2,7,9-19), cyclophosphamide (2,11), mycophenolate mofetil (10,12,14,15,20,21), 6-mercaptopurine (14,22), tacrolimus (10,15,16,20) and sirolimus (15,16,23), not always successfully (Table 2).
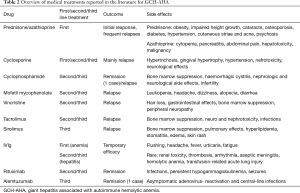
Full table
GCH-AHA has been associated with high mortality, related not only to severe liver failure or anemia, but also to sepsis in the setting of high-dose immunosuppressive drugs use.
In a few children with persistent hemolysis, plasmapheresis (7,17,20) and splenectomy (2,10,17,24,25) have been reported. Plasmapheresis can lead to a temporary improvement in severe AHA, possibly by removing antibodies and toxic substances.
In case of severe liver dysfunction and/or severe anemia, transitory remission has been achieved with intravenous immunoglobulins (IVIg) (2,9-11,13-15,17-23,26-29). IVIg has been used mainly at replacement dose and in single administration, for treating AHA before the onset of liver disease or in case of hepatitis relapses. The largest series of patients treated with IVIg has been reported by Marsalli et al. (16). The authors described 7 patients: three already treated with different immunosuppressive drugs, who received IVIg for GCH-AHA relapse, and four newly diagnosed. All patients received one to three IVIg infusions at variable doses (0.5 to 2 g/kg) and five of the seven patients received sequential IVIg infusions (mean of 13.4 doses) once a month, in addition to their immunosuppressive treatment. Of these latter 5 patients, three normalized ALT activity allowing prednisone tapering, while in the other two, ALT decreased but not normalized and immunosuppressive therapy was maintained, in one case with rituximab. IVIg infusions were well-tolerated with mild, and reversible side effects. This study supports the use of IVIg as second line rescue therapy in the presence of severe liver disease and/or hepatitis relapse, in association with prednisone and other immunosuppressive drugs, due to their temporary efficacy (Table 2).
Since, Whitington et al. (4) hypothesized a humoral immune mechanism for hepatocyte injury in patients with GCH-AHA, encouraging results have been reported in several refractory cases treated with B-cell depletion with chimeric anti-CD20 monoclonal antibody (rituximab) and anti-CD52 monoclonal antibody (alemtuzumab) (2,4,7,12-16,21,23,27,30). Because both of these drugs have anti-B-cell actions, the favorable outcomes of the treated patients seems to confirm that humoral immunity plays a key role in the mechanism of liver injury in GCH-AHA.
As proposed by Paganelli et al. (15), relapses of disease are possible during rituximab treatment because depletion of B lymphocytes is not complete with survival of memory B cells. In fact, in the cases reported, patients often need repeated infusions to treat and or prevent relapses, even if the exact duration of treatment and the frequency of infusions remains to be defined.
As of now 22 patients have been reported in literature as being treated with RTX (2,4,7,12-16,21,23,27,30). RTX was administered either for hepatic and/or hematologic relapse (375 mg/m2 once a week for 4 weeks) often with multiple infusions. Rituximab allowed, in most patients, tapering or discontinuation of others immunosuppressive drugs, most commonly prednisone. Rituximab was usually well tolerated and the most significant side effects include persistent hypogammaglobulinemia during RTX infusions in two patients, one episode of seizures secondary to possible viral encephalitis one year after the last RTX infusion and one asymptomatic adenovirus reactivation with central-line infection in a patient who received rituximab and alemtuzumab treatment (12,15,30) (Table 2).
In cases of liver failure, liver transplantation has been used as a rescue treatment; however, transplant is burdened by risk of recurrence of hepatitis in the graft and death (2,4,9,24,31-33). Of the 11 transplant recipients reported in the literature, 6 are alive (data concerning follow-up were available only for 10 patients) but only 2 did not have recurrence of GCH-AHA (32,33). Moreover, one of these patients developed bullous pemphigoid and autoimmune neutropenia after liver transplant (33).
