Endocytoscopy: technology and clinical application in the lower GI tract
Introduction
Endoscopy has an important role in determining the treatment strategy for colorectal tumors. The technology and endoscopic diagnosis of colorectal lesions has been developed together with magnified endoscopy. On the other hand, endocytoscopy can visualize white-light images at higher resolution. Using this technology, we can recognize and evaluate the histological structure of the colon epithelium, so called “virtual histology” or “optical biopsy” (1).
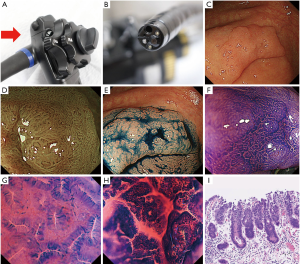
The endocytoscope magnifies images 520× on a 26-inch monitor (2). During endocytoscopic observation, the double staining technique using crystal violet and methylene blue stains cell stroma and nucleus (Figure 1), making it possible to visualize structures at the cellular level in vivo (3,4). Endocytoscopy has become one of the valuable diagnostic tools for colorectal tumors. Numerous studies examined the differential diagnosis of neoplastic and non-neoplastic colorectal lesions (1,5-7), diagnosis of sessile serrated adenoma/polyp (SSA/P) or traditional serrated adenoma (TSA) (8-10), detection of desmoplastic reaction in T1 colorectal cancer (11), or estimation of histological grade of colorectal cancer using endocytoscopy (12). Additionally, several studies has evaluated depth diagnosis and assessment of the depth of submucosal invasion in early colorectal cancer using the technology (6,13-16).
The clinical questions in this review are as follows:
- The current applicability of endocytoscopy in the lower gastrointestinal tract and
- The diagnostic value of endocytoscopy in depth invasion estimation of colorectal neoplasms.
In this review, the current situation of endocytoscopy is discussed, including the diagnostic performance and usefulness of artificial intelligence (AI) or computer assisted diagnosis (CAD) for colorectal tumors, along with the clinical application and future prospect of these technology.
Methods
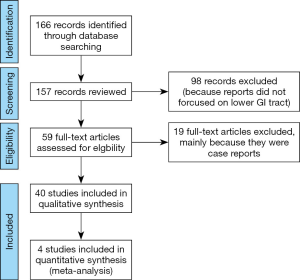
An electronic search via Ovid MEDLINE for articles up to September 2019 was performed using the keywords “endocytoscopy” or “endocytoscopes”. The language was limited to English. After reviewing the titles and abstracts, reports analyzing upper gastrointestinal tract, biliary system, pancreas, liver, bladder, lung, head and neck were excluded. Upon assessing the full text of the remaining articles, reports analyzing inflammatory bowel disease (IBD), juvenile polyps, infection, and parasite were excluded. Case reports were also eliminated. In this meta-analysis, the primary outcome measurement was the diagnostic accuracy of endocytoscopy for colorectal neoplasms compared with magnified chromoendoscopy. The results of binary outcomes were summarized, and comparisons of the diagnostic performance were presented as risk ratios with corresponding 95% confidential intervals. The statistical analysis of the results in this study was calculated using odds ratio, where events represent the number of lesions correctly diagnosed. Comparison of events, in this case the events meant the number of patients who was accurately diagnose, was made between endocytoscopy and magnified chromoendoscopy.
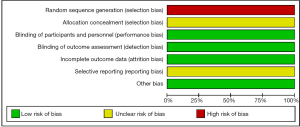
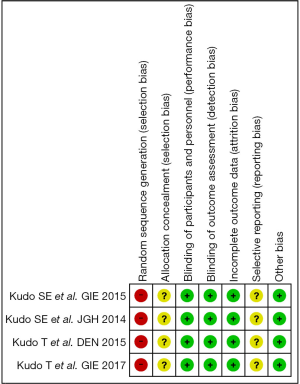
Assessment of the risk of bias was evaluated using a ‘risk’ of ‘bias’ summary figure. A funnel plot was used to assess reporting and selection bias.
Results
The electronic search identified a total of 166 potentially relevant reports. After the abstracts were reviewed, 98 studies were excluded. Fifty-nine studied were obtained for full text review, and 19 articles were subsequently excluded being case reports, studies targeting IBD or other diseases listed above. Ultimately, a total of 40 studies were included in this review. Among them, 4 studies comparing the diagnostic performance of endocytoscopy classification (EC classification) versus pit pattern diagnosis using magnified chromoendoscopy in the depth estimation of colorectal lesions were analyzed (Figure 2).
