
Pancreaticoduodenectomy after Roux-en-Y Gastric Bypass: a novel reconstruction technique
Background
The obesity epidemic continues to increase around the world with its attendant complications of metabolic syndrome and increased risk of malignancies (1), including pancreatic malignancy (2,3). The Roux-en-Y gastric bypass (RYGB) effectively treats obesity and its associated morbidities including metabolic syndrome (4). RYGB creates a gastric pouch with alimentary limb, as well as a biliopancreatic (BP) limb that joins the alimentary limb to form a common channel. Thus, it has both restrictive and mal-absorptive components. Patients with a mass in the head of pancreas are recommended pancreaticoduodenectomy (PD) for suspected malignancy (5). As bariatric surgery is increasingly adopted in recent years (6), more reports of PD following RYGB are published. Post-surgical adhesions and altered anatomy following RYGB poses not only a diagnostic challenge by making endoscopy difficult, but also unique challenge of reconstruction following PD. There are many different techniques of reconstruction proposed. Here we describe a patient where the remnant BP limb was used for a venting anterior gastrojejunostomy. The pancreaticojejunostomy and hepaticojejunostomy was created with a new loop of jejunum and a new distal jejunojejunostomy was performed.
Methods
A 59-year-old male presented with painless obstructive jaundice of one week’s duration. He had a history of RYGB for obesity performed 12 years ago at an overseas institution. His current BMI was 39 and medical comorbidities were type 2 diabetes mellitus, hypertension, hyperlipidaemia, hypertrophic cardiomyopathy and glaucoma. He also had previous Helicobacter pylori related gastric ulcer treated with triple therapy. On examination, there was icterus, and abdomen was non-tender with no masses felt. Serum biochemistry showed following elevated tests: bilirubin 102 µmol/L, alanine transaminase (ALT) 181 U/L, aspartate aminotransferase (AST) 153 U/L, alkaline phosphatase (ALP) 163 U/L and gamma-glutamyl transferase (GGT) 469 U/L. Carbohydrate antigen (CA) 19-9 was elevated at 186 µg/L. Magnetic resonance cholangiopancreatography (MRCP) showed proximal common duct and mild intrahepatic ductal dilatation with a stricture in the distal common bile duct (Figure 1). Computed tomography scan of the thorax, abdomen and pelvis showed no distant metastases. The case was discussed at multidisciplinary hepatopancreatobiliary board meeting and surgery was advised due to suspected malignant stricture. As his previous RYGB was done overseas and we had no access to operative records, definite surgical plans were only possible intra-operatively.
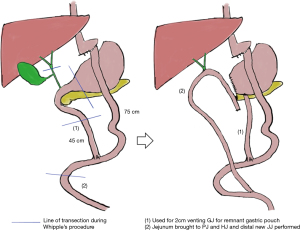
Open PD via a reverse-L incision was performed. Antecolic antegastric 75 cm long Roux limb and 45 cm BP limb were noted. Cholecystectomy and nodal dissection of hepatoduodenal ligament was done and the distal antrum transected. The common duct was isolated and divided above the cystic duct junction and hilar lymph nodes dissected. The pancreas was transected at level of pancreatic neck. Reconstruction was performed with an antecolic end-to-side hand-sewn two-layer duct-to-mucosa pancreatico-jejunostomy (PJ) over a custom-made pancreatic stent from a 4 Fr infant feeding tube and hand-sewn single layer interrupted end-to-side hepaticojejunostomy (HJ) was performed 7–8 cm downstream from PJ. The remnant jejunum from the resected BP limb was used to create a 2 cm hand-sewn anterior gastrojejunostomy for venting of the remnant isolated stomach which is not in continuity with gastrointestinal system. A new jejuno-jejunostomy (JJ) was created downstream to the previous JJ anastomosis. Figure 2 illustrates the original post-RYGB anatomy, line of transection during PD and the final anatomy. At the end of the surgery, the BP limb measured 50 cm, alimentary limb measured 150 cm and the common channel measured 140 cm. Operative duration was 545 minutes, blood loss was 700 mL and length of stay 7 days. Histology revealed choledocholithiasis with chronic ulceration and a neuroendocrine tumour within the head of pancreas. Patient remains well at 30 months follow-up.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
With the global epidemic of diabesity and metabolic syndrome (2), bariatric surgery is increasingly performed (6). Although sleeve gastrectomy (SG) is the most performed bariatric surgical procedure, many patients still undergo the RYGB (6). In patients with previous RYGB, the anatomic reconstruction after PD remains a challenge due to altered anatomy. Similar to various techniques of reconstruction in ‘classical’ PD, there is no consensus on the best method of reconstruction in PD following RYGB. Reports on PD after a previous RYGB are limited to single patient case reports and case series of handful patients. A review of the international literature revealed a total of 121 patients (7-15). A systematic review by Morano et al. identified 25 patients from three case reports and five case series, with authors describe five different reconstruction techniques (13). More recently, Trudeau et al. reported 96 patients with a previous RYGB who underwent PD from 55 pancreatic surgeons in 28 centres (16). In this cohort, 20 different reconstruction methods were described out of 37 possible choices, with no significant difference in outcome between the different reconstructive options. These choices include variations of the following main decisions by the pancreatic surgeon: (I) resection/preservation of remnant stomach and (II) resection/preservation of original biliopancreatic limb. The advantages and disadvantages of each option is summarized in Table 1.