Proposed management
Two forms of GCH-AHA can be identified: a mild form that usually responds to first line treatment with prednisone and azathioprine and a more severe form often refractory to conventional therapy and characterized by multiple relapses, requiring second line treatment with different immunosuppressive drugs and/or rescue liver transplant and, unfortunately, often with a rapidly fatal course.
Immunosuppressive therapy should be instituted as soon as possible and tapered very slowly due to the high risk of relapses. No clear indications regarding the duration of immunosuppressive therapy are reported. Based on literature data and similarly to JAIH prednisone and azathioprine withdrawal could be attempted after at least 3 to 5 years of persistent and stable remission, monitoring serum transaminases before each attempt of tapering. Periodic controls of blood cell counts, direct Coombs test and serum aminotransferase activities are recommended for several years after treatment discontinuation.
We proposed the following approach based on the current state of knowledge:
- Immediately start first line therapy with prednisone and azathioprine. Prednisone should be maintained at standard dose until normalization of serum transaminases, followed by a very slowly steroid tapering while keeping azathioprine at maintenance dose. Steroids should be stopped only after several years of persisting normal aminotransferases because early or rapid discontinuation can result in severe relapse;
- In light of the humoral mechanism of GCH-AHA and the reported efficacy of rituximab, the anti-CD20 monoclonal antibody can be used as the treatment of choice from the outset in case of severe presentation or when steroids and azathioprine appear ineffective. At least 4 doses of RTX are initially required (375 mg/m2 once a week for 4 weeks), with additional doses in case of relapses or incomplete remission. Early administration of rituximab can lead to complete remission, allowing for early steroid withdrawal, thus decreasing steroid-related side effects. In case of partial response to the initial treatment, or in case of a relapse after an initially complete remission of hepatitis repeating RTX, increasing the dose of prednisone and/or adding cyclosporine or IVIg infusions are options that can be used to allow for a complete remission of hepatitis. In case of lack of response but preserved liver function, rituximab could be continued with the hope of a slow improvement over time while in case of liver failure, liver transplantation should be considered;
- Although IVIg treatment is limited by its temporary efficacy, IVIg could be administered as a rescue therapy both at diagnosis, in case of severe liver function impairment prior to RTX administration, or in case of relapse, for instance while attempting discontinuation of immunosuppressive drugs.
Medium and long-term outcomes
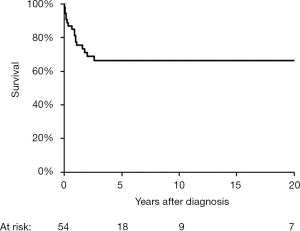
Despite the fact that GCH-AHA has a severe and progressive nature as other autoimmune liver disorders, it differs significantly from JAIH in that a true cure can occur after several years of intensive treatment in a portion of patients. Of the 54 children described in literature who underwent treatment, 36 are alive at the time of report (Figure 2). Deaths occurs within 3 years of the diagnosis of hepatitis, secondary to liver failure, sepsis, hemophagocytosis, status epilepticus or liver transplantation (1,2,4,5,9,14,20,22,27,28). In about a third of the survivors immunosuppressive treatment is withdrawn without relapse of the liver disease after a median treatment duration of 6 years (range, 1.5–19 years) (2,6,7,11,12). Liver histology prior to treatment withdrawal in 10 children shows a range of finding from a virtually normal liver, to residual portal or septal fibrosis and an occasional giant cells (2,11,12). Although numbers are small, such results point toward a cautiously hopeful prognosis for some of these patients.
Conclusions and future directions
In conclusion, GCH-AHA is a rare disease of early childhood, which is still poorly understood and likely underdiagnosed. A dysregulation of the immune response to multiple organs and tissues is thought to be at the root of this disorder, which can be transient and limited to two targets (red blood cells and hepatocytes), or can persist over time and possibly extend to other organs. Optimal treatment of GCH-AHA is still controversial, although recent progress in the understanding of its pathogenetic mechanisms have positively influenced its treatment and in turn its prognosis.
