
Well differentiated arginase-1 negative hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most common primary carcinoma occurring in the liver. Cholangiocarcinoma and metastatic malignant tumors can mimic HCC which can result in a diagnostic dilemma. Immunohistochemical markers, hepatocyte paraffin-1 (Hep-Par-1), glypican-3, polyclonal carcinoembryonic antigen (pCEA), CD10, and α-fetoprotein are useful diagnostic tools in the detection of HCC (1-3). However, their expression has also been reported in non-HCC malignant neoplasms, which can be a potential source of false positives (2,4,5). Arginase is manganese containing enzyme that catalyzes the final step in the urea cycle (6). In normal and neoplastic liver, arginase-1 (Arg-1) is expressed in the hepatocytes, most especially in the periportal hepatocytes (7,8), but not in the bile ducts, endothelial and Kupffer cells with a higher degree of sensitivity and specificity (9-11). Yan and colleagues (12) demonstrated sensitivities for Arg-1 in well, moderately, and poorly differentiated HCCs as 100%, 96.2%, and 85.7%, respectively, while HepPar-1 demonstrated sensitivities of 100%, 83.0%, and 46.4% in these tumors, respectively. Although, poorly differentiated HCC, can lose Arg-1 expression, well differentiated HCCs are rarely negative for arginase immunostaining. A recent study (13), reported 7 cases of well differentiated HCCs that were arginase negative but Hep-Par-1 positive. However, clinical correlation of these tumors was not available. Of all the 174 well differentiated HCC reported in the English literature, only 8 have been negative for Arg-1 (13,14). In this report, we performed a retrospective study and investigated the sensitivity and specificity of Arg-1 in the detection of well differentiated HCC resection specimens using a highly specific Arg-1 monoclonal antibody. We also determined the clinicopathologic correlation of arginase negative well differentiated HCCs which were not performed in the previous studies, perhaps this can help in the future to better understand the role of arginase negative HCC, the possible use of targeted therapy in HCC as well as in the prevention of false positives of arginase expression.
Methods
Case selection
The cases included in this study were obtained from the archived surgical pathology files of the Medstar Georgetown University Hospital between June 2013 and December 2016. A total of 64 neoplasms (Standard paraffin block tissue sections from 40 well differentiated HCCs and 24 liver resections of non-HCC (including 12 cholangiocarcinoma, 8 colon carcinoma, 2 pancreatic endocrine tumors, 1 pancreatic adenocarcinoma and 1 renal cell carcinoma) were evaluated. In order to expand the non-HCC tumors and detect the specificity of our antibody, tissue microarray with 200 samples of non-liver primary tumors including 100 small cell carcinomas of the lung, 3 adenocarcinoma of the lung, 1 carcinoid tumor of the duodenum, 5 ductal carcinoma of the breast, 3 thyroid carcinoma, 50 thymoma, 3 gastric adenocarcinoma, 10 colorectal carcinoma, 12 cholangiocarcinoma, 7 pancreatic adenocarcinoma, 1 adrenocortical carcinoma, 1 prostate carcinoma, 1 renal cell carcinoma and 3 non-Hodgkin lymphomas involving the lymph nodes were also examined for expression of Arg-1. This method allows for increased numbers of non-HCC tumors to be evaluated for Arg-1 expression. The diagnosis of the carcinoma were based on the combination of the clinical and pathology reports and hematoxylin and eosin (H&E) stained slides for the cases and the histologic grade of HCC was established using the World Health Organization criteria (15,16), characterized by enlarged polygonal cells arranged in plates 2 to 5 cells in width with occasional pseudoacinar structures containing bile with minimal cytologic atypia. The diagnosis of HCC was determined by the detection of the histologic morphology of HCC and expression of at least one marker of hepatocytic differentiation such as HepPar-1 and canalicular expression of pCEA. This study was approved by the Georgetown University Institutional board review (IRB #2018-1025).
Tissue micro-array (TMA)
TMA construction was carried out using formalin fixed paraffin embedded tissue blocks of non-HCC. The representative tumor areas were identified within blocks. Four microns thick sections of the block were cut and cores (diameter: 1.5 mm) were punched out and embedded unto microscopic slides in a systematic manner.
