
CT and MRI imaging and interpretation of hepatic arterioportal shunts
Introduction
Hepatic arterioportal shunts (HAPS) occur due to organic or functional fistulization of blood flow from the arterial hepatic vasculature to the venous portal system. It is a type of hemodynamic abnormality of the liver.
HAPS do not occur in isolation, but rather in association with other hepatic abnormalities. When a HAPS is present, the appearance of these abnormalities on imaging studies is always atypical. The presence of HAPS, independently of underlying liver pathology, is frequently asymptomatic. Nonetheless, over time, and as a function of degree of fistulae, symptoms and potential life-threatening complications from the HAPS, regardless of the underlying pathology, may arise in patients. These systemic complications may include the development of portal hypertension, splenomegaly, as well as accelerated metastasis in patients with malignant tumors (1).
Given the potential for HAPS to be associated with severe underlying liver pathology as well as result in severe complications, a careful evaluation of all patients with HAPS remains critical. Herein, we discuss various etiologies that lead to HAPS and review pertinent radiographic imaging, particularly computed tomography (CT) scan and magnetic resonance imaging (MRI), in order to familiarize the reader to understand the pathophysiology of HAPS, the potential underlying causes for its development, and hopefully translate this to timelier approaches for patients with a potentially life-threatening problem (2,3).
Pathophysiology of HAPS development
Although most frequently associated with an underlying hepatocellular carcinoma (HCC), HAPS may occur as a result of a number of other distinct underlying hepatic pathologies. Conceptually, HAPS may be subdivided into acquired causes, such as secondary to intrahepatic tumors or cirrhosis, post-traumatic causes, such as following liver injury or transhepatic stenting, and congenital causes from vascular malformations. Recognition of these underlying pathologic processes and identification of the presence of HAPS remains critical to directing subsequent care.
Anatomic points of HAPS development
The liver receives a dual blood supply, both from the hepatic artery (25%) and portal vein (75%) (4-7). The hepatic arterial and portal venous supplies to the liver run together within Glisson’s capsule, thus these two structures are immediately adjacent. Furthermore, there are several potential and actual vascular communications between them. HAPS can occur by several pathophysiologic mechanisms that result in the formation of a vascular shunt, including transsinusoidal, transvasal, transtumoral, and transplexal (peribiliary) routes (Figure 1) (8). In other instances, a macroscopic arterioportal fistula can cause direct blood flow from high pressure hepatic arteries to lower pressure portal veins. Furthermore, in some instanced there may be multiple of these etiologies contributing to the formation of HAPS.
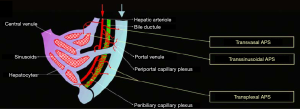
Transsinusoidal route (shunt connecting microscopic interlobular hepatic arterioles and portal venules)
In this route, abnormal flow arises from the hepatic artery connecting to the hepatic sinusoids. Such abnormal connections result in retrograde filling of portal vein branches due to high flow resistance within hepatic venules (9). This mechanism has been used to explain nontumorous HAPS arising in conditions such as cirrhosis and Budd-Chiari syndrome (10).
Tumor thrombus induced transvasal route
In this route of HAPS, vasa vasorum hepatic artery system branches shunt blood from arterial inflow to portal vein branches resulting in arterial shunting into the portal venous system, generally secondarily to the formation of a tumor thrombus (11).
Transtumoral shunt
HAPS can also occur via its draining vein in the setting of hypervascular tumors. Most liver tumors derive the majority of their blood supply from the hepatic arterial supply. This may commonly be observed in the setting of HCC or hemangioma (12).
Transplexal route (peribiliary route)
HAPS may also be observed through arterial shunt involving the peribiliary vascular plexus. Vascular supply is provided by small branches from the hepatic artery and drains into either the portal vein via interlobular veins or into the sinusoids veins (13). In the setting of portal vein occlusion, this pathway appears to be commonly responsible for significant arterioportal communication (14).
Arterioportal fistula
A macroscopic arterioportal fistula may be observed following hepatic trauma, particularly penetrating liver injuries (15).
