
Associated liver partition and portal vein ligation for staged hepatectomy: a review
Introduction
Liver surgery has witnessed slow but steady growth over past seven decades. Earlier experiences of liver surgery were restricted to military or trauma-related indications with high mortality. Couinaud’s work on segmental anatomy and advances in radiological imaging, fueled the growth of modern liver surgery (1,2). Surgical principles continue to undergo refinement and patients continue to reap benefits of technological innovation, improvements in critical care and expansion of resectability criteria. With such advances, outcomes have improved; however, morbidity and mortality following liver resection continue to plague surgeons. Post-hepatectomy liver failure (PHLF) is an important cause of morbidity and mortality following liver resection (3). Future liver remnant (FLR) is an important consideration for any patient undergoing liver resection; an adequate volume and function of liver is required to prevent PHLF. PHLF is defined as the impaired ability of the liver to maintain its synthetic, excretory and detoxifying functions, which are characterised by an increased international normalized ratio and hyperbilirubinemia on or after postoperative day 5 (4). FLR is measured by computed tomography (CT) or magnetic resonance (MR), with 3D reconstruction, and expressed as total estimated liver volume (TELV) (5-10). TELV is calculated using a formula based on body surface area (BSA) which has been validated in a multicenter study (9). The use of artificial intelligence has transformed liver surgery; software like HepaVision2 (MeVis Medical Solutions AG, Bremen, Germany) provides help in segmental volumetry and 3D reconstruction which allows surgical planning (11). Current guidelines for extended hepatectomies require a FLR of >20–25% in healthy patients, >30% in patients with liver steatosis or exposure to chemotherapy and >40% in patients with cirrhosis to prevent PHLF or “small-for-size” syndrome (SFSS) (12-17).
Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) is a novel two-staged procedure which aims to induce rapid hypertrophy of the FLR (18,19). ALPPS was first formally reported in a poster presentation by de Santibañes et al. in 2011 (20), and subsequently described in a landmark study involving 25 patients by Schnitzbauer et al. in 2012 (21). However, skepticisms were raised due to its high 90-day mortality (12%). Since then, several studies have reported improvements of morbidity (14–90%) (21-29), and mortality (0–28.7%) (26,30,31). More recently, there have been reports with no mortality; these improvements have been attributed to careful patient selection and refinements in surgical techniques (23,28,31).
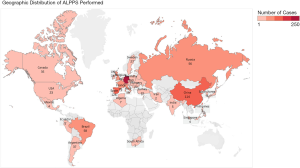
In 2012, an international ALPPS registry (web-based data entry system) was formed with the aims of knowledge sharing, audit, promoting research and identifying applications of ALPPS with collaborative efforts. This was essential to pool data from individual centers with small case series (32). In 2014, the ALPPS registry reported the first multicenter series from 41 centers including 202 patients with a 90-day mortality of 9% and concluded that outcomes of patients with colorectal liver metastases (CRLM) were similar to conventional two-staged hepatectomy and ALPPS was associated with higher mortality in older patients (32). Since then, the ALPPS registry has grown. Figure 1 provides a global overview of the ALPPS registry. In this chapter, we will review the scientific basis, progress and recent advances with regards to ALPPS.
Pathophysiology of liver regeneration
The liver is unique not only because it is largest internal organ but also being the only organ with ability to “regenerate”. After removal of part of liver, the remaining liver undergoes hypertrophy to compensate for lost liver tissue. This increase in liver volume is due to hyperplasia (increase in numbers) of hepatocytes, rather than hypertrophy (increase in size) (33). Retrospective studies have demonstrated increased mortality with decreasing FLR volumes (34,35). Adequate FLR is required for extended hepatectomies to prevent PHLF or SFSS. Hence, it is paramount to understand the pathophysiology of liver regeneration. There are two theories which describe the liver’s regenerative abilities: humoral factors and haemodynamic factors.
Humoral factors
The liver is known to generate cytokines, growth factors and hormones; the interplay between these factors result in initiation, propagation and termination of liver regeneration (36-39). The inflammatory response due to tissue injury generates cytokines and growth factors which induces hepatic regeneration. Certain cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-6 are upregulated after partial hepatectomy and have been demonstrated to have role in liver regeneration (33,40).
Haemodynamic factors
Portal blood flow is a key determinant of liver regeneration. Increase in portal blood flow leads to a relative imbalance between available portal blood and number of hepatocytes which results in hyperplasia of the hepatocytes (41,42). Existing techniques manipulate and redistribute portal venous blood flow to the FLR using various methods of portal vein (PV) occlusion to induce hypertrophy.
The mechanism behind the enhancement of rapid liver hypertrophy in ALPPS remains unclear. A few mechanisms have been postulated (28): (I) portal vein ligation (PVL) redistributes hepatotrophic factors to the FLR which induces hypertrophy; (II) disruption of the “cross portal” circulation via transection between the normal perfused and deportalised liver parts which increases portal flow to the FLR (26,29,43); (III) the diseased arterialised hemiliver allows the FLR to tolerate the haemodynamic stress and modulates the double hepatic vascular inflow, unlike one-staged major hepatectomies; and (IV) transection of the liver parenchyma in ALPPS generates an inflammatory response and releases growth factors, inducing liver hypertrophy (39). Animal models support the theory that the accelerated regeneration observed in ALPPS is due to the release of inflammatory mediators and growth factors: (I) ALPPS resulted in a marked increase in expression of early mediators of regeneration such as IL-6 and TNF-α unlike PVL alone; (II) accelerated regeneration was observed in models undergoing PVL alone after injection of plasma from models after first stage of ALPPS (39). In addition, a recent study by Chan et al. found no substantial increase in FLR portal flow after the liver parenchymal split, which supports the theory that the main mechanism behind the rapid FLR hypertrophy in ALPPS is due to the release of growth factors (44).
Guide on patient selection
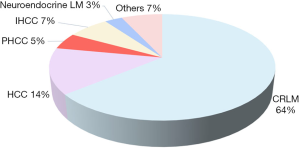
Liver resection is usually performed for patients with malignancies: primary liver cancer such as hepatocellular carcinoma (HCC) or cholangiocarcinoma, and secondary liver cancers, which includes CRLM, neuroendocrine tumours (NETs) or gallbladder cancer. Figure 2 summarizes the prevalence of the underlying malignancy for which ALPPS is performed. Studies have shown that outcomes of ALPPS differ according to pathology. CRLM confers the best clinical outcomes; a series by Hernandez-Alejandro involving 14 patients who underwent ALPPS for planned liver resection of CRLM demonstrated low morbidity (14% Clavien-Dindo ≥ Grade IIIB) and no mortality (23). In addition, a recent study by Joechle et al. has demonstrated that there is no difference between ALPPS and standard liver resection on tumour cell proliferation, apoptosis and vascularization in CRLM (18).