Table 1
| Component | Surgical options | Advantages | Disadvantages |
|---|---|---|---|
| Remnant gastric stump | Preserving the remnant gastric stump | Nutritional access by gastrostomy tube is possible; possible importance to body’s endocrine and exocrine function; can use stomach for reconstruction, e.g., PG | Possible need to consider venting gastrostomy; risk of anastomotic leak if used for additional anastomosis; risk of marginal ulceration; the remnant gastric stump may obscure the operating field; risk of delayed gastric emptying |
| Resecting the remnant gastric stump | Greater exposure of the operating field; no additional anastomosis required; no risk of delayed gastric emptying; removes potential site for marginal ulceration or bleeding; eliminates risk of future gastric malignancy | Increased complexity of the surgery—additional dissection required to resect remnant stomach, with risk of injury to gastric pouch or Roux limb | |
| The original BP limb after specimen resection | Use for reconstruction (i.e., use for HJ, PJ) | Elimination of an added JJ anastomosis; avoids injury to the alimentary limb | Require sufficient length for original BP limb or anastomotic tension is imminent |
| Resecting original BP limb and new BP limb created | Ensures adequate length for tension-free PJ and HJ anastomosis | Additional dissection required to resect the original BP limb; need to decide fate of JJ anastomosis | |
| Use for other purpose, e.g., venting GJ and new BP limb created | Utilize the original BP limb as venting GJ—avoids the use of a gastrostomy tube | Additional JJ anastomosis may increase the risk of anastomotic leak |
PD, Pancreaticoduodenectomy; RYGB, Roux-en-Y gastric bypass; BP, Biliopancreatic; PJ, Pancreaticojejunostomy; PG, Pancreaticogastrostomy; HJ, Hepaticojejunostomy; JJ, Jejunojejunostomy; GJ, Gastrojejunostomy.
In our case, we described a method in which the remnant gastric stump was preserved, and BP drainage was accomplished with a new limb from a segment of normal jejunum distal to original JJ anastomosis. The distal part of original BP limb and original JJ anastomosis were preserved. The gastric remnant was drained into the distal portion of the original BP limb as an anterior venting gastrojejunostomy. This method was partly similar to that previously described by Rutkoski et al. (11), resulting into the formation of a “double-Y” configuration except that Rutkoski et al. described a posterior venting gastrojejunostomy with good short term outcomes. Their patient developed symptoms of Roux-limb obstruction and had a short overall survival of 9 months due to metastatic cancer. Despite the increased level of technical complexity associated with altered anatomy in RYGB patients, this did not significantly impact on peri-operative outcomes.