Radiographic location of shunts
HAPS is divided into three types according to the anatomic location of a shunt within the liver. (I) Central HAPS: when the shunt is located in the porta hepatis. Such central HAPS are manifest as earlier enhancement and arterial-phase opacification of the central portal veins during CT scanning. (II) Peripheral HAPS: when the shunt is located in peripheral liver parenchyma. Given the smaller hepatic vascular size in this region, early contrast into the portal veins generally does not occur. Shunts in this region of the liver usually manifest as transient hepatic enhancement differences (THED). (III) Mixed HAPS: alternatively, HAPS may demonstrate both central and peripheral findings.
Based upon the timing of the appearance of HAPS during CT scan, HAPS may be divided into three degrees of severity: (I) severe HAPS demonstrating early portal vein or first-order branch opacification early in the arterial phase; (II) moderate HAPS demonstrating main portal vein or first-order branch opacification later in the hepatic arterial phase; and (III) mild HAPS revealing second-order or smaller portal vein branches opacification of the in the late HAP.
Central HAPS are usually associated with the highest flow and are associated with surgical shunts through macroscopic arterioportal fistulae or along the transvasal route. These are considered moderate or severe shunts and likely will be associated with symptoms, potentially including GI bleeding, splenomegaly, or an abdominal bruit. In contrast, peripheral HAPS are generally functional shunts that follow the transplexal or transsinusoidal routes, and are usually asymptomatic or result in very mild symptoms. Nonetheless, there are exceptions for both shunt types, and considerable overlap is observed (16,17).
CT and MRI appearance
Given the clinical significance of HAPS, both with regard to the potential for underlying pathology and due to the small, but real risk of developing complications from shunt, it remains critical to understand and be vigilant to diagnose HAPS. Critical findings of HAPS seen by CT and MRI appearances follow. When performing CT scans for this condition, it is critical to obtain a three-phase (triple) CT, where images are captured during the arterial, venous, and equilibrium phases following bolus administration of intravenous contrast.
Early enhancement of the portal vein
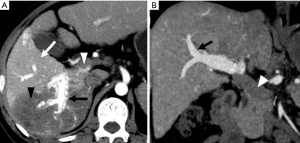
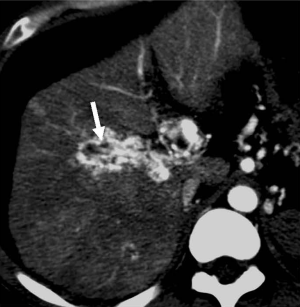
This is a direct demonstration of a HAPS, usually due to a high-flow arterial fistula, generally a macroscopic arterioportal fistula, transvasal or transtumoral shunt. Two practical findings make this observation easier to diagnose: (I) early main portal vein enhancement, occurring before either the splenic (SV) or superior mesenteric vein (SMV); or (II) early enhancement of peripheral portal vein branches before the common portal vein (Figure 2). Frequently early enhancement will also demonstrate early venous opacification, which can be temporally close to the timing and intensity of the abdominal aorta in the setting of a large shunt.
THED
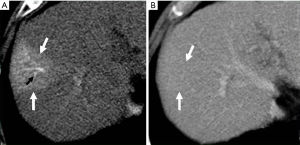
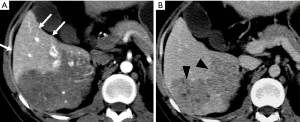
THED indicate transient increased attenuation or signal intensity differences during the HAP, which return to normal or near-normal in the portal venous phase (PVP) and equilibrium phase. It typically manifests as a peripheral, wedge or cone shaped area with a straight margin, and contains normal vessels (Figure 3). THED usually occur in functional HAPS (transsinusoidal shunts and transplexal shunts) caused by decreased portal or hepatic venous flow due to contrast material passage from the high-pressure arterial blood into a low-pressure portal vein branch, thus enhancing a focal area of the liver before the adjacent parenchyma is enhanced through flow from the normal portal venous system (18,19).