Qualitative diagnosis by endocytoscopy
To characterize colorectal lesions by endocytoscopy, the endocytoscopic classification (EC classification) was described and adopted (6). The diagnostic accuracy in the discrimination between neoplasm and non-neoplasm, including those less than 5mm in size, was reported to be 93.3–96.8% (1,5-7). Moreover, some studies reported higher diagnostic accuracy for hyperplastic polyp by endocytoscopy utilizing the EC-V classification. The technology is also effective in the differential diagnosis of SSA/P, and detection of desmoplastic reaction in T1 colorectal cancer (8,11,17). Sako et al. reported that the cribriform-like pattern named as “fused gland formations on endocytoscopy (FGFE)”, can be used to diagnose histological grade (12). Table 1 summarized the studies which reported on characteristic diagnosis using endocytoscopy.
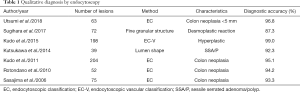
Full table
Quantitative diagnosis by endocytoscopy
A handful of studies reported on the depth diagnosis of early colorectal cancer using magnified chromoendoscopy with crystal violet staining to date (18-20). For observation by endocytoscopy, double staining using both crystal violet and methylene blue is recommended. The EC classification with double staining facilitates visualization of the cellular structures and nucleus, allowing for depth diagnosis. Additionally, endocytoscopy with narrow band imaging (NBI) has also been evaluated in other studies. In this endocytoscopic vascular classification (EC-V), ultra-magnified NBI images without crystal violet or methylene blue visualizes the micro vessels. Table 2 listed the studies which reported on depth diagnosis using endocytoscopy.

Full table
In these reports, 4 studies analyzed the diagnostic performance of depth assessment using endocytoscopy versus pit pattern diagnosis. All of the 4 studies were retrospectively in nature, and selection bias is likely. However, the endoscopists and pathologists who evaluated the endoscopic and histological findings were blinded, hence performance and detection bias were less likely. The risk of bias summary and graph are shown in Figures 3 and 4, respectively.
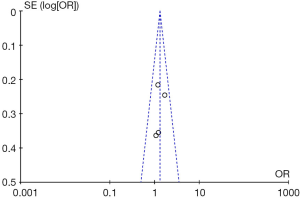
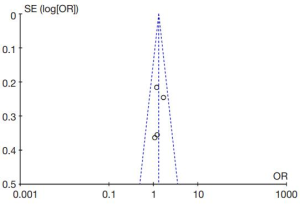
The diagnostic performance of endocytoscopy compared to pit pattern diagnosis was better and the result was statistically significant (odds ratio =1.31; 95% CI, 1.00–1.71; I2=0%; P=0.05; Figure 5). The funnel plot of the studies is almost symmetrical suggesting the intervention effect of each study is similar (Figure 6).
Artificial intelligence (AI)/computer aided diagnosis (CAD)
There are studies investigating AI or CAD-assisted endocytoscopy in the differentiation between neoplastic and non-neoplastic colonic lesion, diagnosis of adenomatous lesion using EC-V, and depth diagnosis of T1 colorectal cancer with crystal violet and methylene blue double staining (21-26). The diagnostic accuracy of CAD for neoplastic colorectal lesion using double staining was 89.0% to 98.1% in the studies (21-23), while CAD with NBI showed accuracy of 87.8–95.2% (23,25,26). Takeda et al. reported that the accuracy of CAD in detecting invasive colorectal cancer was 94.1% (24). In several studies the reported diagnostic performance of CAD was superior to that of non-experts and comparable to experts.
CAD-assisted endocytoscopy, with its application to differentiate between neoplastic or non-neoplastic colorectal lesions, has recently been approved by the Japanese Pharmaceuticals and Medical Devices Agency. The technology has become commercially available.
Discussion
To the extent of our knowledge, this is the first review of endocytoscopy which includes a meta-analysis. Endocytoscopy provide real time cellular imaging and enables the assessment of epithelial structure, the so called “virtual histology”. One of the clinical questions regarding endocytoscopy was the diagnostic ability compared with magnified chromoendoscopy, and several studies have attempted to clarify this. Although there are limited number of studies to date, endocytoscopy has been demonstrated to show a trend towards effective colorectal tumor depth diagnosis.