Immunohistochemistry procedure
Four-micron thick sections of the formalin-fixed, paraffin-embedded tissue blocks and tissue microarray slides of all the studied cases were investigated for the presence of a mouse monoclonal antibody against Arg-1 (clone SL6ARG, Invitrogen, SanDiego, CA, dilution 1:4,000). The identified arginase negative HCC were also evaluated for reactivity for a rabbit polyclonal antibody against pCEA [Dako, Glosttrup, Denmark, ready to use (RTU)], and a mouse monoclonal antibody against HepPar-1 (clone OCH1E5, Dako, Glosttrup, Denmark, RTU), using an automated method (DAKO EnVision + Dual Link System-HRP). Pre-treatment of the formalin-fixed, paraffin-embedded tissue sections with heat-induced epitope retrieval (HIER) were performed using diluted Envision FLEX Target Retrieval Solution, Low pH (×50) (K8004). Deparaffinization, rehydration and epitope retrieval are performed in DAKO PT Link (PT100/PT101). The following parameters are used for PT Link: Pre-Heat temperature: 85 °C, epitope retrieval temperature and time: 97 °C for 20 minutes, cool down to 65 °C. Racks are placed in diluted Envision Flex Wash Buffer (×20) (code K8007) for 5 minutes. The slides are treated with Flex Peroxidase Blocking solution (SM801) for 5 minutes, followed by incubation with the primary antibody (Arg-1, pCEA or HepPar-1) for 20 mins, The slides are treated sequentially with Flex Mouse Linker (SM804) for 15 min, Flex HRP (SM802) for 20 min and Flex diaminobenzidine (DAB) with Substrate-Chromogen (SM803) for 10 min. Sections were counterstained with hematoxylin for 5 mins before checked under microscope. Normal liver tissues were used as positive control, while negative control was done using the same tissue (normal liver), omitting the primary antibody. In addition, non-liver tumors in the TMA were also used as negative controls for Arg-1. Cytoplasmic staining with or without nuclear staining in the tumor cells was considered positive. Positive staining was classified as diffuse (when ≥90% of the tumor cells were positive), regional (when ≥50% but ≤90% of the tumor cells were positive) and focal (when ≤50% of the tumor cells were positive). The intensity of immunostaining was scored as 0 (no staining), 1+ (weak staining), 2+ (moderate staining), and 3+ (intense staining). Immunoreactivity was semiquantitatively scored by 2 pathologists.
Results
Immunohistochemical findings
The patients included 44 men and 20 women with an age range at diagnosis of 17–78 years (mean age 62 years). The patients with HCC included 32 males and 8 females with an age range of 17–72 years and a mean age of 63.5 years. All the 24 non-HCC neoplasms [including 12 cholangiocarcinoma (Figure 1), 8 colon carcinoma, 2 pancreatic endocrine tumors, 1 pancreatic adenocarcinoma and 1 renal cell carcinoma] were all negative for Arg-1. In addition, all benign liver tissues adjacent to non-HCC (Figure 1C) (n=24) as well as liver tissue adjacent to the HCC tumors (n=40) showed diffuse and strong (3+) immunostaining for Arg-1. The tissue microarray with the 200 non-hepatic tumors were consistently negative for Arg-1 staining, thus showing 100% specificity of our Arg-1 antibody. Arg-1 immunostaining was positive in 36 of 40 cases (diffusely positive with 3+ intensity in 34 cases and regionally positive with 3+ intensity in 2 cases). In 4 cases (10%) Arg-1 immunostaining was negative (with a score of 0) within the tumor cells (Figures 2,3). The background non-neoplastic liver parenchyma was present in 4 of 4 cases, and Arg-1 was strongly and diffusely positive within the background liver parenchyma in all 4 cases (Figure 1D).
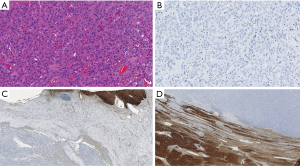
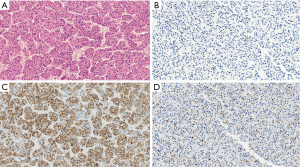
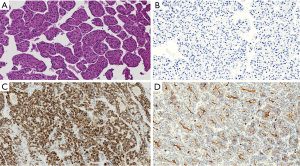
Arginase negative well differentiated HCC
The four Arg-1-negative HCCs arose in 2 females and 2 males (Table 1). The average age at resection was 72±6.7 years. The underlying liver disease was chronic hepatitis C infection in 50% (2 cases) of the well differentiated HCC cases and two of the tumors arose in a background of cirrhosis. Serum a-fetoprotein was within normal limits in 2 cases, and high (>20 ng/mL) in the remaining 2 cases. Seventy-five percent of the tumors were unifocal and two of four cases had microvascular invasion. Only one case had TNM stage III, while the remaining cases had stages I–II. All Arg-1-negative well-differentiated HCCs were positive for HepPar-1 (Figures 2C,3C). Polyclonal CEA was performed on all 4 cases, and they were all positive for its characteristic canalicular pattern of expression in HCC (Figures 2D,3D).
