
Clinical and laboratory parameters associated with li-rads as diagnostic of liver nodule in patients with cirrhosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common types of cancer in the world (1). Cirrhosis of the liver is the main risk factor for its development; chronic viral hepatitis the most common cause of HCC (2). Most patients are diagnosed at the advanced and intermediate stages of the Barcelona Clinic Liver Cancer (BCLC) classification, for which palliative treatments are advised (3).
Cirrhotic patients with clinical decompensation may raise suspicion of HCC. The clinical presentation depends on the stage of the liver disease and on the stage of the tumor. In general, the symptomatology may be related to tumor growth (abdominal pain or constitutional symptoms—weight loss, abdominal distension, nausea, anorexia) or liver disease decompensation, increased alkaline phosphatase (ALP), bilirubin (BT), and alpha-fetoprotein (AFP), recent-onset ascites, acute intra-abdominal bleeding, encephalopathy and hematemesis/melena (4,5). AFP has a prognostic value, but is not sufficiently accurate for screening and diagnosis. The American Association for the Study of Liver Diseases (AASLD) recommends screening using ultrasonography with or without AFP for HCC in cirrhotic patients every 6 months (6).
The diagnosis of hepatic lesions can be made using imaging methods (7). The liver imaging reporting and data system (LI-RADS) was developed with the aim of standardizing the interpretation of computed tomography (CT) and magnetic resonance imaging (MRI) of patients at risk of developing HCC cancer. CT and MRI are the definitive diagnostic methods for HCC. LI-RADS aims to aid interpretation and communication among physicians (8). LI-RADS also provides an algorithm that categorizes liver nodules from definitely benign (LR 1) to clearly HCC (LR 5). The definition of indeterminate nodules may be broad and could be benign or require follow-up without a biopsy. LR 1 and LR 2 include benign and probably benign categories, which encompass cysts, hemangiomas, perfusion abnormalities, hepatic fat deposition, and areas of fibrosis or scarring. LI-RADS expands the “indeterminate” category in relation to intermediate and probable HCC (LR categories 3 and 4, respectively). Other classifications of LI-RADS, such as LR-NC, involve cases in which categorization is not possible, owing to degradation or omission of the imaging sequence. LR-TIV categorizes a definition of tumors in veins, LR-M categorizes nodules that are probably cancer but is not specific for HCC. LR 5 involves hepatic nodules that are definitely HCC (9). The main imaging features characterized and used in this diagnostic algorithm include image hyper-reduction in the arterial phase, tumor size (diameter), portal phase washout, enhancement and nature of the capsule and the growth pattern of the nodule (8). LI-RADS has already been validated in Brazil by the Brazilian College of Radiology and Diagnostic Imaging, but is still very rarely used. The aim of the present study was to identify the clinical and laboratory characteristics of cirrhotic patients with hepatic nodules applying the LI-RADS algorithm categorization.
Methods
Patient selection
A cross-sectional and observational study and group comparison were carried out with 74 (Figure 1) cirrhotic patients with hepatic nodules undergoing CT or MRI with contrast between 2017 and 2018, at Hospital Oswaldo Cruz/University, Pernambuco and the Liver and Transplantation Institute of Pernambuco.
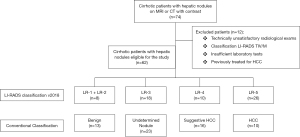
Patients aged under 18 years were excluded from the study, along with those classified according to the LI-RADS as LR-M or LR-TIV algorithm, those who did not provide the clinical, laboratory and radiological data needed to analyze the proposed variables and those who underwent any treatment of the hepatic nodule, be it surgery, chemoembolization or radio-ablation.
The radiological findings used for diagnosis of hepatic cirrhosis were presence of heterogeneity and hepatic surface nodularity, hypertrophy of the caudate lobe, segmental atrophy in the right lobe, signs of portal hypertension, and others described in the literature (10,11).
Patients eligible for the study were categorized according to the LI-RADS algorithm and classified into four groups: Group 1 (LR 1 + LR 2, definitely and probably benign, respectively) 13% (n=8), Group 2 (LR 3, n=18), Group 3 (LR 4, probably HCC), 16% (n=10) and Group 4 (LR 5, definitely HCC) 42% (n=26).
