
Trends and outcomes of fungal infections in hospitalized patients of inflammatory bowel disease: a nationwide analysis
Introduction
Inflammatory bowel disease (IBD) is chronic inflammatory disorder comprising of Crohn’s disease and ulcerative colitis (UC). The pathogenesis is complex with various genetic, immunological and environmental factors playing role in the development of the disease (1). There is an emerging data over the last decade on the role of mycobiome of the gut in the pathogenesis of IBD (2,3). IBD itself is not considered an immunocompromised state however its treatment increases the risk of serious and opportunistic infections significantly (4,5). IBD treatment includes immunomodulators like steroids and azathioprine and biologic agents like anti-tumor necrosis factor (TNF) and anti-integrin monoclonal antibodies. Use of anti TNF agent doubles the risk of opportunistic infections in the IBD patients (6). Several studies have shown that the risk is highest with the anti TNF agents especially when they are combined with steroids or thiopurines (6-8). Studies on Vedolizumab however have shown the risk of opportunistic fungal infection was not higher compared to placebo (9).
Opportunistic fungal infections include but are not limited to Histoplasmosis, Pneumocystis, Cryptococcosis, Aspergillosis, Blastomycosis, Mucormycosis, Candidiasis and Coccidiomycosis (8). With prompt diagnosis and early administration of anti-fungal agents these opportunistic fungal infections can be treated successfully in most cases. However, opportunistic fungal infections in immunocompromised patients carry high mortality, increased health care related cost and increased days of hospitalizations (10). Study in IBD patients who were treated with anti-TNF agents showed that candidiasis and histoplasmosis are frequent opportunistic fungal infections in these patients (11). Current guidelines do not recommend giving primary prophylaxis for opportunistic fungal infections in IBD patients (5).
The incidence and prevalence of opportunistic fungal infections is lacking due to lack of large population data and rarity of the infections and thus obviating the need for large population-based study. We conducted this study to identify the epidemiologic factors in the development of opportunistic fungal infections in hospitalized patients having IBD. We also wanted to study the mortality trends and the factors which predicts the development of opportunistic fungal infections in IBD.
Methods
Database
We did retrospective analysis of National Inpatient Sample database. We used NIS data sets from 2002 to 2014 for our study. The NIS data set is a part of Healthcare Cost and Utilization Project (HCUP) which belongs to health care databases established by the Agency for Healthcare Research and Quality and each year contains >7 million hospitalizations. HCUP databases are based on data collected and composed by state level organizations, government, and private organizations. From 2012 NIS data has been restructured to represent 20% of stratified sample of all discharges from US community hospitals. Rehabilitation and long-term acute care hospitals have been excluded from the database. State Inpatient Databases (SIDs) provide all in-patient data to HCUP national database (12).
Study participants
We studied all the subjects with the discharge diagnosis of IBD (UC and Crohn’s disease) and fungal infections as primary or secondary diagnosis via ICD code 9. ICD-9 is the 9th revision of the International Statistical Classification of Diseases and Related Health Problems, a medical classification list by the World Health Organization. Fungal infections included were histoplasmosis, pneumocystis, cryptococcosis, aspergillosis, blastomycosis, candidiasis and Coccidiomycosis. The study period included from January 2002 to December 2014.
Study analyses
All analyses were performed with the use of PROC SURVEY MEANS, SURVEYFREQUENCY and SURVEYLOGISTIC procedures of SAS statistical software, version 9.4 (SAS institute). Where appropriate, we used χ2 for categorical data frequency and t test for comparing means. P value of <0.05 was regarded as statistically significant. As per HCUP data-use agreement all observations ≤10 were not reported.