Changes of parenchymal enhancement in the liver and spleen
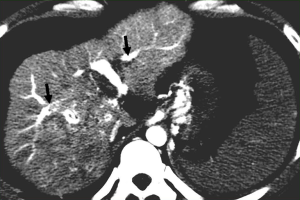
Reversed portal flow shunting of arterial contrast caused by HAPS leads to decreased splenic enhancement and increased hepatic enhancement on HAP images (Figure 4). Therefore, (I) the enhancement of the liver is significant, while that of the spleen is decreased during the HAP; and (II) the density (signal intensity) of the liver is close to or even higher than that of the spleen.
Different causes, different HAPS
HAPS can be classified as tumorous and non-tumorous. The causes of tumorous HAPS include HCC, hemangioma, cholangiocarcinoma, and other rare malignant neoplasms. The non-tumorous HAPS include cirrhosis, Budd-Chiari syndrome, inflammation, trauma, iatrogenic, and congenital abnormalities (Table 1).
Full table
HAPS secondary to HCC
HAPS is most frequently observed in the setting of HCC (16). In patients with large HCCs the incidence may be as high as 63% (12). The presence of HAPS may also cause earlier and wider spread of HCC. It may also potentially affect the ability to administer transarterial chemoembolization (TACE) and should be a consideration in the scope of surgical resection or other liver-directed therapies that might be considered. Furthermore, macroscopic portal vein invasion substantially worsens prognosis. Determination of portal vein invasion is critically important prior to consideration for resection or other therapies (10,20).
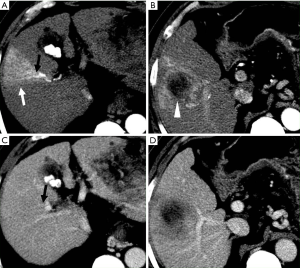
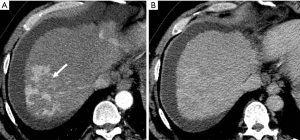
HAPS secondary to HCC may occur through the following routes: (I) transvasal: early invasion and tumor thrombi formation in the branches of the portal vein is frequent in HCC. Since the hypervascular tumor thrombi are supplied by the vasa vasorum of the portal vein wall (21), hepatic arterial flow may drain into the lumen of the portal vein from tumor thrombi (11). These usually manifest as a central and severe HAPS (Figure 5). (II) Transplexal: HCC may sometimes be associated with compromised portal vein flow, and a subsequent HAPS via the transplexal route may be involved (2,16). Arterial blood shunts to the portal venous and hepatic sinusoids via the peribiliary plexus in these cases and usually presents with moderate HAPS (Figure 6). (III) Transsinusoidal: less frequently, retrograde arterial blood shunts into branches of the portal vein via the hepatic sinusoids because of the compromised hepatic venous flow in HCC, which generally constitutes a mild and peripheral HAPS. (IV) Transtumoral: the arterial blood supply to the HCC can be shunted through the tumor venous into peripheral portal veins (10).
Evaluation of HCC with HAPS
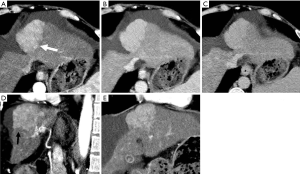
HAP results in the heterogeneous enhancement of the hepatic parenchyma. The enhancement of HCC will be significantly reduced in the situation of serious HAPS due to a large amount of contrast medium flowing into the portal vein through the shunt. On the equilibrium phase, the characteristic washout and extension of the tumor can nonetheless be identified accurately. The equilibrium phase is most useful for diagnosis and delineation of HCC as an area of heterogeneous low density (intensity) (Figures 7,8). The presence of HAPS is not necessarily a contraindication to resection, ablative therapies or other liver-directed therapies, and the degree of resection or other therapy can best be determined in the equilibrium phase (22).