Patients with non-CRLM undergoing ALPPS have been described as an independent predictor of complications (32). ALPPS with concomitant biliary reconstruction (usually performed for hilar cholangiocarcinoma or gallbladder cancer) confers higher morbidity and mortality (21,24,26). Use of ALPPS in HCC with liver cirrhosis has been suggested to be safe with low morbidity (0% had Clavien-Dindo ≥ Grade IIIB complications) and no mortality over a median follow-up duration of 9 months (45). However, a study by D’Haese et al. comparing the outcomes of ALPPS in patients with CRLM vs. HCC demonstrated that HCC results in a 90-day mortality of 31%, which is about fivefold that of CRLM; this study concluded that only patients less than 60 years old with low grade fibrosis in HCC should undergo ALPPS to maximise the risk-benefit ratio (46). Use of ALPPS in all patients with HCC and underlying liver cirrhosis may be less beneficial as liver cirrhosis results in poorer regenerative capacity and HCC is associated with neoplastic thrombosis of the PV, hepatic vein or bile duct, which confers poor prognosis (47,48). However, a recent study by Chan et al. evaluated the use of ALPPS in hepatitis-related HCC in comparison with hepatectomy after portal vein embolization (PVE), ALPPS offered a higher resection rate with comparable post-operative morbidity and mortality, as well as long-term survival regardless of tumour staging (44). It is imperative to note that although the extent of FLR hypertrophy is significantly higher in ALPPS than PVE, the recovery of FLR function is still slower in cirrhotic livers; a longer time interval is still required between the two stages to permit adequate hepatocyte regeneration (44). A study by Linecker et al. also demonstrated that younger patients and ALPPS indicated for CRLM resulted in a reduction in mean predicted mortality for stage 1 of ALPPS (49).
In patients with neuroendocrine liver metastases (NELM), patient selection is also important. An ALPPS registry study by Linecker et al. has shown high morbidity 29% after stage 1 and 52% after stage 2 (50). High disease recurrence was also reported (1-year disease free survival (DFS) 73.2%, 2-year DFS 41.8%), an issue also experienced in conventional two-stage hepatectomy (51). Hence, patient selection with exclusion of poorly-differentiated NETs (Ki67 >20%) and use of complimentary adjuvant therapies such as peptide receptor radionuclide therapy (PRRT) are integral to multimodal management to reduce disease recurrence (50).
Although ALPPS offers the best outcomes for patients with CRLM, there are some factors which may increase the risk of poor outcomes in this group: model for end-stage liver disease (MELD) score >10 before the second stage of hepatectomy (52), and pre-treatment systemic chemotherapy (53). Hence, careful patient selection is essential. Various morbidity prediction tools have been developed to predict post-operative morbidity in liver resection, such as the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) surgical risk calculator and the Physiological and Operative Severity Score in the enUmeration of Mortality and Morbidity (POSSUM). A study by Madhavan et al. demonstrated that ACS-NSQIP is superior to POSSUM in the prediction of morbidity post-liver resection because (I) it can be computed pre-operatively, facilitating patient and surgeon decision-making (II) makes a distinction between the types of liver resection and (III) use of clearly defined variables (54). Patient selection can also be guided by the ALPPS Risk Score which is developed from the ALPPS registry: a scoring system which uses age, tumour type, bilirubin and creatinine levels to determine risk of futile outcomes (defined as 90-day mortality or in-hospital mortality) before the first stage and second stage of ALPPS to guide patient selection (55).
Advantages and complications
Significant increase in FLR volume of 61–93% in a short time interval of 9–14 days are two of the most cited benefits ALPPS confers over other techniques (21-26,28,30-32,36,56-58). This is unlike conventional two-stage hepatectomy which involves PVE or PVL (first stage) to induce FLR hypertrophy, followed by an interval period of 4–8 weeks, and a definitive liver resection (second stage) (59). However, there is a possibility of tumour progression and inadequate hypertrophy during the interval period which may preclude patients (~30%) from subsequent curative resection (60,61). A shorter time interval to definitive surgery has been suggested to improve oncological outcomes: tumour progression is unlikely and tumour resection is less challenging as definitive hepatectomy can be performed before the development of adhesions and may permit faster recovery (20,62). Studies have shown that almost all patients who underwent ALPPS were able to complete their second definitive surgery as compared to conventional two-stage hepatectomies (failure rate of 28–34%) (56,63). A recent multi-centre randomized control trial by Sandström et al. demonstrated that resection rates of ALPPS are superior to two-stage hepatectomy (92% vs. 57%, P<0.0001) (64).
The main complications of ALPPS are high morbidity (Clavien-Dindo ≥ Grade IIIB complications) (23,26,30) and 90-day mortality. Morbidity rates range from 14–50% (22-24); however, incidence of all postoperative complications has been reported to range from 53–90% (21,24-29). 90-day mortality rates are 0–28.7% (26,30,31). However, more recent studies have reported lower mortality rates with careful patient selection and technical modifications (23,28,31). CRLM patients had lower morbidity and mortality rates as compared to other cancers (23,31). Certain technical modifications have also been described to improve mortality rates: (I) avoiding routine ligation of diseased hemiliver bile duct which results in increased morbidity and mortality due to bile leaks, without any benefit of FLR hypertrophy (29); (II) usage of an anterior approach to help in liver transection and reduce tumour manipulation (21,65); (III) use of less invasive techniques such as Partial-ALPPS (66,67), Laparoscopic ALPPS (68), Tourniquet-ALPPS (57), and Mini-ALPPS (49,69). These variations are discussed later in the chapter.
Although one of the main benefits of ALPPS is the rapid hypertrophy in a short time interval, some patients may still experience inadequate increase in FLR. These may be due to poorer regenerative capacity in chronic liver disease or cirrhotic livers or shunting of arterial blood to the underlying tumour (45). A case of salvage transarterial embolization (TAE) for shunting of blood away from the FLR was reported (70). Salvage TAE may be considered in cases of large HCC and chronic liver disease with inadequate hypertrophy after the first stage of ALPPS. It has also been suggested that the shorter time interval to definitive resection may improve oncological outcomes by earlier removal of tumour burden (20,62). Initial data suggests that ALPPS has comparable 2-year DFS with two-stage hepatectomy (32,71). A recent meta-analysis in 2018 by Moris et al. (72) comparing ALPPS vs. two-stage hepatectomy concluded that ALPPS is not superior in oncological outcomes; three studies reported oncological outcomes. Adam et al. (59) (ALPPS n=17, two-stage hepatectomy n=41) reported a lower median overall survival in ALPPS (ALPPS 20 months, two-stage hepatectomy 37 months, P=0.006), but DFS was comparable (P=0.843). Ratti et al. (22) (ALPPS n=12, two-stage hepatectomy n=36) reported similar 1-year overall survival (ALPPS 92%, two-stage hepatectomy 94%, P=1) and 1-year DFS (ALPPS 67%, two-stage hepatectomy 80%, P=0.43) between the two groups. Kambakamba et al. (73) (ALPPS n=43, two-stage hepatectomy n=31) also reported comparable mean overall survival (ALPPS 24.7±2.3 months, two-stage hepatectomy 29.3±5.1 months, P=0.965). Due to the limited number of studies reporting oncological outcomes, further studies are required to validate the finding (72).