The necessary clinical, radiological and laboratory patient information was collected from medical records. These were registered age, ascites, encephalopathy, portal hypertension, diabetes, history of alcoholism and/or smoking, and MELD and CHILD calculations performed. Laboratory tests were carried out within a maximum of 30 days between collection and radiological imaging. The laboratory analysis included aspartate aminotransferase (AST), alanine aminotransferase (ALT), ALP, gamma glutamyl transferase (GGT), BT, AFP, platelets and albumin (ALB). The serological profile included serology for hepatitis B (HBsAg, anti-HBs, total anti-HBc) and hepatitis C (anti-HCV). Diagnosis of hepatitis C was carried out using HCV-RNA screening in patients testing positive for anti-HCV. Patients were also evaluated for nodule size and number hepatic nodules being classified as uni- or multinodular (≥2 nodules).
All patients agreed to participate in the study by signing a consent form. The present study was approved by the local ethics committee (UPE-HUOC) under CAAE number: 82265517.4.0000.5207.
Radiological images
The radiological examinations were conducted using the following contrast devices: a Philips MRI, Model: Achieva 1.5, Channels: 16 and a Philips CT, Model: Ingenuity, Channels: 128. The digital storage system was PACS (Onis®, JP).
Interpretation of images
Conventional radiological analysis versus LI-RADS v2018 algorithm
Two radiologists, with radiology experience and no access to the clinical and laboratory results of the patients, analyzed the radiological images. The classification of hepatic nodules was performed on two different occasions after MRI or CT with contrast. The first evaluation was performed by conventional diagnosis, classifying the hepatic nodule according to AASLD guidelines, currently the most commonly used in Brazil, and the second classification, conducted by another radiologist, followed the LIRADS/v2018 algorithm.
According to AASLD criteria, HCC can be diagnosed radiologically by CT or MRI scans without biopsy if typical imaging features are present (6). HCC is detected in contrast-enhanced CT or MRI scans when nodular hepatic lesions present a specific vascular profile of hyper-enhancement during the arterial phase and washout during the venous and/or late portal phases. In the arterial phase, the HCC shows greater intensity compared to the surrounding liver parenchyma, whereas in its venous phase it has lower intensity (12,13).
LI-RADS version 2018 provides updated criteria for small LR-5 nodules (10–19 mm and a simplified definition of threshold growth. These updates represent an important milestone in achieving consistency and integration of the clinical practice guidelines for AASLD hepatocellular carcinoma in 2018. The classification of hepatic nodules according to the LI-RADS algorithm includes five major criteria. (I) Hyper-enhancement of the nodule in the arterial phase reflects the angiogenesis process, a key component of HCC pathogenesis (14,15). (II) Washout can be evaluated in the portal or late venous phase if an extracellular contrast agent is administrated in MRI or CT This is one of the most reliable features. (III) “Capsule appearance” or “capsule” is defined as a uniform and pointed hyper-enhancement ring around most or all of a nodule (16). The degree of enhancement typically increases from early phases to late, reflecting the slow flow of intracapsular vessels. (IV) Size of the hepatic nodule is defined as the dimension of the greatest length from external to outer border including the capsule, if present. (V) Threshold growth is defined as ≥50% increase in mass size in ≤6 months (14,17).
Statistical analysis
The existence of associations between categorical variables was evaluated using Pearson’s Chi-square test and Fisher’s exact test. Differences were considered significant at P<0.05. The magnitude of these associations was estimated by odds ratio (OR), using 95% confidence intervals. For comparison of continuous variables, between the two groups, Student’s t-test or the non-parametric Mann-Whitney test was applied, and for comparison between more than two groups were applied ANOVA or Kruskal Wallis test was used, as appropriate. The PRISMStatistics v.6.0 program (GraphPad Software, San Diego, California, USA) was used for these analyzes. Logistic regression was employed to adjust the OR for possible confounding factors, using the SPSS V.22 program. The Kappa concordance coefficient was used to describe the agreement between the radiological reports performed by the conventional method and the reports performed following the LI-RADS v2018 algorithm on the same sample. A kappa value of 0 indicates no agreement, kappa values of 0.01–0.20 represent slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 good, almost perfect agreement 0.81–0.99 and 1 perfect agreement (18).