Even though the funnel plot was symmetrical, one should still take into consideration the potential of publication bias. Almost all studies with large numbers of cases reported were from the same institution. This means external validation of the diagnostic performance of endocytoscopy is limited. One of the reasons was because endocytoscopy was not commercially available until recently, and many studies were conducted using only the endocytoscope prototype.
The initial prototype was mainly fiber bundle endocytoscope, while the more recent commercially available endocytoscope are single-charge coupled device integrated type endocytoscopy. The magnifying power of the two types are different thus potentially affecting the diagnostic ability, and this has to be taken into account when interpreting the result. Given the new integrated type endocytoscope is now commercially available, validation studies with the same condition can be expected in the near future.
Furthermore, recent reports demonstrated high diagnostic performance using endocytoscopy with NBI (EC-V classification) for diagnosing neoplastic and invasive colorectal lesions. Because the accessibility of staining dye agents for endocytoscopy (EC classification) is limited in certain counties, EC-V can be better adopted into clinical practice there (14,17,23).
Another related topic is AI/CAD automated diagnosis. Applying AI/CAD technology can potentially resolve the so called “frame problem” during endocytoscopy (27). In general, AI/CAD performance is difficult if the region of interest (ROI), number of lesions, or the margin of the lesion are uncertain. The image depicted by endocytoscopy assumes the entire image is the lesion in question, therefore ROI is not considered. On the other hand, it means the images processed by AI/CAD is completely dependent on the image captured by the endoscopists.
Endocytoscopy will have a more definite role when endoscopic submucosal dissection (ESD) becomes widespread. The existing studies reported on the diagnostic performance of the technique are not only limited to small polyps, but also larger lesions including lateral spreading tumor (LST). Kudo et al. demonstrated the diagnostic accuracy of estimating invasion depth in the different morphological types of colorectal tumors. Endocytoscopy was more accurate in diagnosing invasion depth of LST-non-granular (NG) type lesions, while pit pattern diagnosis performed better in protruding lesions (15).
As mentioned, studies have shown that AI/CAD-assisted endocytoscopy provided higher diagnostic accuracy of colorectal lesions for non-expert endoscopists comparable to experts. In terms of the clinical application, AI/CAD technology may be advantageous, especially for novice endoscopists.
Conclusions
This article systematically reviewed comparative studies to investigate the current clinical applicability of endocytoscopy, and its diagnostic performance compared with magnified chromoendoscopy. Bearing in mind the limited number of studies, this systematic review and meta-analysis supports the finding of the statistically significant diagnostic performance of endocytoscopy for colorectal neoplasm depth diagnosis.
AI/CAD-assisted endocytoscopy also aids in the differential diagnosis of colorectal lesions, especially for non-expert endoscopists. Endocytoscopy and AI/CAD-assisted endocytoscopy is now commercially available. Future studies will be able to provide validation on the efficacy of endocytoscopy in clinical practice.
Acknowledgments
I would like to express my deepest gratitude to Dr. Shigeki Sekine for clinical and histological suggestions for my study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Sasajima K, Kudo SE, Inoue H, et al. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc 2006;63:1010-7. [Crossref] [PubMed]
- Maeda Y, Kudo SE, Mori Y, et al. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc 2019;89:408-15. [Crossref] [PubMed]
- Ichimasa K, Kudo SE, Mori Y, et al. Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: an in vivo prospective pilot study. Dig Endosc 2014;26:403-8. [Crossref] [PubMed]
- Kodashima S, Fujishiro M, Takubo K, et al. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy 2006;38:1115-21. [Crossref] [PubMed]