Acknowledgments
The authors thank professor Olivier Bernard for suggestions in writing the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Luca Fabris and Mario Strazzabosco) for the series “Recent Advances in Rare Liver Diseases” published in Translational Gastroenterology and Hepatology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tgh.2020.03.10). The series “Recent Advances in Rare Liver Diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bernard O, Hadchouel M, Scotto J, et al. Severe giant cell hepatitis with autoimmune hemolytic anemia in early childhood. J Pediatr 1981;99:704-11. [Crossref] [PubMed]
- Maggiore G, Sciveres M, Fabre M, et al. Giant cell hepatitis with autoimmune hemolytic anemia in early childhood: long-term outcome in 16 children. J Pediatr 2011;159:127-32.e1. [Crossref] [PubMed]
- Nastasio S, Sciveres M, Maggiore G. Should Giant Cell Hepatitis With Autoimmune Hemolytic Anemia Be Considered a Pediatric Autoimmune Liver Disease? J Pediatr Gastroenterol Nutr 2018;66:e137. [Crossref] [PubMed]
- Whitington PF, Vos MB, Bass LM, et al. Humoral immune mechanism of liver injury in giant cell hepatitis with autoimmune hemolytic anemia. J Pediatr Gastroenterol Nutr 2014;58:74-80. [Crossref] [PubMed]
- Brichard B, Sokal E, Gosseye S, et al. Coombs-positive giant cell hepatitis of infancy: effect of steroids and azathioprine therapy. Eur J Pediatr 1991;150:314-7. [Crossref] [PubMed]
- Raj S, Stephen T, Debski RF. Giant cell hepatitis with autoimmune hemolytic anemia: a case report and review of pediatric literature. Clin Pediatr (Phila) 2011;50:357-9. [Crossref] [PubMed]
- Gorelik M, Debsky R, Frangoult H. Autoimmune hemolytic anemia with giant cell hepatitis: case report and review of the literature. J Pediatr Hematol Oncol 2004;26:837-9. [PubMed]
- Eroğlu Y, Düzovali O, Kavukçu S, et al. Combination of steroid with azathioprine in treatment of giant cell autoimmune hepatitis. Turk J Pediatr 1997;39:565-71. [PubMed]
- Melendez HV, Rela M, Baker AJ, et al. Liver transplant for giant cell hepatitis with autoimmune haemolytic anaemia. Arch Dis Child 1997;77:249-51. [Crossref] [PubMed]
- Hadzic N, Portmann B, Lewis I, et al. Coombs-positive giant cell hepatitis - a new feature of Evans’ syndrome. Arch Dis Child 1998;78:397-8. [Crossref] [PubMed]
- Vajro P, Migliaro F, Ruggeri C, et al. Life saving cyclophosphamide treatment in a girl with giant cell hepatitis and autoimmune haemolytic anaemia: case report and up-to-date on therapeutical options. Dig Liver Dis 2006;38:846-50. [Crossref] [PubMed]
- Rovelli A, Corti P, Beretta C, et al. Alemtuzumab for giant cell hepatitis with autoimmune hemolytic anemia. J Pediatr Gastroenterol Nutr 2007;45:596-9. [Crossref] [PubMed]
- Baran M, Özgenç F, Berk Ö, et al. Giant cell hepatitis and autoimmune hemolytic anemia after chickenpox. Turk J Gastroenterol 2010;21:448-51. [Crossref] [PubMed]
- Bakula A, Socha P, Klaudel-Dreszler M, et al. Giant cell hepatitis with autoimmune hemolytic anemia in children: proposal for therapeutic approach. J Pediatr Gastroenterol Nutr 2014;58:669-73. [Crossref] [PubMed]
- Paganelli M, Patey N, Bass LM, et al. Anti-CD20 treatment for giant cell hepatitis with autoimmune hemolytic anemia. Pediatrics 2014;134:e1206-10. [Crossref] [PubMed]
- Marsalli G, Nastasio S, Sciveres M, et al. Efficacy of intravenous immunoglobulin therapy in giant cell hepatitis with autoimmune hemolytic anemia: a multicenter study. Clin Res Hepatol Gastroenterol 2016;40:83-9. [Crossref] [PubMed]