Results
Clinical features
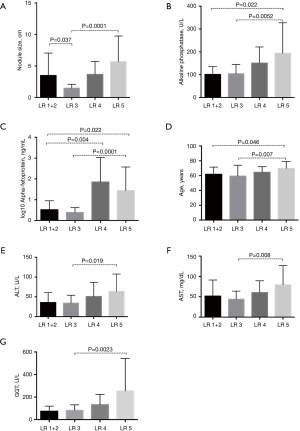
The final study sample included 62 cirrhotic patients with a hepatic nodule, 30 (48%) women and 32 (52%) men. Patients were divided into groups according to categorization of radiological images according to the LI-RADS algorithm (LR 1+2, LR 3, LR 4 and LR 5). clinical characteristics (gender, portal hypertension, ascites, diabetes mellitus, smoking and/or alcoholism, HBV, HCV, CHILD and MELD), and number of nodules, and possible association with HCC was investigated. Of the patients covered, HCV was found in 41.93% (n=26), HBV in 8% (n=5), ascites in 43.5% (n=27), portal hypertension in 64.5% (n=40), encephalopathy in 1.6% (n=1), DM in 29% (n=18), a history of alcoholism in 46.7% (n=29) and a history of smoking in 19.3% (n=12). The mean age in years for the groups was LR 1 and 2: 62±9.8 years, LR 3: 59±14.9 years, LR 4: 64±7.7 years and LR 5: 70±9.4 years (Table 1). There was a statistically significant positive correlation for the age variable in LR 5 group compared to the LR 1+2 groups (P=0.046) and also when compared to LR 3 (P=0.007). Nodule size showed a statistically significant correlation with larger nodules in LR 1+2 vs. LR 3 group (P=0.037) and in LR 3 vs. LR 5. There were larger nodules in the LR 5 group when compared to the LR 3 group (P=0.0001).
Table 1
| Clinical features (N=62) | LR 1+2 (N=8) | LR 3 (N=18) | LR 4 (N=10) | LR 5 (N=26) | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| LR 1+2 vs. LR 3 | LR 1+2 vs. LR 4 | LR 1+2 vs. LR 5 | LR 3 vs. LR 5 | LR 4 vs. LR 5 | |||||
| Males (n=32), n [%] | 4 [50] | 9 [50.0] | 5 [50] | 14 [53.8] | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Age, years, mean ± SD | 62±9.8 | 59±14.9 | 64±7.7 | 70±9.4 | 0.667 | 0.530 | 0.046 | 0.007 | 0.118 |
| Nodules, n [%] | 0.667 | 0.99 | 0.99 | 0.75 | 0.99 | ||||
| Uninodular | 4 [50] | 12 [66.7] | 6 [60] | 15 [57.7] | |||||
| Multinodular | 4 [50] | 6 [33.3] | 4 [40] | 11 [42.3] | |||||
| Size larger nodule (cm), median [range] | 2.55 [1–12] | 1.4 [0.6–2.5] | 3.05 [0.9–7.2] | 4.5 [1–17] | 0.037 | 0.614 | 0.065 | 0.0001 | 0.208 |
| HBV (n=5), n [%] | 0 | 3 [16.7] | 1 [10] | 1 [3.8] | – | – | – | – | 0.484 |
| HCV (n=26), n [%] | 2 [25] | 4 [22.2] | 4 [40] | 16 [61.5] | 0.99 | 0.638 | 0.110 | 0.370 | 0.285 |
| Ascites (n=27), n [%] | 4 [50] | 7 [38.9] | 3 [30] | 13 [50.0] | 0.682 | 0.630 | 0.99 | 0.546 | 0.456 |
| PH (n=40), n [%] | 6 [75] | 12 [66.7] | 7 [70] | 15 [57.7] | 0.99 | 0.99 | 0.443 | 0.750 | 0.706 |
| Encephalopathy (n=1), n [%] | 0 | 1 [5.6] | 0 | 0 | – | – | – | – | – |
| DM (n=18), n [%] | 2 [25] | 6 [33.3] | 2 [20] | 8 [30.8] | 0.601 | 0.99 | 0.99 | 0.99 | 0.689 |
| Alcoholic/ex-alcoholic (n=29), n [%] | 4 [50] | 9 [50.0] | 6 [60] | 10 [38.5] | 0.67 | 0.541 | 0.285 | ||
| Smoking/ex-smoker (n=12), n [%] | 0 | 3 [16.7] | 3 [30] | 6 [23.1] | – | – | – | – | 0.685 |
| MELD, median [range] | 7 [6–12] | 9 [6–17] | 10 [6–20] | 9 [6–16] | 0.205 | 0.177 | 0.114 | 0.95 | 0.495 |
| Child#, n [%] | |||||||||
| A (n=34) | 4 [50] | 11 [61.1] | 6 [60] | 13 [50.0] | |||||
| B (n=17) | 2 [25] | 3 [16.7] | 2 [20] | 10 [38.5] | 0.99 | 0.99 | 0.99 | 0.419 | 0.438 |
| C (n=2) | 0 | 1 [5.6] | 1 [10] | 0 | |||||
#, Child-Pugh score. LI-RADS, liver imaging reporting and data system; HCV, hepatitis C virus; HBV, hepatitis B virus; PH, portal hypertension; DM, diabetes mellitus.