In addition, there is a paucity of data on the impact of ALPPS on the quality of life (QoL) of patients. QoL is an important long-term outcome measure in surgical oncology which plays a valuable role in clinical decision making (74,75). Measurement of QoL is difficult due to the need for disease-specific instruments to better assess the impairments caused by the disease and/or intervention. In addition, this is compounded by the relative novelty of ALPPS, where majority of the studies focused on safety, feasibility and short-term outcomes such as morbidity and mortality. The first study on QoL using the European Organization for Research and Treatment for Cancer (EORTC) Quality of Life Core Questionnaire (QLQ-C30) following ALPPS was conducted by Wanis et al. in 2018 in patients with CRLM and concluded that ALPPS can be performed with excellent postoperative QoL: patients reported high median global health (7-point scale on overall quality of life ranging from “much worse” to “much better”) and functional scores (higher score indicates better QoL) with low symptom scale scores (such as pain, dyspnea, constipation, diarrhoea) which are comparable to the general population (76). Reporting of long-term outcomes of ALPPS is difficult as the technique is relatively new (21).
Surgical technique
ALPPS is a two-staged procedure; the first stage aims to induce hypertrophy of the FLR, while the second stage is a definitive surgery to remove the underlying malignancy. The second stage is usually performed within 2 weeks. The surgical technique of ALPPS is as follows (21,26):
First stage
The first stage of ALPPS involves the following:
- Surgical exploration;
- Ipsilateral (tumour side) PVL;
- In-situ splitting (ISS) of liver parenchyma.
Explorative laparotomy is performed to exclude metastatic disease or progression of disease that precludes resection. Intra-operative ultrasound is done to establish resectability. The common bile duct, PV and common hepatic artery (HA) are then exposed close from their bifurcations, starting from the right part of the hepatoduodenal ligament. The following description is for right trisectionectomy by ALPPS. The right PV is divided and left PV is exposed centrally to identify segment I and IV branches (Figure 3A). In the presence of PV trifurcation, the anterior and posterior branches are divided separately (26). Segment IV PV, arterial and biliary branches are identified along the right rim of the round ligament and divided. Segment I branches are preserved, unless there is a need for caudate lobe resection. Right liver lobe is mobilized from the inferior vena cava (IVC). An alternative approach to this is the use of combination of liver hanging manoeuvre and anterior approach to prevent adhesion formation (65,77,78). Total or nearly total parenchymal dissection is performed with the cavitron ultrasonic suction aspirator (CUSA, Valleylab, Boulder, CO, USA). Test for bile leakage (white gauze test) is performed (79,80). Techniques to minimise blood loss include low central venous pressure and continuous inflow occlusion of the portal triad. Blood loss directly impacts liver regeneration. Ischemic preconditioning enhances liver regeneration and is associated with lower transaminitis (81). Ischemic preconditioning involves a short period of ischemia, followed by a brief period of reperfusion, before a prolonged period of ischemic insult (81). The field is prepared for the second stage using vessels loops to allow easy identification of HA (Figure 3A), middle HV and BD and using silicon sheeting between the split liver to prevent adhesions (Figure 3B). A drain is placed in the liver hilum before closing of the abdomen. Any small daughter lesions in FLR need to be resected or ablated.
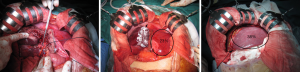
Interval phase
An abdominal CT scan is usually performed a week after the first stage to compute the volume of the FLR and median volume gained. If FLR is adequate, the patient is scheduled for the second stage (Figure 3C) (24,26). Inadequate FLR hypertrophy may warrant hepatic angiography to determine any shunting of blood to the tumour and; if present, a salvage transhepatic arterial embolization may be required (70). During the interval phase, nutritional support is important. Patient is usually monitored for complications like nosocomial infections or bile leak. Vigilant monitoring and proactive approach to deal with any complications is essential.
Second stage
The right HA, right HV and right BD (left BD in the context of hilar cholangiocarcinoma) are divided. Complete right trisectionectomy is performed; remaining parenchymal bridges are divided if present, and atypical resections for small tumour nodules may be required. In patients with hilar cholangiocarcinoma, segment I is resected and Roux-en-Y hepaticojejunostomy is performed. The remaining left lateral lobe is fixed to the anterior abdominal wall to prevent torsion. A drain is placed at the resection surface before closing of the abdomen.
Alternatives for inducing liver regeneration
In addition to ALPPS, there are other conventional techniques that induce liver hypertrophy and reduce PHLF. Several studies have been done to compare the effectiveness (percentage increase in FLR and time interval between intervention and definitive surgery), morbidity and mortality of these interventions. Alternatives to ALPPS are as follows:
- Interventional radiology
- PVE;
- Transarterial chemoembolization (TACE);
- Transarterial radioembolization (TARE) or selective internal radiation therapy (SIRT);
- Hepatic vein embolisation (HVE).
- Surgical
- PVL;
- Splenectomy.
PVE
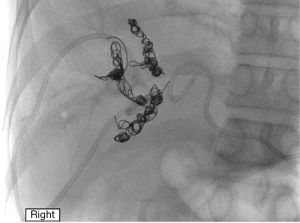
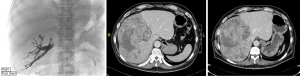
PVE causes occlusion of the portal blood flow to the areas of the liver planned for resection and redirects flow to the FLR which causes hypertrophy (82). Figure 4 shows a patient who completed right and segment IV PVE, and Figure 5A shows a patient who underwent right PVE. Contraindications for PVE include: (I) patient factors, such as uncorrected coagulopathy and end-stage renal disease; (II) disease factors, such as extension of tumour into FLR or PV, or tumour precluding safe transhepatic access (83). A meta-analysis by Isfordink et al. in 2017 demonstrated comparable mean FLR hypertrophy rates (PVE 43.2%, PVL 38.5%, P=0.386), morbidity (PVE 3.9%, PVL 5.2%, P=0.397) and 30-day mortality (PVE 3.8%, PVL 2.8%, P=0.795) (84). However, PVE should be considered over PVL as it is minimally-invasive. When explorative laparotomy is performed with consideration of a two-stage hepatectomy, PVL may be performed. When compared with TACE, PVE is superior in extent of FLR hypertrophy (PVE 61.5%, TACE 29%, P<0.001) (85). However, studies have reported possibilities of tumour progression during the waiting interval after PVE (86-88).
TACE
The role of TACE is an adjunct to PVE. It improves the regenerative response and prevents interval progression of tumour, especially in the context of HCC (38). Figure 5 shows a patient who underwent PVE and TACE prior to an extended right hepatectomy. Figures 5B,C show the CT scans of the same patient who underwent a 1st TACE and 2nd TACE respectively and the extent of FLR hypertrophy. A combination of TACE and PVE has been shown to have a greater increase in FLR over a shorter time interval, lower recurrence, and improved overall survival in HCC as compared to PVE alone (89-91). A study by Yoo et al. involving 71 patients with TACE + PVE and 64 patients with PVE only reported a 5-year DFS of 61% and 38% in the TACE + PVE group and PVE-only group respectively (P=0.001) (89). No significant differences in morbidity (PHLF in TACE + PVE 4% vs. PVE-only 12%, P=0.185) and mortality (TACE + PVE group 0% vs. PVE-only group 3%, P=0.210) were observed.