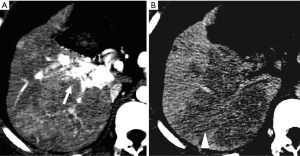
HAPS secondary to hemangioma
HAPS is not infrequently observed in patients with hepatic hemangiomas. It has a reported prevalence of up to 26% in these benign tumors. HAPS are more frequently found in small (<2 cm) hemangiomas with rapid enhancement (23).
HAPS are diagnosed when imaging demonstrates (I) wedge-shaped or irregular homogeneous liver parenchyma enhancement adjacent to tumor, (II) isoattenuation/isointensity or slight hyperattenuation/hyperintensity on PVP scan (Figure 9), or no other demonstrable cause of attenuation or intensity differences is noted (23).
HAPS secondary to hemangioma usually occur through the transtumoral route, which means that the high flow in the rapid enhancement hemangiomas is shunting into the between the hepatic and portal systems. The pathogenesis has not been fully clarified, but it is assumed that slow fill tumors have relatively large vascular spaces, and that rapidly enhancing tumors have smaller vascular spaces and large interstitium. Therefore, it has been proposed that high flow into smaller intravascular spaces is more likely to produce shunting from the hepatic artery to the portal vein (11,18,24).
HAPS secondary to cirrhosis
Liver cirrhosis is caused by chronic hepatocellular injury, necrosis and fibroblast activation. Long-term this results in deposition of connective tissue, nodular hepatocyte regeneration, lobular distortion, as well as abnormal vascular hepatic architecture and flow, including increased post-sinusoidal portal vascular resistance and pressure (25,26). Liver cirrhosis is associated with increased hepatic arterial flow with decreased portal venous flow to the liver. Cirrhosis alone is a well-known cause of non-tumorous HAPS. Angiography will demonstrate nontumorous HAPS in liver cirrhosis in up to 13% of patients (18).
Nontumorous HAPS in the setting of liver cirrhosis are has been attributed to the secondary occlusion of small hepatic venules resulting in the retrograde filling of small portal vein branches via an arterioportal anastomotic (transsinusoidal route) (13,14). Under such conditions, the portal vein functions as a draining rather than a supplying vein to the liver, explaining the compensatory increase in hepatic arterial flow to maintain perfusion (14).
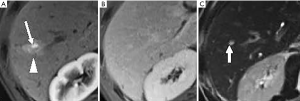
In the progression of cirrhosis and portal hypertension, HAPS generally will occur as a pan-hepatic process due to the decreased portal venous flow and compensatory increase of hepatic arterial flow in patients. This manifests radiologically as a diffusely inhomogeneous parenchymal enhancement and is highly suggestive of advanced cirrhosis (Figure 10) (27-29). These early vascular enhancements observed in HAPS are typically wedge or oval-shaped parenchymal changes, with the base along the liver surface (Figure 11). HAPS that are more centrally located in the hepatic parenchyma are unlikely to make a wedge-shaped enhancement (29). Nonetheless, such wedge-shaped enhancements will follow and demonstrate the axial direction of the shunt vasculature (14,30). Moreover, in the setting of advanced macronodular cirrhosis with its resultant considerable architectural distortion, HAPS-induced THED will become further abnormal, tending to be more prominent and diffuse.
HAPS secondary to Budd-Chiari syndrome
Budd-Chiari syndrome results in an outflow obstruction of the hepatic veins resulting in a dramatic redirection of liver flow. The compromised hepatic venous flow, from webs or thrombosis, result in dramatically increased sinusoidal pressure that reverses hepatic drainage into the portal venous system (5,8). The portal vein becomes a major draining hepatic vein. As in other venous disorders, perfusion remains maintained through a compensatory increase in hepatic arterial flow.
In such cases, HAP images demonstrate early-phase transient hepatic enhancement in the area of obstructed hepatic venous drainage system consistent with the increased arterial flow. The vertex of this wedge-shaped hyperattenuating area has been noted to point to the inferior vena cava (17). This wedge pattern changes on PVP imaging, when a reticular, more heterogeneous, or “mosaic” pattern will be observed (31). Later, delayed images will nonetheless change to a more homogeneous pattern of enhancement (Figure 12) (5).