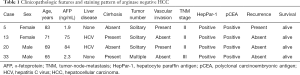
Full table
Clinicopathologic features of Arg-1 positive well differentiated HCC
Eleven females and 25 males had Arg-1 positive well differentiated HCC with a mean age at resection of 65.6±10.2. Twenty-two patients had hepatitis C infection and 23 of the tumors arose in a background of cirrhosis. Serum a-fetoprotein was high (>20 ng/mL) in 13 cases, while the remaining cases were within normal limits. Thirty-nine percent of the tumors (14 cases) were multifocal and four cases had microvascular invasion. Thirty-three cases had TNM stage I–II, while the remaining cases had stages III-IV.
Recurrence and survival
All four Arg-1 negative HCC cases were treated with partial hepatectomy, three had no recurrence and are currently alive and stable with a clinical follow up of 24, 52 and 54 months. However, one patient had a recurrence of HCC at 28 months. The patient had TNM stage II and there was multiple microvascular invasion in the tumor. The recurrent tumor was resected and the patient is stable at 14 months’ post resection. The patients with arginase negative HCCs had a mean recurrence free survival of 39.5 months and a mean overall survival of 43 months. On the other hand, for the Arg-1 positive well differentiated tumors, 8 had recurrent tumors while 5 patients expired. These patients had a mean recurrence free of 37.7 months and a mean overall survival of 40.6 months.
Discussion
Arginase is the last enzyme in the urea cycle that is responsible for the conversion of arginine to urea. Arginase occurs in two isoforms, including Arg-1 and Arginase-2. Arg-1 is primarily found in the cytosol of hepatocytes, whereas Arg-2 expression is high in the kidney and pancreas, but low in the liver (17). Arg-1 shows a high degree of specificity for hepatocellular differentiation and have shown superior sensitivity and specificity to other hepatocellular markers such as HepPar1 and pCEA. Previous studies have shown that well differentiated HCC were always arginase positive, however, more recently that assumption has been shown to be false (13). Because they were the only study that used monoclonal Arg-1 antibody, Clark and colleagues (13) reviewed all reports on arginase expression in well differentiated HCC and discovered that all prior studies used polyclonal Arg-1 antibody which showed an overall sensitivity of 92% with increased negative arginase staining observed in the poorly differentiated HCC. In addition, all reported well differentiated HCCs were positive for Arg-1. It is possible that the use of polyclonal Arg-1 antibody was detecting either weaker expression of the protein or nonspecific cross reactivity with other proteins. In our study we used a highly specific monoclonal Arg-1 antibody that was completely negative in 274 non-HCC tumors with a 100% specificity. The presence of positive staining in adjacent normal liver tissue in non-HCC tumors and in the arginase negative well differentiated HCC supports that the negative staining is a natural property of the tumor cells and not an artifact.