TARE/SIRT
TARE is traditionally used in the management of primary and secondary liver tumours with good response (92,93); it involves the delivery of high-dose radiation via the hepatic arterial circulation through the infusion of 90Y labeled glass or resin microspheres. However, there is also notable hypertrophy in the contralateral untreated liver lobes. A matched study by Garlipp et al. demonstrated that TARE is inferior to PVE in inducing liver hypertrophy (mean FLR increase from baseline = 29%, vs. PVE 61.5%, P<0.001) (85). However, TARE may be an alternative to PVE in selected group of patients with tumour at risk of being unresectable, as PVE has been reported to increase the rate of tumour growth (86), while TARE has the ability to reduce the risk of tumour progression.
HVE
HVE is used as an adjunct after PVE to avoid development of PV collaterals; this combination is coined as liver venous deprivation (LVD), or hepatic and portal vein embolisation (HPVE). Current studies demonstrate that LVD achieves liver hypertrophy that is comparable to ALPPS; however, LVD requires a longer time interval (mean 23 days) before definitive liver resection (94). A recent systematic review by Esposito et al. on 6 studies with 68 patients showed a FLR increase of 54.4% and 43.7% in simultaneous HPVE and sequential HPVE (HVE performed 1 to 4 weeks after PVE due to insufficient FLR increase) respectively (95). Posthepatectomy morbidity (Clavien-Dindo ≥ Grade III) is 10.3%, and 90-day mortality of 5.1% (95). Variations to the technique have been reported: extended LVD, which also involves embolisation of the middle HV, also achieves a safe, rapid and adequate increase in liver function (96).
PVL
PVL involves the ligation of the PV using a laparoscopic or open approach. A meta-analysis by Isfordink et al. in 2017 demonstrated that PVE and PVL are comparable in extent of FLR increase, morbidity and mortality (84). However, the extent of liver hypertrophy when PVL is performed as part of ALPPS is superior to PVE alone (mean FLR increase in ALPPS 69% vs. PVE 50%) (97). PVL is indicated in the absence of interventional radiological facilities, when patient requires a two-staged resection where the first stage involves removing the tumour from the FLR, or when performed as part of ALPPS (97,98).
Splenectomy
Unlike other techniques which prevents PHLF through the induction of liver hypertrophy, the role of splenectomy is to reduce portal venous pressure (PVP), which is a predictor of liver failure and mortality after hepatectomy (99). PHLF is characterised by hepatocellular dysfunction due to a mismatch between an excessive portal flow and reduced liver volume (100). Interventions for PVP modulation are indicated when PVP >20 mmHg (99). Pre-operative and intra-operative splenectomy have been reported to reduce hepatocyte damage, microvascular steatosis and improve survival in animal models with 90% hepatectomy (101). It is also reported to improve function (increase in prothrombin time, platelet and albumin levels) in patients with HCC and concomitant hypersplenism (102). However, the role of splenectomy post-hepatectomy is unclear, but may have a therapeutic potential to treat PHLF (103). Splenic artery ligation (SAL) is an alternative to splenectomy intra-operatively to reduce PVP in PHLF or SFSS. A study by Yoshizumi et al. compared SAL and splenectomy in living donor liver transplantation (LDLT): both techniques are comparable in reduction in PVP (104). Preoperative reduction in PVP can also be performed using splenic artery embolisation (SAE) a day before surgery: a study by Umeda et al. in LDLT demonstrated that preoperative SAE resulted in a significantly lower postoperative portal flow velocity and in-hospital mortality (SAE 3.3%, non-SAE 13.3, P=0.0364) as compared to no intervention (105).
Progress of ALPPS
Variations in techniques are shown to reduce 90-day mortality rates and reduction in inter-stage complications (49). These are the minor and major modifications of the conventional ALPPS.
Minor modifications
These include (I) replacing the plastic bag or sheet wrapped around the liver with an absorbable material in the event that the patient is unable to undergo the second stage (106); (II) usage of an anterior approach with or without hanging manoeuvre to reduce tumour manipulation and adhesion formation (21,65,77,78); (III) preservation of the middle HV to avoid ischemia to segment IV (23).
Major modifications
Partial-ALPPS
Partial splitting of liver parenchyma, defined as 50–80% of the complete transection surface, has been suggested to reduce morbidity and yet achieve comparable FLR hypertrophy in non-cirrhotic and non-cholestatic patients. Lower morbidity and 0% post-operative mortality was observed in both studies which compared partial-ALPPS with complete-ALPPS (66,67): Linecker et al. showed that partial-ALPPS had lower incidence of any complications (partial-ALPPS 61%, complete-ALPPS 91%, P=0.017) and with 0% 90-day mortality (complete ALPPS n=6/22, 27.3%) (67). Favourable outcomes in partial-ALPPS have been postulated to be due to the preservation of central outflow structures and the strict avoidance of potential intra-operative complications caused when approaching these structures (67). However, these outcomes were not reproducible in patients with chronic liver disease: complete-ALPSS resulted in a significantly greater FLR hypertrophy than partial-ALPPS, with a shorter interval period between the two stages and quicker recovery of liver function (107).
Minimally-invasive ALPPS (laparoscopic ALPPS and robotic assisted ALPPS)
Open surgery is more prone to development of adhesions which may cause difficulty during second stage (108). Various techniques have been described to reduce adhesion development in the interval between the first and second stage, such as the use of a combination of liver hanging manoeuvre and anterior approach and the placement of a silicon sheeting before abdomen closure (65,77,78). In recent years, there have been reports of ALPPS performed successfully and safely by totally laparoscopic (68,109) and robotic assisted approach (110,111). This is consistent with recent study by Shelat et al. who showed that laparoscopic approach is safe, feasible and has comparable oncologic outcomes for hepatic resection in liver tumours ≥5 cm, although associated with longer operative time (112). In addition, laparoscopic liver resection has been demonstrated to confer lower morbidity and a shorter length of hospital stay in elderly patients (113). More data is needed to establish the role of minimal access surgery in ALPPS.