- Rotondano G, Bianco MA, Salerno R, et al. Endocytoscopic classification of preneoplastic lesions in the colorectum. Int J Colorectal Dis 2010;25:1111-6. [Crossref] [PubMed]
- Kudo SE, Wakamura K, Ikehara N, et al. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy 2011;43:869-75. [Crossref] [PubMed]
- Utsumi T, Sano Y, Iwatate M, et al. Prospective real-time evaluation of diagnostic performance using endocytoscopy in differentiating neoplasia from non-neoplasia for colorectal diminutive polyps (</= 5 mm). World J Gastrointest Oncol 2018;10:96-102. [Crossref] [PubMed]
- Kutsukawa M, Kudo SE, Ikehara N, et al. Efficiency of endocytoscopy in differentiating types of serrated polyps. Gastrointest Endosc 2014;79:648-56. [Crossref] [PubMed]
- Mori Y, Kudo SE, Ogawa Y, et al. Diagnosis of sessile serrated adenomas/polyps using endocytoscopy (with videos). Dig Endosc 2016;28 Suppl 1:43-8. [Crossref] [PubMed]
- Ogawa Y, Kudo SE, Mori Y, et al. Use of endocytoscopy for identification of sessile serrated adenoma/polyps and hyperplastic polyps by quantitative image analysis of the luminal areas. Endosc Int Open 2017;5:E769-74. [Crossref] [PubMed]
- Sugihara Y, Kudo SE, Miyachi H, et al. In vivo detection of desmoplastic reaction using endocytoscopy: A new diagnostic marker of submucosal or more extensive invasion in colorectal carcinoma. Mol Clin Oncol 2017;6:291-5. [Crossref] [PubMed]
- Sako T, Kudo SE, Miyachi H, et al. A novel ability of endocytoscopy to diagnose histological grade of differentiation in T1 colorectal carcinomas. Endoscopy 2018;50:69-74. [PubMed]
- Kudo T, Kudo SE, Mori Y, et al. Classification of nuclear morphology in endocytoscopy of colorectal neoplasms. Gastrointest Endosc 2017;85:628-38. [Crossref] [PubMed]
- Nakamura H, Kudo SE, Misawa M, et al. Evaluation of microvascular findings of deeply invasive colorectal cancer by endocytoscopy with narrow-band imaging. Endosc Int Open 2016;4:E1280-5. [Crossref] [PubMed]
- Kudo T, Kudo SE, Wakamura K, et al. Diagnostic performance of endocytoscopy for evaluating the invasion depth of different morphological types of colorectal tumors. Dig Endosc 2015;27:754-61. [Crossref] [PubMed]
- Kudo SE, Mori Y, Wakamura K, et al. Endocytoscopy can provide additional diagnostic ability to magnifying chromoendoscopy for colorectal neoplasms. J Gastroenterol Hepatol 2014;29:83-90. [Crossref] [PubMed]
- Kudo SE, Misawa M, Wada Y, et al. Endocytoscopic microvasculature evaluation is a reliable new diagnostic method for colorectal lesions (with video). Gastrointest Endosc 2015;82:912-23. [Crossref] [PubMed]
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008;103:2700-6. [Crossref] [PubMed]
- Kudo SE, Kashida H. Flat and depressed lesions of the colorectum. Clin Gastroenterol Hepatol 2005;3:S33-6. [Crossref] [PubMed]
- Kudo S, Rubio CA, Teixeira CR, et al. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy 2001;33:367-73. [Crossref] [PubMed]
- Mori Y, Kudo SE, Wakamura K, et al. Novel computer-aided diagnostic system for colorectal lesions by using endocytoscopy (with videos). Gastrointest Endosc 2015;81:621-9. [Crossref] [PubMed]
- Mori Y, Kudo SE, Chiu PW, et al. Impact of an automated system for endocytoscopic diagnosis of small colorectal lesions: an international web-based study. Endoscopy 2016;48:1110-8. [Crossref] [PubMed]
- Mori Y, Kudo SE, Misawa M, et al. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann Intern Med 2018;169:357-66. [Crossref] [PubMed]
- Takeda K, Kudo SE, Mori Y, et al. Accuracy of diagnosing invasive colorectal cancer using computer-aided endocytoscopy. Endoscopy 2017;49:798-802. [Crossref] [PubMed]
- Misawa M, Kudo SE, Mori Y, et al. Accuracy of computer-aided diagnosis based on narrow-band imaging endocytoscopy for diagnosing colorectal lesions: comparison with experts. Int J Comput Assist Radiol Surg 2017;12:757-66. [Crossref] [PubMed]
- Misawa M, Kudo SE, Mori Y, et al. Characterization of Colorectal Lesions Using a Computer-Aided Diagnostic System for Narrow-Band Imaging Endocytoscopy. Gastroenterology 2016;150:1531-32.e3. [Crossref] [PubMed]
- McCarthy J, Hayes P. Some Philosophical Problems from the Standpoint of Artificial Intelligence. Readings in Artificial Intelligence 1969;4:463-502.
Cite this article as: Takamaru H, Wu SY, Saito Y. Endocytoscopy: technology and clinical application in the lower GI tract. Transl Gastroenterol Hepatol 2020;5:40.