Results
Mortality trends
Among discharged patients with diagnosis of IBD the proportion of patients with fungal infections was small with range varying from 1.84% to 3.11% over the course of studied period. There was no significant difference between the 12 years of study. The overall mortality of patients with IBD showed increasing trend from 1.19% to 1.52%. The mortality data showed that there was increased mortality among IBD patients with Fungal infections as compared to those without IBD. The trend of mortality was decreasing from 4.27% to 2.15% in 2006 and started to increase in 2007 and peaked in 2008 to 5.6%. Since then Mortality steadily started to trend down as shown in the Figure 1A,B.

Demographics in UC
Table 1 shows the detailed demographics features in patients with UC for the studied fungal infections. The prevalence of aspergillosis, Blastomycosis, Candidiasis and Coccidiomycosis was higher among patient’s aged above 50. Except Candidiasis all other fungal infections were more common in male patients. All fungal infections were more common in whites.
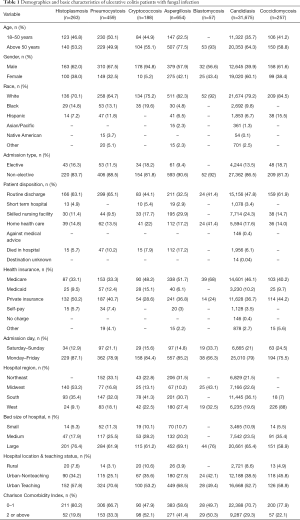
Full table
Demographics Crohn’s disease
Table 2 shows the demographic features in patients with Crohn’s disease for the studied fungal infections. Blastomycosis and Coccidiomycosis were more common in younger patients between 18–50 years of age while cryptococcosis and aspergillosis were more common in people with age above 50. Aspergillosis was more common in males while candidiasis and coccidiomycosis was more common in Female. All studied fungal infections were more prevalent in whites.
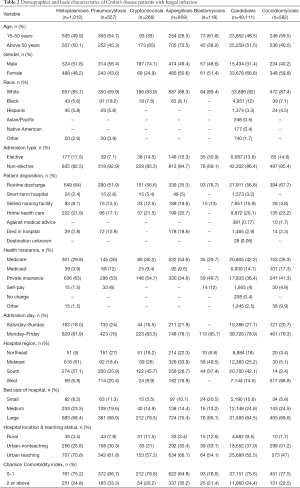
Full table
Geographic distribution
Histoplasmosis was more common in Midwest in both UC and Crohn’s disease. Coccidiomycosis was predominantly in West 88.8% in CD and 88% in UC. Other diseases were distributed in other states as well as shown in the Tables 1,2.
Predictors of fungal infections in IBD
Different demographic variables were studied for prediction of Fungal infections in IBD (Table 3). Age above 50 showed increased risk. The highest risk of developing fungal infection was associated with concomitant AIDS HR 5.99 (CI, 4.99–7.21). Other risk factors which showed significantly increased risk were congestive heart failure, anemia, diabetes mellitus COPD, pulmonary circulation disorders, rheumatoid arthritis/collagen vascular disease. solid organ malignancy, metastatic malignancy, lymphoma and weight loss were also found to increase the risk of opportunistic fungal infections in IBD patients.
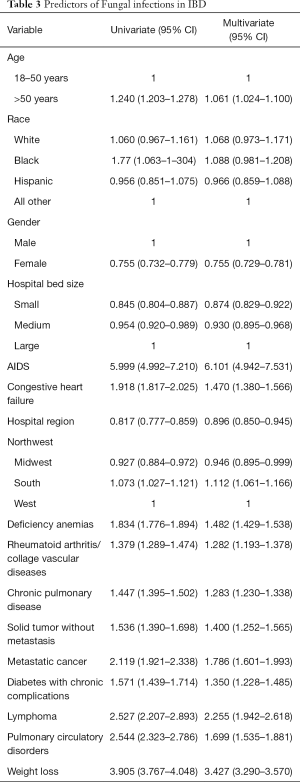
Full table
Discussion
Our study is one of the largest population-based study to study the epidemiology of the fungal infections in hospitalized patients with the IBD. We found out that the overall prevalence of the opportunistic fungal infections in hospitalized patients with IBD is low around 2%. Mortality due to opportunistic fungal infections in patients with IBD is higher than without them. There was an interesting mortality trend noted where there was a sudden increase in mortality in 2008 followed by steady downward trend. We believe the initial rise was due to increased used of the anti-TNF agents. In April 2008, FDA issued warning about the increased risk of opportunistic infections with the use of ant-TNF agents and the delays in diagnosis which resulted in mortality especially in cases with histoplasmosis.