Tourniquet-ALPPS (T-ALPPS)
T-ALPPS, also termed Associating Liver Tourniquet and Portal vein ligation for Staged hepatectomy (ALTPS), is a variant of ALPPS where liver bipartition is performed in the second stage rather than the first stage of ALPPS (114): a tourniquet is placed within the main portal fissure or umbilical fissure to occlude vascular communication between both lobes without the need for liver transection. A propensity-score matched study on 42 patients with CRLM by Robles-Campos et al. in 2019 demonstrated comparable oncological outcomes between T-ALPPS and two-stage hepatectomy: median overall survival was 36 months and 41 months in T-ALPPS and two-stage hepatectomy respectively (P=0.925), and median DFS was 9 months and 16 months in T-ALPPS and two-stage hepatectomy respectively (P=0.930) (115). Exclusion of liver bipartition during the first stage reduces blood loss and operation time. Traditionally, patients with Barcelona Clinic Liver Cancer (BCLC) stage B HCC were excluded from curative liver resection or transplantation (116,117). However, recent studies suggest that surgical treatment may improve survival in advanced HCC (117-120); hence ALPPS should be considered in patients with BCLC stage B with variations in technique to reduce ALPPS-related morbidity and mortality.
Monosegment ALPPS
Monosegment ALPPS, as the name suggests, leaves the patient with a single Couinaud segment (± segment I, which is considered an accessory segment) upon completion of the second stage (1,48). Traditionally, patients should be left with ≥2 liver segments (usually segment II and III), even with PVE or PVL. Prior to the advent of ALPPS, single remnant Couinaud segment was only possible with two-stage hepatectomy performed with intervals of several months in between two stages and has high risk (121). Monosegment resection opens the window for curative resection in patients with extensive tumour burden. Four types of feasible resections have been described (Table 1). A retrospective analysis of 12 patients (majority were <60 years old) with CRLM showed 0% 90-day mortality and 33.3% experienced PHLF, though all of them recovered. Although there is a paucity of evidence for its use, monosegment ALPPS remains a viable option for carefully selected patients with extensive tumour burden and/or lesions in close proximity to vascular structures (122).
Mini-ALPPS
Mini-ALPPS involves partial liver parenchymal transection and intra-operative PVE in the first stage, with minimal liver mobilization and avoidance of hilar plate or hilum dissection. The second stage is performed via an anterior approach. This modified technique was reported in the 1st ALPPS International Consensus Meeting in 2015 (123), and published by de Santibañes et al. in 2016 (n=4): mini-ALPPS had comparable FLR hypertrophy rates (mean FLR hypertrophy 62.6%) with classic ALPPS with lower morbidity (0% incidence of major complications; 1 patient developed abdominal wound infection (Clavien-Dindo grade II)) and nil 90-day mortality (69). Mini-ALPPS can also be performed totally laparoscopically: this was first described by Pekolj et al. in 2018 on a patient with HCC who had an uneventful post-operative recovery and was discharged in 5 days following the second stage of the surgery (124).
Radiofrequency-assisted liver partition with portal vein ligation (RALPP)
RALPP replaces the need for physical parenchymal transection of the liver with the use of a radiofrequency ablation device to create a line of coagulative necrosis in the liver parenchyma. The obsolescence of physical transection may potentially reduce the incidence of post-operative complications and the traditionally high morbidity described in ALPPS (125,126). In addition, RALPP can be performed via an open or laparoscopic approach. The use of a totally laparoscopic approach may provide additional benefit in morbidity reduction and reduced length of hospitalisation stay (126-128). A case series on five patients with CRLM showed that RALPP is comparable to ALPPS in FLR hypertrophy (median FLR increase 62.3%), and has low morbidity (n=1 (20%), one patient developed multiple pulmonary emboli) and nil 90-day mortality (43).
Left, rescue and right ALPPS modification
These variations were described in 2013 by Gauzolino et al. (129), which differ in the method of in-situ liver splitting. In Left ALPPS, the first stage involves limited resection of the right anterior and posterior section, left PVL and in-situ liver splitting along the main portal fissure. The second stage involves completion of left hemihepatectomy with segment I resection. Rescue ALPPS is a salvage technique for patients with inadequate liver hypertrophy after conventional techniques: the first stage involves ISS along the main portal fissure (right PV has already been ligated in the conventional methods); the second stage involves completion of right hemihepatectomy. In Right ALPPS, the first stage involves a left lateral sectionectomy, ligation of the posterolateral branch of the right PV, multiple resections on the right anterior and left medial section and splitting along the right portal fissure. The second stage involves completion of right posterior sectionectomy. These variations have been shown to be comparable to conventional ALPPS.
Conclusions
Since 2012, several studies have established safety and feasibility of ALPPS. ALPPS achieves FLR hypertrophy in short time interval and almost 100% of the patients complete definitive surgery (56,63). Modifications and variations in surgical technique continue to evolve. Evidence on long-term outcomes such as DFS and Quality of Life supporting the use of ALPPS is scarce (72,76). ALPPS may be a superior choice than conventional techniques in carefully selected group of patients. Use of the ALPPS Risk Score may help in patient selection (55).
Key points for clinical practice:
- ALPPS leads to accelerated increase in the FLR in short time (<2 weeks);
- ALPPS has higher morbidity and hence patient selection is important;
- Oncological outcomes of ALPPS are at least comparable to existing techniques;
- The ALPPS Risk Score may be used to guide patient selection;
- PVE also induces liver hypertrophy, but takes longer time with risk of tumour progression.
Acknowledgments
The authors acknowledge the ALPPS Registry for sharing of the data.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Couinaud C. Liver lobes and segments: notes on the anatomical architecture and surgery of the liver. La Presse médicale 1954;62:709. [PubMed]
- Gosink BB. Evaluation of hepatic neoplasms. AJR Am J Roentgenol 1980;134:621. [Crossref] [PubMed]
- Kabir T, Ye M, Mohd Noor NA, et al. Preoperative Neutrophil-to-Lymphocyte Ratio Plus Platelet-to-Lymphocyte Ratio Predicts the Outcomes after Curative Resection for Hepatocellular Carcinoma. Int J Hepatol 2019;2019:4239463. [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision‐making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176-81. [PubMed]
- Heymsfield SB, Fulenwider T, Nordlinger B, et al. Accurate Measurement of Liver, Kidney, and Spleen Volume and Mass by Computerized Axial Tomography. Ann Intern Med 1979;90:185-7. [Crossref] [PubMed]
- Ogasawara K, Une Y, Nakajima Y, et al. The significance of measuring liver volume using computed tomographic images before and after hepatectomy. Surg Today 1995;25:43-8. [Crossref] [PubMed]
- Henderson JM, Heymsfield SB, Horowitz J, et al. Measurement of liver and spleen volume by computed tomography. Assessment of reproducibility and changes found following a selective distal splenorenal shunt. Radiology 1981;141:525-7. [Crossref] [PubMed]
- Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 2002;8:233-40. [Crossref] [PubMed]
- Ribero D, Chun YS, Vauthey JN, editors. Standardized liver volumetry for portal vein embolization. Seminars in interventional radiology. Thieme Medical Publishers, 2008.
- Bourquain H, Schenk A, Link F, et al. HepaVision2—a software assistant for preoperative planning in living-related liver transplantation and oncologic liver surgery. CARS 2002 Computer Assisted Radiology and Surgery. Springer, 2002:341-6.