The Child classification was evaluated in only 53 patients, owing to incomplete data, and the patients were classified as follows: Child A 64.2% (n=34), B 32% (n=17) and C 3.8% (n=2) (Table 1). No statistically significant difference between the groups in this study was observed for the other clinical variables.
Laboratory characteristics
The laboratory variables classified according to groups classified after radiological imaging using the LI-RADS algorithm presented statistical significance with progressively higher serum levels for the LR 5 group when compared to the LR 3 group for GGT (P=0.0023), AFP (P=0.0001), ALP (P=0.0052), AST (P=0.008) and ALT (P=0.019) (Table 2).
Table 2
| Laboratory data | LR 2+1 (N=8) | LR 3 (N=18) | LR 4 (N=10) | LR 5 (N=26) | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| LR 1+2 vs. LR 3 | LR 1+2 vs. LR 4 | LR 1+2 vs. LR 5 | LR 3 vs. LR 5 | LR 4 vs. LR 5 | |||||
| Albumin (g/dL), mean ± SD | 4±0.5 | 3.9±0.7 | 3.7±0.5 | 3.65±0.7 | 0.814 | 0.227 | 0.221 | 0.168 | 0.936 |
| Bilirubin total, (mg/dL), median (range) | 0.93 (0.46–6.7) | 1.03 (0.3–3.7) | 1.47 (0.4–6.9) | 1.11 (0.59–8.4) | 0.987 | 0.714 | 0.473 | 0.362 | 0.977 |
| Alkaline phosphatase (U/L), median [range] | 92.7 [73–173] | 100 [36–164] | 152 [54–233] | 160 [72–577] | 0.857 | 0.228 | 0.0228 | 0.0052 | 0.664 |
| GGT (U/L), median [range] | 80 [28–756] | 72.5 [19–191] | 99 [12–284] | 159 [21–1,298] | 0.962 | 0.489 | 0.117 | 0.0023 | 0.192 |
| INR, mean ± SD | 1.07±0.13 | 1.22±0.19 | 1.30±0.28 | 1.15±0.21 | 0.079 | 0,065 | 0.364 | 0.207 | 0.098 |
| AFP (ng/mL), median [range] | 2.92 [0.92–23] | 2.65 [0.84–6.18] | 30.7 [3.0–4.498.0] | 19.5 [0.9–51,000.0] | 0.503 | 0.004 | 0.022 | 0.0001 | 0.371 |
| ALT (U/L), median [range] | 35 [12,9–87] | 30 [11–86] | 35 [15–113] | 48 [17–159] | 0.964 | 0.403 | 0.073 | 0.019 | 0.431 |
| AST (mg/dL), median [range] | 43.5 [15.5–125] | 43 [22–71] | 62.5 [17–94,7] | 57 [22–191] | 0.883 | 0.468 | 0.140 | 0.008 | 0.248 |
| Platelets (mg/dL), median [range] | 111,000 |
147,000 |
135,000 |
123,000 |
0.424 | 0.982 | 0.584 | 0.337 | 0.413 |
LI-RADS, liver imaging reporting and data system; GGT, gamma glutamyl transferase; INR, international standard ratio; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The ALP analysis presented statistical significance of higher serum levels for the LR 5 group when compared to LR 1+2 (P=0.0228) and LR 3 (P=0.0052). The AFP in the present study showed higher serum levels for the LR 4 group compared to LR 1+2 (P=0.004) and higher serum levels for the LR 5 group compared to LR 1+2 (P=0.022) and LR 3 (P=0.0001) (Figure 2).