Another possible way to utilize the remnant stomach is for reconstruction. Younan et al. described a method in which the gastric remnant is preserved and used for pancreaticogastrostomy (PG) reconstruction and subsequently drained by the same jejunal limb used for the HJ (17). Tsamalaidze et al. reported that out of seven patients with stomach preservation, the remnant stomach was used for pancreatic reconstruction in three patients and two patients had insertion of transgastric jejunal feeding tube for nutrition (18). One of the potential benefits is a tension-free anastomosis due to the proximity of the posterior gastric wall to the pancreas with reduced risk of post-operative pancreatic fistula. However, meta-analysis of randomized controlled trials comparing PJ versus PG after PD found no significant difference in rates of post-operative pancreatic fistula but a higher rate of postpancreatectomy haemorrhage in the PG group (19). We routinely place a stent across PJ anastomosis along with two abdominal drains and are guided by drain fluid amylase for peri-operative care (20). Some authors advocate for remnant gastrectomy as the preferred option in patients with previous RYGB undergoing PD. Peng et al. reported 11 patients and recommend remnant gastrectomy with reconstruction using the BP limb (9), eliminating the need for an additional anastomosis and hence postoperative morbidity related to enteric leak or delayed gastric emptying. Trudeau et al. reported 96 patients in which 54.7% underwent gastric resection with no significant difference in peri-operative outcomes and morbidity (16). Gastric resection requires additional dissection with an increase in operating time. Moreover, in patients with prior RYGB, the presence of adhesions may render additional dissection difficult. Hence, the decision to perform to preserve or resect the remnant stomach should depend on the following factors: (I) ability to improve the exposure within operative field, and (II) presence of adhesions and difficulty of dissection.
After deciding on whether to perform gastric resection, the surgeon then has to decide on the BP limb. If the original BP limb is preserved, it may be utilized for reconstruction for PJ and HJ. An adequate length is required to ensure a tension-free anastomosis, and this should be decided by the surgeon intraoperatively. Kawamoto et al. described PD in seven patients with previous gastric resection, with three out of seven having Roux-en-Y configurations (21). The authors recommended at least 50cm of afferent limb for PJ and HJ to avoid afferent limb syndrome. Should there be insufficient length, then a new BP limb should be created from distal jejunum and a new jejuno-jejunostomy created. The original BP limb can then either be resected or utilized as a venting gastrojejunostomy (as in our patient) should the gastric remnant be preserved, hence avoiding the need for a tube gastrostomy.
Our case also highlights the difficulty in evaluating suspicious biliary lesions after RYGB reconstruction. Both surgical and non-surgical options exist. Non-surgical options include enteroscopy-assisted ERCP (e-ERCP) and endoscopic ultrasound (EUS)-guided gastrogastrostomy ERCP (EUS-GG-ERCP), although both require special equipment and technical expertise (22). Image-guided percutaneous biopsy may also be attempted. Surgical options include laparoscopic assisted ERCP (LAERCP) (23) and laparoscopically assisted EUS (LAEUS) or a combination of both (24). The decision to proceed with surgery in our patient was based on suspicious imaging findings and endorsed by multidisciplinary board. Up to 20% of patients undergoing surgery for suspected biliary malignancy can have benign pathology (5,25). In the cohort by Trudeau et al., ERCP was attempted in 20.8% of patients but successfully completed in less than half (46.7%) (16). This highlights the importance of thorough preoperative discussion with the patient on the available diagnostic and therapeutic options.
Conclusions
With the increase in number of bariatric procedures performed worldwide, pancreatic surgeons should be aware of the varied surgical reconstruction options available. There are pro and cons to each option and there is no “one-size-fits-all” approach and instead this should be tailored to the patient’s anatomy and pathology. We describe a novel technique of gastric preservation, using the distal original biliopancreatic limb for anterior venting gastrojejunostomy, creating a new Roux-limb from jejunum for pancreaticojejunostomy and hepaticojejunostomy and performing a second jejunojejunostomy distal to index jejunojejunostomy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh.2020.02.11/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794-8. [Crossref] [PubMed]