Anti TNF agents are one of the main therapeutic options for patients with IBD. Anti TNF agents block TNF alpha and hence block the cell mediated response required for the generation of granulomatous inflammation (13). The increased risk of tuberculosis and opportunistic fungal infections due to use of these agents has been supported by several studies (6,11). The downward trend of mortality from 2008 afterwards may be due to FDA warning that may have raised awareness among physicians to detect the opportunistic infections early. In a systematic review on opportunistic fungal infections in patients with IBD which was recently published the authors found only two major studies to study the incidence and characteristics of fungal infections (6,14,15). There is lack of data on mortality due to opportunistic fungal infections in IBD patients with scattered case reports of fatal cases.
In UC, the prevalence of all fungal infections was higher in whites which is consistent with the fact that IBD is more prevalent in whites in USA. Age is known to be an independent risk factors for developing opportunistic infections and our study showed that the patients with age above 50 had higher prevalence of aspergillosis, blastomycosis, candidiasis, coccidiomycosis in UC and cryptococcosis and aspergillosis were more common in Crohn’s disease. Only aspergillosis was common in aged above 50 in both UC and Crohn’s disease. In Crohn’s disease however infections were more common in younger patients. It may be because younger patients are the main population affected with the disease.
Certain Fungal infections are endemic in different region of the United States (16). The geographic distribution of endemic fungal infections in our study was consistent with the previous well-established territories. In IBD patients who were hospitalized Histoplasmosis was most common in Midwest region, Cryptococcosis in South region, Coccidiomycosis in West region and Blastomycosis in Midwest region.
The strongest predictor of having fungal infections in our study was having AIDS. AIDS is an immunocompromised state and a well-known risk factor for the development of opportunistic infection due to impaired T cell mediated immunity (17,18). Having AIDS with IBD together increased the risk of having opportunistic fungal infection by six-fold. There is no previous data to suggest such an increased risk. There is lack of good studies but available case report and series have shown that anti-TNF agents and azathioprine can be used safely in HIV patients (18,19). The increased risk can be possibly explained by inability to mount significant host T cell response by HIV infected cells along with use of immunosuppressive drugs for IBD treatment. Due to lack of information on the treatment details in these patients the exact reason of such increased risk of opportunistic fungal infections is not clear.
IBD patients with Congestive heart failure patient had higher prevalence of Fungal infections with OR of 1.9 (CI, 1.81–2.02). There is no obvious explanation for this as heart failure itself does not lower the T cell immunity. Leake et al. also found in their case control study that Heart failure was independent risk factors for Coccidiomycosis in elderly patient above 65 with increased risk of infections on both univariate and multivariate analysis (20). Other significant risk factors include metastatic cancers, lymphoma and autoimmune conditions. Diabetes mellitus (DM) is considered as a risk factor for development of opportunistic fungal infections with the best association with mucormycosis (21,22). However, some investigators did not find DM to be a significant risk factor for the development of cryptococcosis. They found that AIDS, Malignancy and autoimmune diseases were independent risk factors for development of cryptococcosis (23).
Iron deficiency anemia (IDA) is common in patients with IBD (24). IDA in IBD commonly occurs due to chronic blood loss from the ulcerated surface of the bowel, malnutrition with reduced iron intake, or impaired iron absorption through the intestinal mucosa (25). European consensus guidelines on the management of iron deficiency and anemia recommends that all patients with IBD be assessed for presence of anemia (26). IDA is known to affect the cell mediated immunity through various mechanisms as described by Oppenheimer and predisposing to granulomatous infections (27). We found that presence of anemia increased the risk of infection by 1.8-fold. Anemia itself has not been previously described as risk factor for opportunistic fungal infection in IBD. However Hemoglobin less than 9 g/dL was found to be an independent predictor of severe outcomes in patients with Clostridium difficile infection in IBD patients (28).