Kappa analysis
Kappa index analysis was performed to ascertain whether there was concordance correlation between conventional diagnosis and LI-RADS. The result for Group D (conventional diagnosis—HCC vs. LR 5), was kappa 0.328, P=0.007 and kappa 0.369, P<0.0001 was observed for Group C (conventional-suspected diagnosis HCC vs. LR 4) and Group D (Tables 3,4).
Table 3
| Conventional radiological diagnosis | LR 1+2 | LR 3 | LR 4 | LR 5 | P value Kappa category |
|---|---|---|---|---|---|
| Benign nodule, N=13 [%] | 4 [30.7] | 7 [53.8] | 2 [15.5] | 0 | 0.061 |
| Undetermined nodule, N=23 [%] | 5 [22] | 9 [39] | 2 [8.6] | 7 [30.4] | 0.179 |
| Suggestive HCC, N=16 [%] | 0 | 1 [6] | 6 [38] | 9 [56] | 0.007 |
| HCC, N=10 [%] | 0 | 1 [10] | 0 | 9 [90] | <0.001 |
| Total =62 | 9 [14.5] | 18 [29] | 10 [16.1] | 25 [40.4] |
LI-RADS, liver imaging reporting and data system; HCC, hepatocellular carcinoma.
Table 4
| Classification | A | B | C | D | Kappa general |
|---|---|---|---|---|---|
| Kappa category (95% CI) | 0.232 (0.475–0.011) | 0.168 (0.413–0.077) | 0.328 (0.567–0.09) | 0.369 (0.576–0.162) | 0.274 (0.409–0.139) |
| P value Kappa | 0.061 | 0.179 | 0.007 | <0.001 | <0.001 |
Group A: Conventional (Undetermined)
Multivariate analysis
A binary logistic regression of the forward stepwise type was performed to check for possible predictors of HCC in the LR 5 and LR 3 groups, including the variables that were significant in univariate analysis (age, GGT, AST, ALT, AFP, ALP and nodule size). After the test, only nodule size (P=0.047) and ALP (P=0.027) were predictors of HCC diagnosis (Table 5).
Table 5
| Variables* | P value | OR | 95% CI |
|---|---|---|---|
| Size of nodule | 0.047 | 1.104 | 1.001–1.218 |
| Alkaline phosphatase | 0.027 | 1.036 | 1.004–1.069 |
*, variables included in the equation: age, GGT, AST, ALT, AFP. HCC, hepatocellular carcinoma; GGT, gamma glutamyl transferase; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
The present study evaluated the relation between clinical, laboratory and radiological factors and the LI-RADS algorithm on diagnosis of HCC. This is one of the first studies to evaluate these factors together. Recently, the incidence of HCC has been increasing exponentially. Clinical practice shows that analysis of individual patients is fundamental. Factors related to liver disease, liver function, portal hypertension, comorbidities and the tumor should all be considered.
Univariate analysis showed that the occurrence of HCC was statistically significant for age >50 years, hepatic nodule size and heightened levels of enzymes such as ALP, GGT, AFP, ALT and AST. Age presented data similar to those described in literature, revealing a progressive increase in age for groups with the greatest likelihood of occurrence of HCC. Another study evaluating HCC mortality conducted in Brazil revealed that 84.7% of patients were aged 50 years or older (19). The multivariate analysis of the present study also revealed that a larger size of the hepatic nodule and higher levels of ALP were strong predictors of HCC occurrence.
The size of the nodule presented a statistical correlation for LR 1+2 vs. LR 3 and LR 3 vs. LR 5, although the size of the nodule in the LR 1+2 group was higher in relation to LR 3. This may be explained by the presence of a 12 cm hepatic hemangioma in a patient with LR 1 classification. Multivariate analysis of LR 5 and LR 3 patients demonstrated that larger nodule size was associated with the occurrence of HCC, independent of other confounding factors. Pawlik et al. in a multicenter study of 1,073 HCC patients, observed that tumor size and number of nodules were associated with microvascular invasion, capsular invasion, tumor size, satellite nodules, invasive behavior, and that tumor size can predict its histological grade (20). Tumor size is an independent risk factor for HCC patient survival and prognosis. Genetic alterations and pathological features are associated with tumor size in HCC (21,22). The presence of tumor recurrence and metastasis may be more commonly observed in patients with larger HCC.