- Imgrueth M, Wagner HP, Pipczynski-Suter K, et al. Plasma exchange: an important part of the therapeutic procedure in a small child with autoimmune hemolytic anemia. Acta Paediatr Scand 1986;75:1037-41. [Crossref] [PubMed]
- Saadah OI, Smith AL, Hardikar W. Long-term outcome of autoimmune hepatitis in children. J Gastroenterol Hepatol 2001;16:1297-302. [Crossref] [PubMed]
- Lega S, Maschio M, Taddio A, et al. Giant cell hepatitis with Coombs-positive haemolitic anemia: steroid sparing with high dose intravenous immunoglobulin and cyclosporine. Acta Paediatr 2013;102:137-9. [Crossref] [PubMed]
- Hartman C, Berkowitz D, Brik R, et al. Giant cell hepatitis with autoimmune hemolytic anemia and hemophagocytosis. J Pediatr Gastroenterol Nutr 2001;32:330-4. [Crossref] [PubMed]
- Shores D, Kobak G, Pegram LD, et al. Giant cell hepatitis and immune thrombocytopenic purpura:reversal of liver failure with rituximab therapy. J Pediatr Gastroenterol Nutr 2012;55:e128-30. [Crossref] [PubMed]
- Perez-Atayde AR, Sirlin SM, Jonas M. Coombs-positive autoimmune hemolytic anemia and postinfantile giant cell hepatitis in children. Pediatr Pathol 1994;14:69-77. [Crossref] [PubMed]
- Miloh T, Manwani D, Morotti R, et al. Giant cell hepatitis and autoimmune hemolytic anemia successfully treated with rituximab. J Pediatr Gastroenterol Nutr 2007;44:634-6. [Crossref] [PubMed]
- Adrian-Casavilla F, Reyes J, Tzakis A, et al. Liver transplantation for neonatal hepatitis as compared to the other two leading indications for liver transplantation in children. J Hepatol 1994;21:1035-9. [Crossref] [PubMed]
- Choulot JJ, Parent Y, Etcharry F, et al. Giant cell hepatitis and autoimmune hemolytic anemia: efficacy of splenectomy on hemolysis. Arch Pediatr 1996;3:789-91. [Crossref] [PubMed]
- Kashyap R, Sarangi JN, Choudhry VP. Autoimmune hemolytic anemia in an infant with giant cell hepatitis. Am J Hematol 2006;81:199-201. [Crossref] [PubMed]
- Ünal Ş, Kuşkonmaz B, Balamtekin N, et al. Autoimmune hemolytic anemia and giant cell hepatitis: Report of three infants. Turk J Haematol 2010;27:308-13. [Crossref] [PubMed]
- Bouguila J, Mabrouk S, Tilouche S, et al. Giant cell hepatitis with autoimmune hemolytic anemia in a nine month old infant. World J Hepatol 2013;5:226-9. [Crossref] [PubMed]
- Lachaux A, Bertrand Y, Bouvier R, et al. Intravenous immunoglobulin therapy in an infant with autoimmune haemolytic anemia associated with necrotic hepatits and peliosis. J Pediatr Gastroenterol Nutr 1996;22:99-102. [Crossref] [PubMed]
- Matarazzo L, Di Chio T, Nastasio S, et al. B-cell depletion induces prolonged remission in patients with giant cell hepatitis and autoimmune hemolytic anemia. Clin Res Hepatol Gastroenterol 2020;44:66-72. [Crossref] [PubMed]
- Pappo O, Yunis E, Jordan JA, et al. Recurrent and de novo giant cell hepatitis after orthotopic liver transplantation. Am J Surg Pathol 1994;18:804-13. [Crossref] [PubMed]
- Akyildiz M, Karasu Z, Arikan C, et al. Successful liver transplantation for giant cell hepatitis and Coombs-positive hemolytic anemia: a case report. Pediatr Transplant 2005;9:630-3. [Crossref] [PubMed]
- Kerkar N, Cohen S, Dugan C, et al. Bullous pemphigoid after liver transplantation for liver failure. Liver Transpl 2006;12:1705-10. [Crossref] [PubMed]
Cite this article as: Nastasio S, Matarazzo L, Sciveres M, Maggiore G. Giant cell hepatitis associated with autoimmune hemolytic anemia: an update. Transl Gastroenterol Hepatol 2021;6:25.