HCC depends on arginine from extracellular sources, for cellular proliferation and survival (18). Deprivation of the tumor cells of arginine results in apoptosis and regression of the cancer (19-21). Recombinant human arginase has been shown to have antitumor effect in HCC by inducing arginine deficiency and causing cell cycle arrest (22,23). However, clear clinical benefits of these agents have not been demonstrated in clinical trials and it is possible that treating HCC with recombinant arginase may be the wrong strategy. Mao and coworkers (24) showed that HCCs with low arginase expression have poor prognosis when compared with those with high arginase expression. Due to the fact that there are conflicting studies of the role of arginase in HCC, it is important to correctly identify arginase expression in HCC and determine the clinicopathologic parameters in rare arginase negative HCC. We demonstrated 4 cases of well differentiated HCC that were negative for Arg-1 expression. Although only two cases demonstrated microvascular invasion, only one case recurred and that was after 28 months. In contrast, the Arg-1 positive well differentiated HCC tumors had more incidences of vascular invasion, multiple tumors, high AFP levels, recurrent tumors and decreased survival. However, due to the small numbers and uneven population in both groups, meaningful statistical significance could not be obtained. Hence the true prognostic value of Arg-1 in well differentiated HCC requires further evaluation in large clinical studies. Since most HCC will express Arg-1, it is difficult to believe that arginase expression will be associated with good prognosis. Clear stratification of Arg-1 into high and low expression using a monoclonal antibody is necessary. In our study, our monoclonal antibody clearly identified all Arg-1 negative well differentiated HCC which should fall into the low arginase expression whereas all our arginase positive tumors showed high expression of arginase expression. However, the number of the arginase negative HCC population is small, therefore precluding any significant conclusion. Besides, Chrzanowska et al. (25) demonstrated high serum arginase activity in patients with HCC, which decreased drastically following curative surgery; thus suggesting a role for arginase in monitoring patients with HCC following hepatectomy. Therefore, it is possible that Arg-1 is involved in the tumorigenesis of HCC and may require other factors, such as having a higher histologic grade or TNM stage, which will result in the progression of HCC. Molecular studies to examine the ARG-1 gene is required to determine if certain mutations or methylation are present which may better serve as predictive markers. Limitations of our study is the small study size of arginase negative tumors due to the rare nature of the entity, hence further studies to fully characterize the implications of arginase negative well differentiated HCC is warranted. Furthermore, it is important to determine that arginase negative well differentiated HCC are indeed of hepatic origin. Although morphology still remains a major key factor to diagnosis, some cases of cholangiocarcinoma (Figure 1A) or metastatic carcinomas to the liver can have a well differentiated hepatoid morphology. Our cases of arginase negative well differentiated HCC were all positive for other hepatocellular markers which include, HepPar-1 and pCEA. This correlates with other reports of arginase negative well differentiated HCC which were all positive for HepPar-1 (13,14). Hence it is important to perform additional markers to prevent false negatives. HepPar-1, which recognizes carbamoyl synthetase, an enzyme in the urea cycle and pCEA are among those commonly used (1). Sensitivities of 100% and 92% has been reported respectively with HepPar-1 and pCEA in well differentiated HCC (26). Glypican 3, a heparin sulfate proteoglycan can also be used in the detection of well differentiated HCC (26), however limitations include lower sensitivity (62%) and expression in other tumors such as lung squamous cell carcinoma and yolk sac tumor (5). Other hepatic immunohistochemical markers include CD10, which yields a canalicular pattern and AFP. Similarly, they are affected by the same limitations that is observed with Glypican 3. Although Arg-1 is the most sensitive marker for the detection of HCC, it is important to use at least 2 hepatic markers to prevent misdiagnosis in Arg-1 negative HCC.
Conclusions
In conclusion, we have demonstrated that well differentiated HCC can be negative for arginase expression. Although, only 8 arginase negative well differentiated HCC tumors have been described, the clinicopathologic outcomes have been poorly described. This entity has been rarely demonstrated possibly due to the use of polyclonal antibodies which has led to false positives in prior studies. Future studies are necessary to fully understand the clinical behavior of arginase negative well differentiated HCC.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Georgetown University Institutional board review (IRB #2018-1025) and written informed consent was waived.