Analysis of prognostic factors or biomarkers that help predict survival and make clinical decisions is urgently needed. Some serum hepatic enzymes, ALT, AST, ALP, GGT and AFP, are routinely required in cirrhotic patients with hepatic nodules. Of these, ALP and GGT are not as widely used as AFP, which has been reported as a predictor in the diagnosis of HCC, although with low sensitivity (23,24). ALP, a hydrolase enzyme, may be associated with tumor patient prognosis and is found mainly in the liver, bile duct and bone (24). Serum ALP levels increase during pregnancy or under some pathological conditions, including cholangiocarcinoma, HCC, biliary cirrhosis, and liver injury (25). Some tumor cells exhibited heightened ALP activity in the nucleolus during cell cycles, and ALP may play an important role in cell cycle regulation, cell proliferation and tumor formation (26). The participation of ALP in tumor formation represents both a direct and an indirect inflammatory reaction and this may be predictive of the prognosis in HCC patients (27).
Xu et al. in a study with 172 HCC patients undergoing hepatic resection, followed up for 10 years, found that ALP, GGT and tumor size were independent predictors of lower overall survival and tumor-free survival. A further in-depth analysis showed that patients with heightened levels of ALP and GGT had significantly greater risk of death and tumor recurrence according to the Kaplan-Meier analysis, indicating a potential predictive role of ALP and GGT in the prognosis of HCC patients after liver resection (28). ALP is also one of the fundamental elements in the HCC staging system used by the Chinese University’s Prognostic Index (CUPI) (29).
Abnormal expression of GGT has been found in a number of human tumors (30). GGT is found in healthy adults and is secreted primarily by Kupffer hepatic cells and endothelial cells of the bile duct. Its expression increases in tissue with HCC. It has been reported that GGT sensitivity is 74.0% for the detection of larger size HCC and 43.8% for smaller-size HCC. Sensitivity can be significantly increased by simultaneous determination of GGT and AFP (31). GGT is an oxidative stress flag related to the origin of pro-oxidant reactions and producing endogenous reactive hydrogen species in tumor cells, and plays an important role in the formation of tumors, cell proliferation and apoptosis (32-34). GGT catalyzes the transpeptidation and hydrolysis of the glutamyl group of glutathione and participates in biotransformation, nucleic acid metabolism and genesis (35). In addition to being easily obtained in routine tests, ALP and GGT can also predict the prognosis in patients with HCC (36). Overall, the results of the studies showed that high levels of ALP and GGT had a close relationship with tumor recurrence, formation and progression (27).
The relative risk of HCC increases with the severity of liver damage, indicated by heightened levels of ALT and AST. A cohort study conducted in 2013 with 5,555 men in Taiwan showed that increased levels of liver enzymes were associated with overall mortality and cancer mortality, in particular HCC-related mortality. Increased liver enzymes, AST or ALT or GGT, were independently associated with higher overall cancer mortality and HCC-specific mortality (37).
AFP is a fetal component protein produced during the embryonic period by the visceral endoderm of the gestational sac and subsequently by the liver (38). In the present study, AFP-related univariate analysis showed a statistically significant difference between LR 1+2 groups vs. LR 4, LR 1+2 vs. LR 5, and LR 3 vs. LR 5. AFP has been routinely used to aid diagnosis of HCC. Heightened levels may be a poor prognostic factor in HCC patients. Progressively higher levels of AFP have been associated with a more aggressive molecular subclass of HCC and abnormal or altered genesis of hepatic cells (39,40).
The present study also found a concordance correlation between conventional diagnosis and LI-RADS using Kappa index analysis. A significant correlation was observed only in group C (conventional-suspected diagnosis HCC vs. LR 4) and Group D (conventional diagnosis—HCC vs. LR 5), kappa 0.328, P=0.007 and kappa 0.369, P<0.0001, respectively (Tables 3,4). The 2018 version of LI-RADS is more judicious regarding AASLD criteria alone for non-invasive diagnosis of HCC in high-risk patients, providing important and complementary information on the likelihood of HCC and enabling possible changes in management of these patients.