The main strength of our study is that it is one of the first and largest population-based data providing the insights into the epidemiology of the opportunistic fungal infections in patients with IBD. It is also the first study providing the mortality trends for a duration of more than 10 year in patients having IBD. There are several limitations to our study. The data was taken from the data base and relied on the reporting of the diagnosis on discharge and hence is subject to reporting bias. We also limited our study to the seven common fungal infections. Our study lacked the details of the severity of the IBD and the exact nature of therapy patient was at the time of fungal infection. Hence the need for developing an active surveillance database for IBD patients for development of opportunistic fungal infection is needed.
Conclusions
Patients with IBD who are hospitalized have a steady incidence of opportunistic fungal infections around 2%. The mortality increased between 2006 and 2008 and a significant difference remains between IBD patients with and without fungal infections. One explanation of rise in mortality but a consistent incidence could be due to the use of biologics that did not increase but compromised the ability of IBD patients to fight opportunistic fungal infections. Reduced mortality after 2008 suggests the increased awareness and early recognition of the infections after FDA warning regarding opportunistic fungal infections in users of anti-TNF agents. We described the various factors predictive of opportunistic fungal in IBD patients with AIDS being the strongest. The prevalence of opportunistic fungal infections in different US regions is consistent with previously published reports.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Zhang YZ, Li YY. Inflammatory bowel disease: Pathogenesis. World J Gastroenterol 2014;20:91-9. [Crossref] [PubMed]
- Li Q, Wang C, Tang C, et al. Dysbiosis of Gut Fungal Microbiota is Associated With Mucosal Inflammation in Crohn’s Disease. J Clin Gastroenterol 2014;48:513-23. [Crossref] [PubMed]
- Richard ML, Lamas B, Liguori G, et al. Gut Fungal Microbiota: The Yin and Yang of Inflammatory Bowel Disease. Inflamm Bowel Dis 2015;21:656-65. [Crossref] [PubMed]
- Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREATTM registry. Am J Gastroenterol 2012;107:1409-22. [Crossref] [PubMed]
- Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443-68. [Crossref] [PubMed]
- Ford AC, Peyrin-Biroulet L. Opportunistic Infections With Anti-Tumor Necrosis Factor-α Therapy in Inflammatory Bowel Disease: Meta-Analysis of Randomized Controlled Trials. Am J Gastroenterol 2013;108:1268-76. [Crossref] [PubMed]
- Toruner M, Loftus EV, Harmsen WS, et al. Risk Factors for Opportunistic Infections in Patients With Inflammatory Bowel Disease. Gastroenterology 2008;134:929-36. [Crossref] [PubMed]
- Dave M, Purohit T, Razonable R, et al. Opportunistic Infections Due to Inflammatory Bowel Disease Therapy. Inflamm Bowel Dis 2014;20:196-212. [Crossref] [PubMed]
- Wang MC, Zhang LY, Han W, et al. PRISMA—Efficacy and Safety of Vedolizumab for Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 2014;93:e326. [Crossref] [PubMed]
- Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohns Colitis 2013;7:107-12. [Crossref] [PubMed]
- Ordonez ME, Farraye FA, Di Palma JA. Endemic fungal infections in inflammatory bowel disease associated with anti-TNF antibody therapy. Inflamm Bowel Dis 2013;19:2490-500. [Crossref] [PubMed]