References
- Chu PG, Ishizawa S, Wu E, et al. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol 2002;26:978-88. [Crossref] [PubMed]
- Fan Z, van de Rijn M, Montgomery K, et al. Hep par 1 antibody stain for the differential diagnosis of hepatocellular carcinoma: 676 tumors tested using tissue microarrays and conventional tissue sections. Mod Pathol 2003;16:137-44. [Crossref] [PubMed]
- Leong AS, Sormunen RT, Tsui WM, et al. Hep Par 1 and selected antibodies in the immunohistological distinction of hepatocellular carcinoma from cholangiocarcinoma, combined tumours and metastatic carcinoma. Histopathology 1998;33:318-24. [Crossref] [PubMed]
- Kakar S, Muir T, Murphy LM, et al. Immunoreactivity of Hep Par 1 in hepatic and extrahepatic tumors and its correlation with albumin in situ hybridization in hepatocellular carcinoma. Am J Clin Pathol 2003;119:361-6. [Crossref] [PubMed]
- Aviel-Ronen S, Lau SK, Pintilie M, et al. Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod Pathol 2008;21:817-25. [Crossref] [PubMed]
- Butler SL, Dong H, Cardona D, et al. The antigen for Hep Par 1 antibody is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab Invest 2008;88:78-88. [Crossref] [PubMed]
- Multhaupt H, Fritz P, Schumacher K. Immunohistochemical localisation of arginase in human liver using monoclonal antibodies against human liver arginase. Histochemistry 1987;87:465-70. [Crossref] [PubMed]
- Sekine S, Ogawa R, McManus MT, et al. Dicer is required for proper liver zonation. J Pathol 2009;219:365-72. [Crossref] [PubMed]
- Ordonez NG. Arginase-1 is a novel immunohistochemical marker of hepatocellular differentiation. Adv Anat Pathol 2014;21:285-90. [Crossref] [PubMed]
- McKnight R, Nassar A, Cohen C, et al. Arginase-1: a novel immunohistochemical marker of hepatocellular differentiation in fine needle aspiration cytology. Cancer Cytopathol 2012;120:223-9. [Crossref] [PubMed]
- Radwan NA, Ahmed NS. The diagnostic value of arginase-1 immunostaining in differentiating hepatocellular carcinoma from metastatic carcinoma and cholangiocarcinoma as compared to HepPar-1. Diagn Pathol 2012;7:149. [Crossref] [PubMed]
- Yan BC, Gong C, Song J, et al. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol 2010;34:1147-54. [Crossref] [PubMed]
- Clark I, Shah SS, Moreira R, et al. A subset of well-differentiated hepatocellular carcinomas are Arginase-1 negative. Hum Pathol 2017;69:90-5. [Crossref] [PubMed]
- Koo M, Lipshutz GS, Cederbaum SD, et al. Biopsy-proven Hepatocellular Carcinoma in a 53-year-old Woman With Arginase Deficiency. Pediatr Dev Pathol 2017;20:517-21. [Crossref] [PubMed]
- Martins-Filho SN, Paiva C, Azevedo RS, et al. Histological Grading of Hepatocellular Carcinoma-A Systematic Review of Literature. Front Med (Lausanne) 2017;4:193. [Crossref] [PubMed]
- Theise N, Curado, MP, Francheschi, S editor. Hepatocellular carcinoma. In: Bosman FT, Carneiro F, Hruban RH, et al. (eds). WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer, 2010:205-16.
- Choi S, Park C, Ahn M, et al. Immunohistochemical study of arginase 1 and 2 in various tissues of rats. Acta Histochem 2012;114:487-94. [Crossref] [PubMed]
- Wheatley DN, Campbell E. Arginine deprivation, growth inhibition and tumour cell death: 3. Deficient utilisation of citrulline by malignant cells. Br J Cancer 2003;89:573-6. [Crossref] [PubMed]
- Feun L, You M, Wu CJ, et al. Arginine deprivation as a targeted therapy for cancer. Curr Pharm Des 2008;14:1049-57. [Crossref] [PubMed]
- Gonzalez GG, Byus CV. Effect of dietary arginine restriction upon ornithine and polyamine metabolism during two-stage epidermal carcinogenesis in the mouse. Cancer Res 1991;51:2932-9. [PubMed]
- Ensor CM, Holtsberg FW, Bomalaski JS, et al. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res 2002;62:5443-50. [PubMed]
- Cheng PN, Lam TL, Lam WM, et al. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res 2007;67:309-17. [Crossref] [PubMed]
- Chow AK, Ng L, Sing Li H, et al. Anti-tumor efficacy of a recombinant human arginase in human hepatocellular carcinoma. Curr Cancer Drug Targets 2012;12:1233-43. [PubMed]
- Mao H, Gao W, Lu G, et al. Clinicopathological and prognostic implications of arginase expression in hepatocellular carcinoma. Clin Lab 2013;59:37-43. [Crossref] [PubMed]
- Chrzanowska A GW, Mielczarek-Puta M, Baranczyk-Kuzma A. Significance of arginase determination in body fluids of patients with hepatocellular carcinoma and liver cirrhosis before and after surgical treatment. Clin Biochem 2014;47:1056-59. [Crossref] [PubMed]
- Nguyen T, Phillips D, Jain D, et al. Comparison of 5 Immunohistochemical Markers of Hepatocellular Differentiation for the Diagnosis of Hepatocellular Carcinoma. Arch Pathol Lab Med 2015;139:1028-34. [Crossref] [PubMed]
Cite this article as: Obiorah IE, Chahine J, Park BU, Ko K, deGuzman J, Kallakury B. Well differentiated arginase-1 negative hepatocellular carcinoma. Transl Gastroenterol Hepatol 2019;4:66.