LI-RADS was created as a dynamic system with regular updates to maintain best practices based on the latest evidence and specialized multidisciplinary consensus. LI-RADS is consistent with the National Comprehensive Cancer Network (NCCN) guidelines. The new version of LI-RADS has now been integrated into the 2018 AASLD guidelines for clinical practice. This represents an important step towards general endorsement of LI-RADS (17).
The fundamental importance of the present study lies in the evaluation of factors associated with HCC and the LI-RADS algorithm. However, the limitation relating to the low number of patients in our series may have affected the power of the analysis and consequently the extrapolation of results. Studies with a larger sample of patients are needed to confirm the role of the markers in HCC evolution to be incorporated in diagnostic and prognostic HCC algorithms.
Acknowledgments
Thanks to the entire team mainly involved and the patients who agreed to participate and made possible this research.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tgh.2020.01.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients agreed to participate in the study by signing a consent form. The present study was approved by the local ethics committee (UPE-HUOC) under CAAE number: 82265517.4.0000.5207. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gomes MA, Priolli DG, Tralhao JG, et al. Carcinoma hepatocelular: epidemiologia, biologia, diagnóstico e terapias. Rev Assoc Med Bras 2013;59:514-24. [Crossref] [PubMed]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Kikuchi LO, Paranagua-Vezozzo DC, Chagas AL, et al. Nodule less than 20mm and vascular invasion are predictors of survival in small hepatocellular carcinoma. J Clin Gastroenterol 2009;43:191-5. [Crossref] [PubMed]
- Ferenci P, Fried M, Labrecque DWorld Gastroenterology Organisation Guidelines and Publications Committee, et al. J Gastrointestin Liver Dis 2010;19:311-7. [PubMed]
- Ginès P, Fernández J, Durand F, Saliba F. Management of critically-ill cirrhotic patients. J Hepatol. 2012;56:S13-24. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Pimenta JF, Massabki PS. Carcinoma hepatocelular: um panorama clínico. Rev Bras Clin Med 2010;8:59-67.
- Liver Imaging Reporting and Data System, version 2013. American College of Radiology. Available online: www.acr.org/quality-safety/resources/lirads. Accessed April 4, 2018.
- Corwin MT, Fananapazir G, Michael J, et al. Differences in Liver Imaging and Reporting Data System Categorization Between MRI and CT. AJR Am J Roentgenol 2016;206:307-12. [Crossref] [PubMed]
- Brancatelli G, Federle MP, Ambrosini R, et al. Cirrhosis: CT and MR imaging evaluation. Eur J Radiol 2007;61:57-69. [Crossref] [PubMed]
- Hodler J, Kubik-Huch RA, von Schulthess GK. editors. Diseases of the Abdomen and Pelvis 2018-2021: Diagnostic Imaging - IDKD Book [Internet]. Cham (CH): Springer; 2018.
- Burrel M, Llovet JM, Ayuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology 2003;38:1034-42. [Crossref] [PubMed]
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104. [Crossref] [PubMed]
- Liver imaging reporting and data system 2018. Available online: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018Core.pdf?la=en. Accessed June 8, 2018.