- Asencio JM, García Sabrido JL, Olmedilla L. How to expand the safe limits in hepatic resections? J Hepatobiliary Pancreat Sci 2014;21:399-404. [Crossref] [PubMed]
- Tanaka K, Shimada H, Matsuo K, et al. Remnant liver regeneration after two-stage hepatectomy for multiple bilobar colorectal metastases. Eur J Surg Oncol 2007;33:329-35. [Crossref] [PubMed]
- Tanaka K, Shimada H, Matsuo K, et al. Regeneration after two-stage hepatectomy vs repeat resection for colorectal metastasis recurrence. J Gastrointest Surg 2007;11:1154-61. [Crossref] [PubMed]
- Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care 2005;11:150-5. [Crossref] [PubMed]
- Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 2007;356:1545-59. [Crossref] [PubMed]
- Vauthey JN, Chaoui A, Do K-A, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000;127:512-9. [Crossref] [PubMed]
- Joechle K, Moser C, Ruemmele P, et al. ALPPS (associating liver partition and portal vein ligation for staged hepatectomy) does not affect proliferation, apoptosis, or angiogenesis as compared to standard liver resection for colorectal liver metastases. World J Surg Oncol 2017;15:57. [Crossref] [PubMed]
- Buac S, Schadde E, Schnitzbauer AA, et al. The many faces of ALPPS: surgical indications and techniques among surgeons collaborating in the international registry. HPB (Oxford) 2016;18:442-8. [Crossref] [PubMed]
- de Santibañes E, Clavien PA. Playing Play-Doh to Prevent Postoperative Liver Failure: The “ALPPS” approach. Ann Surg 2012;255:415-7. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right Portal Vein Ligation Combined With In Situ Splitting Induces Rapid Left Lateral Liver Lobe Hypertrophy Enabling 2-Staged Extended Right Hepatic Resection in Small-for-Size Settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Ratti F, Cipriani F, Gagliano A, et al. Defining indications to ALPPS procedure: technical aspects and open issues. Updates Surg 2014;66:41-9. [Crossref] [PubMed]
- Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, et al. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery 2015;157:194-201. [Crossref] [PubMed]
- Li J, Girotti P, Königsrainer I, et al. ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg 2013;17:956-61. [Crossref] [PubMed]
- Knoefel WT, Gabor I, Rehders A, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two‐stage liver resection. Br J Surg 2013;100:388-94. [Crossref] [PubMed]
- Nadalin S, Capobianco I, Li J, et al. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons learned from 15 cases at a single centre. Zeitschrift für Gastroenterologie 2014;52:35-42. [Crossref] [PubMed]
- Machado MA, Makdissi FF, Surjan RC. Right trisectionectomy with principle en bloc portal vein resection for right-sided hilar cholangiocarcinoma: no-touch technique. Ann Surg Oncol 2012;19:1324-5. [Crossref] [PubMed]
- Alvarez FA, Ardiles V, Claria RS, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg 2013;17:814-21. [Crossref] [PubMed]
- Dokmak S, Belghiti J. Which limits to the “ALPPS” approach? Ann Surg 2012;256:e6. [Crossref] [PubMed]
- Sala S, Ardiles V, Ulla M, et al. Our initial experience with ALPPS technique: encouraging results. Updates Surg 2012;64:167-72. [Crossref] [PubMed]
- Oldhafer KJ, Donati M, Jenner RM, et al. ALPPS for patients with colorectal liver metastases: effective liver hypertrophy, but early tumor recurrence. World J Surg 2014;38:1504-9. [Crossref] [PubMed]
- Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 2014;260:829-36. [Crossref] [PubMed]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 2006;43:S45-53. [Crossref] [PubMed]
- Ferrero A, Viganò L, Polastri R, et al. Postoperative liver dysfunction and future remnant liver: where is the limit? World J Surg 2007;31:1643-51. [Crossref] [PubMed]
- Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540-8. [PubMed]
- Bertens KA, Hawel J, Lung K, et al. ALPPS: challenging the concept of unresectability–a systematic review. Int J Surg 2015;13:280-7. [Crossref] [PubMed]
- Mortensen KE, Revhaug A. Liver regeneration in surgical animal models–a historical perspective and clinical implications. Eur Surg Res 2011;46:1-18. [Crossref] [PubMed]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997;276:60-6. [Crossref] [PubMed]
- Schlegel A, Lesurtel M, Melloul E, et al. ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann Surg 2014;260:839-46. [Crossref] [PubMed]
- Böhm F, Köhler UA, Speicher T, et al. Regulation of liver regeneration by growth factors and cytokines. EMBO molecular medicine 2010;2:294-305. [Crossref] [PubMed]
- Fisher B, Szuch P, Levine M, et al. A portal blood factor as the humoral agent in liver regeneration. Science 1971;171:575-7. [Crossref] [PubMed]
- Fisher B, Russ C, Updegraff H, et al. Effect of increased hepatic blood flow upon liver regeneration. AMA Arch Surg 1954;69:263-72. [Crossref] [PubMed]
- Gall TM, Sodergren MH, Frampton AE, et al. Radio-frequency-assisted liver partition with portal vein ligation (RALPP) for liver regeneration. Ann Surg 2015;261:e45-6. [Crossref] [PubMed]
- Chan A, Zhang WY, Chok K, et al. ALPPS Versus Portal Vein Embolization for Hepatitis-related Hepatocellular Carcinoma: A Changing Paradigm in Modulation of Future Liver Remnant Before Major Hepatectomy. Ann Surg 2019. [Epub ahead of print]. [Crossref]
- Vennarecci G, Laurenzi A, Sandri GL, et al. The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol 2014;40:982-8. [Crossref] [PubMed]
- D’Haese JG, Neumann J, Weniger M, et al. Should ALPPS be used for liver resection in intermediate-stage HCC? Ann Surg Oncol 2016;23:1335-43. [Crossref] [PubMed]
- Wang CC, Jawade K, Yap AQ, et al. Resection of large hepatocellular carcinoma using the combination of liver hanging maneuver and anterior approach. World J Surg 2010;34:1874-8. [Crossref] [PubMed]
- Schadde E, Malagó M, Hernandez-Alejandro R, et al. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery 2015;157:676-89. [Crossref] [PubMed]
- Linecker M, Björnsson B, Stavrou GA, et al. Risk adjustment in ALPPS is associated with a dramatic decrease in early mortality and morbidity. Ann Surg 2017;266:779-86. [Crossref] [PubMed]
- Linecker M, Kambakamba P, Raptis DA, et al. ALPPS in neuroendocrine liver metastases not amenable for conventional resection - lessons learned from an interim analysis of the International ALPPS Registry. HPB (Oxford) 2019. [Epub ahead of print].