- Mitchell NS, Catenacci V, Wyatt HR, et al. Obesity: overview of an epidemic. Psychiatr Clin North Am 2011;34:717-32. [Crossref] [PubMed]
- Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog 2012;51:53-63. [Crossref] [PubMed]
- Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014;CD003641. [PubMed]
- Pandya G, Dixit R, Shelat V, et al. Obstructive jaundice: a manifestation of pancreatic tuberculosis. J Indian Med Assoc 2007;105:133-4, 136. [PubMed]
- Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg 2018;28:3783-94. [Crossref] [PubMed]
- Swain JM, Adams RB, Farnell MB, et al. Gastric and pancreatoduodenal resection for malignant lesions after previous gastric bypass--diagnosis and methods of reconstruction. Surg Obes Relat Dis 2010;6:670-5. [Crossref] [PubMed]
- de la Cruz-Muñoz N, Hartnett S, Sleeman D. Laparoscopic pancreatoduodenectomy after laparoscopic gastric bypass. Surg Obes Relat Dis 2011;7:326-7. [Crossref] [PubMed]
- Peng JS, Corcelles R, Choong K, et al. Pancreatoduodenectomy after Roux-en-Y gastric bypass: technical considerations and outcomes. HPB 2018;20:34-40. [Crossref] [PubMed]
- Nikfarjam M, Staveley-O’Carroll KF, Kimchi ET, et al. Pancreaticoduodenectomy in patients with a history of Roux-en Y gastric bypass surgery. JOP 2009;10:169-73. [PubMed]
- Rutkoski JD, Gagné DJ, Volpe C, et al. Pancreaticoduodenectomy for pancreatic cancer after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 2008;4:552-4; discussion 554-555. [Crossref] [PubMed]
- Theodoropoulos I, Franco C, Gervasoni JE. Pancreaticoduodenectomy for pancreatic carcinoma after complicated open Roux-en-Y gastric bypass surgery: an alternative approach to reconstruction. Surg Obes Relat Dis 2012;8:648-50. [Crossref] [PubMed]
- Morano WF, Shaikh MF, Gleeson EM, et al. Reconstruction options following pancreaticoduodenectomy after Roux-en-Y gastric bypass: a systematic review. World J Surg Oncol 2018;16:168. [Crossref] [PubMed]
- Helmick R, Singh R, Welshans J, et al. Pancreaticoduodenectomy after Roux-en-Y gastric bypass: A single institution retrospective case series. Int J Hepatobiliary Pancreat Dis 2013;17-20. [Crossref]
- Khithani AS, Curtis DE, Galanopoulos C, et al. Pancreaticoduodenectomy after a Roux-en-Y gastric bypass. Obes Surg 2009;19:802-5. [Crossref] [PubMed]
- Trudeau MT, Maggino L, Ecker BL, et al. Pancreatic Head Resection Following Roux-en-Y Gastric Bypass: Operative Considerations and Outcomes. J Gastrointest Surg 2020;24:76-87. [Crossref] [PubMed]
- Younan G, Tsai S, Evans DB, et al. A Novel Reconstruction Technique During Pancreaticoduodenectomy After Roux-En-Y Gastric Bypass: How I do It. J Gastrointest Surg 2017;21:1186-91. [Crossref] [PubMed]
- Tsamalaidze L, Asbun HJ, Costa RM, et al. Pancreaticoduodenectomy in Patients with Previous Roux-en-Y Gastric Bypass: a Matched Case-Control Study. Obes Surg 2020;30:369-73. [Crossref] [PubMed]
- Lyu Y, Li T, Cheng Y, et al. Pancreaticojejunostomy Versus Pancreaticogastrostomy After Pancreaticoduodenectomy: An Up-to-date Meta-analysis of RCTs Applying the ISGPS (2016) Criteria. Surg Laparosc Endosc Percutan Tech 2018;28:139-46. [Crossref] [PubMed]
- Serene TEL, Shelat VG, Padmakumar JS, et al. Predictive value of post-operative drain amylase levels for post-operative pancreatic fistula. Ann Hepatobiliary Pancreat Surg 2018;22:397-404. [Crossref] [PubMed]
- Kawamoto Y, Ome Y, Kouda Y, et al. Pancreaticoduodenectomy following gastrectomy reconstructed with Billroth II or Roux-en-Y method: Case series and literature review. Int J Surg Case Rep 2017;35:106-9. [Crossref] [PubMed]
- Bukhari M, Kowalski T, Nieto J, et al. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest Endosc 2018;88:486-94. [Crossref] [PubMed]
- Saleem A, Levy MJ, Petersen BT, et al. Laparoscopic assisted ERCP in Roux-en-Y gastric bypass (RYGB) surgery patients. J Gastrointest Surg 2012;16:203-8. [Crossref] [PubMed]
- Bowman E, Greenberg J, Garren M, et al. Laparoscopic-assisted ERCP and EUS in patients with prior Roux-en-Y gastric bypass surgery: a dual-center case series experience. Surg Endosc 2016;30:4647-52. [Crossref] [PubMed]
- Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (Oxf) 2015;3:22-31. [Crossref] [PubMed]
Cite this article as: Mak MHW, Shelat VG. Pancreaticoduodenectomy after Roux-en-Y Gastric Bypass: a novel reconstruction technique. Transl Gastroenterol Hepatol 2022;7:11.