- Efremidis SC, Hytiroglou P. The multistep process of hepatocarcinogenesis in cirrhosis with imaging correlation. Eur Radiol 2002;12:753-64. [Crossref] [PubMed]
- Ishigami K, Yoshimitsu K, Nishihara Y, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology 2009;250:435-43. [Crossref] [PubMed]
- Elsayes KM, Kielar AZ, Chernyak V, et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma 2019;6:49-69. [Crossref] [PubMed]
- Zidan M, Thomas RL, Slovis TL. What you need to know about statistics, part II: reliability of diagnostic and screening tests. Pediatr Radiol 2015;45:317-28. [Crossref] [PubMed]
- Amorim TR, Merchán-Hamann E. Mortality due to malignant neoplasms of the liver and intrahepatic bile ducts in Brazil, 1980-2010. Cad Saude Publica 2013;29:1427-36. [Crossref] [PubMed]
- Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086-92. [Crossref] [PubMed]
- Wong N, Lai P, Lee SW, et al. Assessment of genetic changes in hepatocellular carcinoma by comparative genomic hybridization analysis: relationship to disease stage, tumor size, and cirrhosis. Am J Pathol 1999;154:37-43. [Crossref] [PubMed]
- Lu XY, Xi T, Lau WY, et al. Pathobiological features of small hepatocellular carcinoma: correlation between tumor size and biological behavior. J Cancer Res Clin Oncol 2011;137:567-75. [Crossref] [PubMed]
- Lopez JB, Balasegaram M, Thambyrajah V, et al. The value of liver function tests in hepatocellular carcinoma. Malays J Pathol 1996;18:95-9. [PubMed]
- Yu MC, Chan KM, Lee CF, et al. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg 2011;15:1440-9. [Crossref] [PubMed]
- Williamson KD, Chapman RW. Editorial: further evidence for the role of serum alkaline phosphatase as a useful surrogate marker of prognosis in PSC. Aliment Pharmacol Ther 2015;41:149-51. [Crossref] [PubMed]
- Yamamoto K, Awogi T, Okuyama K, et al. Nuclear localization of alkaline phosphatase in cultured human cancer cells. Med Electron Microsc 2003;36:47-51. [Crossref] [PubMed]
- Wu SJ, Lin YX, Ye H, et al. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg 2016;36:143-51. [Crossref] [PubMed]
- Xu XS, Wan Y, Song SD, et al. Model based on γ-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis. World J Gastroenterol 2014;20:10944-52. [Crossref] [PubMed]
- Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer 2002;94:1760-9. [Crossref] [PubMed]
- Hanigan MH, Frierson HF Jr, Swanson PE, et al. Altered expression of gamma-glutamyl transpeptidase in human tumors. Hum Pathol 1999;30:300-5. [Crossref] [PubMed]
- Cui R, He J, Zhang F, et al. Diagnostic value of protein induced by vitamin K absence (PIVKAII) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to α-fetoprotein. Br J Cancer 2003;88:1878-82. [Crossref] [PubMed]
- Fu S, Guo Z, Li S, et al. Prognostic value of pre- operative serum gamma-glutamyltranspeptidase in patients with hepatocellular carcinoma after hepatectomy. Tumour Biol 2016;37:3433-40. [Crossref] [PubMed]
- Corti A, Franzini M, Paolicchi A, et al. Gammaglutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting Anticancer Res 2010;30:1169-81. [PubMed]
- Toyokuni S, Okamoto K, Yodoi J, et al. Persistent oxidative stress in cancer. FEBS Lett 1995;358:1-3. [Crossref] [PubMed]
- Ma H, Zhang L, Tang B, et al. γ-Glutamyltranspeptidase is a prognostic marker of survival and recurrence in radiofrequency-ablation treatment of hepatocellular carcinoma. Ann Surg Oncol 2014;21:3084-9. [Crossref] [PubMed]
- Guiu B, Deschamps F, Boulin M, et al. Serum gamma-glutamyl-transferase independently predicts outcome after trans- arterial chemoembolization of hepatocellular carcinoma: external validation. Cardiovasc Intervent Radiol 2012;35:1102-8. [Crossref] [PubMed]
- Hernaez R, Yeh H, Lazo M, et al. Elevated ALT and GGT predict all-cause mortality and hepatocellular carcinoma in Taiwanese male: a case-cohort study. Hepatol Int 2013;7:1040-9. [Crossref] [PubMed]
- Aoyagi Y, Suzuki Y, Igarashi K, et al. Carbohydrate structures of human alpha- fetoprotein of patients with hepatocellular carcinoma: presence of fucosylated and non-fucosylated triantennary glycans Br J Cancer 1993;67:486-92. [Crossref] [PubMed]
- Arrieta O, Cacho B, Morales-Espinosa D, et al. The progressive elevation of alpha- fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer 2007;7:28. [Crossref] [PubMed]
- Villanueva A, Minguez B, Forner A, et al. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annu Rev Med 2010;61:317-28. [Crossref] [PubMed]
Cite this article as: Cruz CR, Carvalho ARMR, Maranhão ACN, Aroucha DB, Foinquinos GA, Carvalho SRC, Vasconcelos LRS, Pereira LMMB. Clinical and laboratory parameters associated with li-rads as diagnostic of liver nodule in patients with cirrhosis. Transl Gastroenterol Hepatol 2021;6:55.