- Mayo SC, De Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 2010;17:3129-36. [Crossref] [PubMed]
- Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg 2015;262:780-5. [Crossref] [PubMed]
- Vennarecci G, Grazi GL, Sperduti I, et al. ALPPS for primary and secondary liver tumors. Int J Surg 2016;30:38-44. [Crossref] [PubMed]
- Madhavan S, Shelat VG, Soong SL, et al. Predicting morbidity of liver resection. Langenbecks Arch Surg 2018;403:359-69. [Crossref] [PubMed]
- Linecker M, Stavrou GA, Oldhafer KJ, et al. The ALPPS Risk Score. Ann Surg 2016;264:763-71. [Crossref] [PubMed]
- Schadde E, Ardiles V, Slankamenac K, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 2014;38:1510-9. [Crossref] [PubMed]
- Robles R, Parrilla P, López‐Conesa A, et al. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br J Surg 2014;101:1129-34. [Crossref] [PubMed]
- Torres OJ, Fernandes Ede S, Oliveira CV, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): the Brazilian experience. Arq Bras Cir Dig 2013;26:40-3. [Crossref] [PubMed]
- Adam R, Miller R, Pitombo M, et al. Two-stage hepatectomy approach for initially unresectable colorectal hepatic metastases. Surg Oncol Clin N Am 2007;16:525-36. [Crossref] [PubMed]
- Lam VW, Laurence JM, Johnston E, et al. A systematic review of two‐stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB 2013;15:483-91. [Crossref] [PubMed]
- Chua TC, Liauw W, Chu F, et al. Summary outcomes of two‐stage resection for advanced colorectal liver metastases. J Surg Oncol 2013;107:211-6. [Crossref] [PubMed]
- Alvarez FA, Ardiles V, de Santibañes E. The ALPPS approach for the management of colorectal carcinoma liver metastases. Curr Colorectal Cancer Rep 2013;9:168-77. [Crossref]
- Shindoh J, Vauthey JN, Zimmitti G, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 2013;217:126-33. [Crossref] [PubMed]
- Sandström P, Røsok BI, Sparrelid E, et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg 2018;267:833. [Crossref] [PubMed]
- Vennarecci G, Sandri GBL, Ettorre GM. Performing the ALPPS procedure by anterior approach and liver hanging maneuver. Ann Surg 2016;263:e11. [Crossref] [PubMed]
- Petrowsky H, Györi G, de Oliveira M, et al. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg 2015;261:e90-2. [Crossref] [PubMed]
- Linecker M, Kambakamba P, Reiner CS, et al. How much liver needs to be transected in ALPPS? A translational study investigating the concept of less invasiveness. Surgery 2017;161:453-64. [Crossref] [PubMed]
- Machado MAC, Makdissi FF, Surjan RC. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann Surg 2012;256:e13. [Crossref] [PubMed]
- de Santibañes E, Alvarez FA, Ardiles V, et al. Inverting the ALPPS paradigm by minimizing first stage impact: the Mini-ALPPS technique. Langenbecks Arch Surg 2016;401:557-63. [Crossref] [PubMed]
- Wang Z, Peng Y, Sun Q, et al. Salvage transhepatic arterial embolization after failed stage I ALPPS in a patient with a huge HCC with chronic liver disease: A case report. Int J Surg Case Rep 2017;39:131-5. [Crossref] [PubMed]
- Shindoh J, Tzeng CW, Aloia TA, et al. Portal vein embolization improves rate of resection of extensive colorectal liver metastases without worsening survival. Br J Surg 2013;100:1777-83. [Crossref] [PubMed]
- Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta-analysis. World J Surg 2018;42:806-15. [Crossref] [PubMed]
- Kambakamba P, Linecker M, Alvarez FA, et al. Short Chemotherapy-Free Interval Improves Oncological Outcome in Patients Undergoing Two-Stage Hepatectomy for Colorectal Liver Metastases. Ann Surg Oncol 2016;23:3915-23. [Crossref] [PubMed]
- Langenhoff BS, Krabbe PF, Wobbes T, et al. Quality of life as an outcome measure in surgical oncology. Br J Surg 2001;88:643-52. [Crossref] [PubMed]
- Ahmed S, de Souza NN, Qiao W, et al. Quality of life in hepatocellular carcinoma patients treated with transarterial chemoembolization. HPB Surg 2016;2016:6120143. [PubMed]
- Wanis KN, Ardiles V, Alvarez FA, et al. Intermediate-term survival and quality of life outcomes in patients with advanced colorectal liver metastases undergoing associating liver partition and portal vein ligation for staged hepatectomy. Surgery 2018;163:691-7. [Crossref] [PubMed]
- Vennarecci G, Laurenzi A, Santoro R, et al. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg 2014;38:1498-503. [Crossref] [PubMed]
- Fleres F, Piardi T, Sommacale D. How to do: technique of liver hanging maneuver—step by step. J Vis Surg 2018;4:213. [Crossref]
- Nadalin S, Li J, Lang H, et al. The White test: a new dye test for intraoperative detection of bile leakage during major liver resection. Arch Surg 2008;143:402-4. [Crossref] [PubMed]
- Yugasaravanan K, Affirul C, Zamri Z, et al. White gauze test: a novel technique in preventing post-hepatectomy bile leak. Clin Ter 2015;166:e102-4. [PubMed]
- Clavien PA, Yadav S, Sindram D, et al. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000;232:155. [Crossref] [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [Crossref] [PubMed]
- Madoff DC, Abdalla EK, Vauthey JN. Portal Vein Embolization in Preparation for Major Hepatic Resection: Evolution of a New Standard of Care. J Vasc Interv Radiol 2005;16:779-90. [Crossref] [PubMed]
- Isfordink CJ, Samim M, Braat M, et al. Portal vein ligation versus portal vein embolization for induction of hypertrophy of the future liver remnant: A systematic review and meta-analysis. Surg Oncol 2017;26:257-67. [Crossref] [PubMed]
- Garlipp B, de Baere T, Damm R, et al. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology 2014;59:1864-73. [Crossref] [PubMed]
- Hoekstra LT, van Lienden KP, Doets A, et al. Tumor progression after preoperative portal vein embolization. Ann Surg 2012;256:812-7. [Crossref] [PubMed]
- Simoneau E, Aljiffry M, Salman A, et al. Portal vein embolization stimulates tumour growth in patients with colorectal cancer liver metastases. HPB 2012;14:461-8. [Crossref] [PubMed]
- de Graaf W, van den Esschert JW, van Lienden KP, et al. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol 2009;16:423-30. [Crossref] [PubMed]
- Yoo H, Kim JH, Ko GY, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol 2011;18:1251-7. [Crossref] [PubMed]
- Aoki T, Imamura H, Hasegawa K, et al. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg 2004;139:766-74. [Crossref] [PubMed]
- Ogata S, Belghiti J, Farges O, et al. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg 2006;93:1091-8. [Crossref] [PubMed]
- Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012;56:464-73. [Crossref] [PubMed]
- Wasan H, Kennedy A, Coldwell D, et al. Integrating radioembolization with chemotherapy in the treatment paradigm for unresectable colorectal liver metastases. Am J Clin Oncol 2012;35:293-301. [Crossref] [PubMed]