- HCUP Databases. Healthcare Cost and Utilization Project (HCUP). 2016. Available online: www.hcup-us.ahrq.gov/nisoverview.jsp
- Deepak P, Stobaugh DJ, Ehrenpreis ED. Infectious complications of TNF-α inhibitor monotherapy versus combination therapy with immunomodulators in inflammatory bowel disease: analysis of the Food and Drug Administration Adverse Event Reporting System. J Gastrointestin Liver Dis 2013;22:269-76. [PubMed]
- Stamatiades GA, Ioannou P, Petrikkos G, et al. Fungal infections in patients with inflammatory bowel disease: A systematic review. Mycoses 2018;61:366-76. [Crossref] [PubMed]
- McAuliffe ME, Lanes S, Leach T, et al. Occurrence of adverse events among patients with inflammatory bowel disease in the HealthCore Integrated Research Database. Curr Med Res Opin 2015;31:1655-64. [Crossref] [PubMed]
- Chu JH, Feudtner C, Heydon K, et al. Hospitalizations for Endemic Mycoses: A Population-Based National Study. Clin Infect Dis 2006;42:822-5. [Crossref] [PubMed]
- Ho TH, Cohen BL, Colombel JF, et al. Review article: the intersection of mucosal pathophysiology in HIV and inflammatory bowel disease, and its implications for therapy. Aliment Pharmacol Ther 2014;40:1171-86. [Crossref] [PubMed]
- Beltrán B, Nos P, Bastida G, et al. Safe and effective application of anti‐TNF‐α in a patient infected with HIV and concomitant Crohn’s disease. Gut 2006;55:1670-1. [Crossref] [PubMed]
- Chamberlain FE, Dinani N, Jagjit Singh GK, Bower M, Nelson M. Azathioprine can be safely used in HIV-infected individuals. AIDS 2014;28:447. [Crossref] [PubMed]
- Leake JAD, Mosley DG, England B, et al. Risk Factors for Acute Symptomatic Coccidioidomycosis among Elderly Persons in Arizona, 1996–1997. J Infect Dis 2000;181:1435-40. [Crossref] [PubMed]
- Hong HL, Lee YM, Kim T, et al. Risk Factors for Mortality in Patients with Invasive Mucormycosis. Infect Chemother 2013;45:292-8. [Crossref] [PubMed]
- Abidi MZ, Coelho-Prabhu N, Hargreaves J, et al. Mucormycosis in patients with inflammatory bowel disease: case series and review of the literature. Case Rep Med 2014;2014:637492. [PubMed]
- Lin YY, Shiau S, Fang CT. Risk Factors for Invasive Cryptococcus neoformans Diseases: A Case-Control Study. PLoS One 2015;10:e0119090. [Crossref] [PubMed]
- Iron and inflammatory bowel disease - Oldenburg - 2001 - Alimentary Pharmacology & Therapeutics - Wiley Online Library [Internet]. [cited 2019 Jan 4]. Available online: https://onlinelibrary.wiley.com/doi/full/10.1046/j.1365-2036.2001.00930.x
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol 2015;6:62-72. [Crossref] [PubMed]
- Dignass AU, Gasche C, Bettenworth D, et al. European Consensus on the Diagnosis and Management of Iron Deficiency and Anaemia in Inflammatory Bowel Diseases. J Crohns Colitis 2015;9:211-22. [Crossref] [PubMed]
- Oppenheimer SJ. Iron and Its Relation to Immunity and Infectious Disease. J Nutr 2001;131:616S-33S. [Crossref] [PubMed]
- Ananthakrishnan AN, Guzman-Perez R, Gainer V, et al. Predictors of severe outcomes associated with Clostridium difficile infection in patients with Inflammatory Bowel Disease. Aliment Pharmacol Ther 2012;35:789-95. [Crossref] [PubMed]
Cite this article as: Mushtaq K, Khan Z, Aziz M, Alyousif ZA, Siddiqui N, Khan MA, Nawras A. Trends and outcomes of fungal infections in hospitalized patients of inflammatory bowel disease: a nationwide analysis. Transl Gastroenterol Hepatol 2020;5:35.