- Guiu B, Chevallier P, Denys A, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol 2016;26:4259-67. [Crossref] [PubMed]
- Esposito F, Lim C, Lahat E, et al. Combined hepatic and portal vein embolization as preparation for major hepatectomy: a systematic review. HPB (Oxford) 2019;21:1099-106. [Crossref] [PubMed]
- Guiu B, Quenet F, Escal L, et al. Extended liver venous deprivation before major hepatectomy induces marked and very rapid increase in future liver remnant function. Eur Radiol 2017;27:3343-52. [Crossref] [PubMed]
- Pandanaboyana S, Bell R, Hidalgo E, et al. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery 2015;157:690-8. [Crossref] [PubMed]
- Aussilhou B, Lesurtel M, Sauvanet A, et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg 2008;12:297-303. [Crossref] [PubMed]
- Allard MA, Adam R, Bucur PO, et al. Posthepatectomy portal vein pressure predicts liver failure and mortality after major liver resection on noncirrhotic liver. Ann Surg 2013;258:822-9. [Crossref] [PubMed]
- Golse N, Bucur PO, Adam R, et al. New Paradigms in Post-hepatectomy Liver Failure. J Gastrointest Surg 2013;17:593-605. [Crossref] [PubMed]
- Arakawa Y, Shimada M, Uchiyama H, et al. Beneficial effects of splenectomy on massive hepatectomy model in rats. Hepatol Res 2009;39:391-7. [Crossref] [PubMed]
- Sugawara Y, Yamamoto J, Shimada K, et al. Splenectomy in patients with hepatocellular carcinoma and hypersplenism11No competing interests declared. J Am Coll Surg 2000;190:446-50. [Crossref] [PubMed]
- Takamatsu Y, Hori T, Machimoto T, et al. Intentional modulation of portal venous pressure by splenectomy saves the patient with liver failure and portal hypertension after major hepatectomy: is delayed splenectomy an acceptable therapeutic option for secondary portal hypertension? Am J Case Rep 2018;19:137. [Crossref] [PubMed]
- Yoshizumi T, Taketomi A, Soejima Y, et al. The beneficial role of simultaneous splenectomy in living donor liver transplantation in patients with small-for-size graft. Transplant International 2008;21:833-42. [Crossref] [PubMed]
- Umeda Y, Yagi T, Sadamori H, et al. Preoperative proximal splenic artery embolization: a safe and efficacious portal decompression technique that improves the outcome of live donor liver transplantation. Transpl Int 2007;20:947-55. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Lang H, et al. ALPPS: response to letter to the editor. Ann Surg 2012;256:e16-7. [Crossref]
- Chan AC, Chok K, Dai JW, et al. Impact of split completeness on future liver remnant hypertrophy in associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in hepatocellular carcinoma: Complete-ALPPS versus partial-ALPPS. Surgery 2017;161:357-64. [Crossref] [PubMed]
- Gutt CN, Oniu T, Schemmer P, et al. Fewer adhesions induced by laparoscopic surgery? Surg Endosc 2004;18:898-906. [Crossref] [PubMed]
- Xiao L, Li JW, Zheng SG. Totally laparoscopic ALPPS in the treatment of cirrhotic hepatocellular carcinoma. Surg Endosc 2015;29:2800-1. [Crossref] [PubMed]
- Vicente E, Quijano Y, Ielpo B, et al. First ALPPS procedure using a total robotic approach. Surg Oncol 2016;25:457. [Crossref] [PubMed]
- Krishnamurthy J, Naragund AV, Mahadevappa B. First ever robotic stage one ALPPS procedure in India: for colorectal liver metastases. Indian J Surg 2018;80:269-71. [Crossref] [PubMed]
- Shelat VG, Cipriani F, Basseres T, et al. Pure laparoscopic liver resection for large malignant tumors: does size matter? Ann Surg Oncol 2015;22:1288-93. [Crossref] [PubMed]
- Martínez-Cecilia D, Cipriani F, Vishal S, et al. Laparoscopic versus open liver resection for colorectal metastases in elderly and octogenarian patients: a multicenter propensity score based analysis of short-and long-term outcomes. Ann Surg 2017;265:1192-200. [Crossref] [PubMed]
- López-López V, Robles-Campos R, Brusadin R, et al. Tourniquet-ALPPS is a promising treatment for very large hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Oncotarget 2018;9:28267. [Crossref] [PubMed]
- Robles-Campos R, Brusadin R, López-Conesa A, et al. Long-Term Outcome After Conventional Two-Stage Hepatectomy Versus Tourniquet-ALPPS in Colorectal Liver Metastases: A Propensity Score Matching Analysis. World J Surg 2019;43:2281-9. [Crossref] [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;13:750-1. [Crossref] [PubMed]
- Selby LKE, Tay RX, Woon WW, et al. Validity of the Barcelona Clinic Liver Cancer and Hong Kong Liver Cancer staging systems for hepatocellular carcinoma in Singapore. J Hepatobiliary Pancreat Sci 2017;24:143-52. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. editors. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Seminars in liver disease. Thieme Medical Publishers, 1990.
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Manzini G, Henne-Bruns D, Porzsolt F, et al. Is there a standard for surgical therapy of hepatocellular carcinoma in healthy and cirrhotic liver? A comparison of eight guidelines. BMJ Open Gastroenterol 2017;4:e000129. [Crossref] [PubMed]
- Starzl TE, Putnam C, Groth C, et al. Alopecia, ascites, and incomplete regeneration after 85 to 90 per cent liver resection. Am J Surg 1975;129:587-90. [Crossref] [PubMed]
- Soggiu F, Giovinazzo F, Straiton J, et al. Monosegment ALPPS hepatectomy preserving segment 4 for colorectal liver metastases: literature review and our experience. Hepatobiliary Surg Nutr 2018;7:105. [Crossref] [PubMed]
- Donati M, Basile F, Oldhafer KJ. Present status and future perspectives of ALPPS (associating liver partition and portal vein ligation for staged hepatectomy). Future Oncol 2015;11:2255-8. [Crossref] [PubMed]
- Pekolj J, Alvarez FA, Biagiola D, et al. Totally Laparoscopic Mini-ALPPS Using a Novel Approach of Laparoscopic-Assisted Transmesenteric Portal Vein Embolization. J Laparoendosc Adv Surg Tech A 2018;28:1229-33. [Crossref] [PubMed]
- Alvarez FA, Ardiles V, de Santibañes M, et al. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg 2015;261:723-32. [Crossref] [PubMed]
- Rong Z, Lu Q, Yan J. Totally laparoscopic radiofrequency-assisted liver partition with portal vein ligation for hepatocellular carcinoma in cirrhotic liver. Medicine (Baltimore) 2017;96:e9432. [Crossref] [PubMed]
- Nguyen KT, Marsh JW, Tsung A, et al. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348-56. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Gauzolino R, Castagnet M, Blanleuil ML, et al. The ALPPS technique for bilateral colorectal metastases: three “variations on a theme”. Updates Surg 2013;65:141-8. [Crossref] [PubMed]
Cite this article as: Chan KS, Low JK, Shelat VG. Associated liver partition and portal vein ligation for staged hepatectomy: a review. Transl Gastroenterol Hepatol 2020;5:37.