HAPS secondary to inflammation
HAPS may be observed in inflammatory diseases such as hepatic abscess (32,33), acute cholecystitis (34) and cholangitis (2). Local inflammation in these disorders is associated with an increased hepatic artery perfusion and occlusion or constriction of adjacent portal or hepatic venous structures (2,32-34).
In hepatic abscess, HAPS have been attributed to thrombosis of smaller portal venous branches (33) or to vessel narrowing due to periportal swelling and inflammation (32). Mechanistically, however, it remains unclear if abscess is primary or secondary to pylephlebitis (Figure 13). Similarly, in cholecystitis, HAPS is associated with increased blood flow from the aberrantly dilated cystic artery of the inflamed hypervascular gallbladder (34) and will be associated with thrombosis of regional portal or hepatic veins.
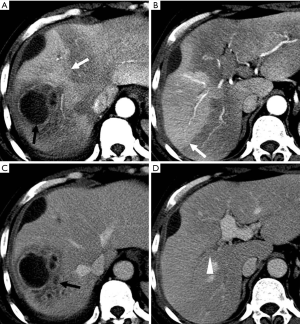
It has been reported that the transplexal route is the most prominent arterioportal communication to arise in the setting of diminished portal venous flow (functional HAPS) secondary to inflammation. It is seen as a transient wedge-shaped hyperattenuated or hyperintense area on HAP images in the early arterial phase that returns to global liver density by the PVP (30). Such early lesions may raise questions for the possibility of tumors. In such circumstances, however, the hyperattenuating/hyperintense pseudolesions can be easily diagnosed by means of their characteristic location around the inflamed area and normal hepatic vessels contained within the hyperattenuating/hyperintensing area (30,33).
HAPS secondary to hereditary hemorrhagic telangiectasia (HHT)
HHT is a rare autosomal dominant disorder of vascular dysplasia occurring with an estimated frequency of approximately 1 per 50,000 (35,36). Also known as Rendu-Osler-Weber syndrome, the disease was originally identified as associated with bleeding disorders but was recognized to be due to underlying vascular dysplasia. Arteriovenous and mucocutaneous telangiectases may affect any organ site. The underlying molecular pathogenesis of the disease is still not clear but could result from genetic mutations in the endoglin gene that interfere with the TGF-beta mediated angiogenesis and its regulatory mechanisms (37).
HHT patients develop mucocutaneous or visceral angiodysplastic lesions (telangiectases and arteriovenous malformations) throughout the body. The prevalence of hepatic involvement in patients with HHT ranges from 8% to 31% in various studies (38-40). Hepatic involvement is characterized by the presence of intrahepatic shunts, vascular lesions, and disseminated intraparenchymal telangiectases (37,40)
HAPS are the most common type of vascular shunt observed in the liver in HHT patients. I may be identified in two-thirds of patients (37). In these patients, abnormal parenchymal perfusion is also observed as THED during HAP. They are characterized by the presence of multiple triangular-shaped, peripheral, high-attenuation segmental areas (Figure 14). These changes reflect the preferential liver perfusion through arterial flow to a hepatic lobe or segments. Decreased portal venous flow occurs secondary to the presence of vascular shunts (2,7). Thus, the demonstration of transient hepatic parenchymal enhancement is evidence for the presence of HAPS. HAPS in the HHT patient may lead to portal hypertension, portosystemic encephalopathy, and possibly atypical cirrhosis (2). Due to these risks, careful examination of liver function by radiologic imaging remains important.
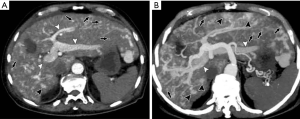
HAPS secondary to trauma
Abdominal trauma including iatrogenic injury (such as percutaneous biopsy, ethanol injection, radiofrequency ablation, and TACE) may cause an arterial to portal fistula or portal vein thrombosis within the liver (31) (Figure 15).
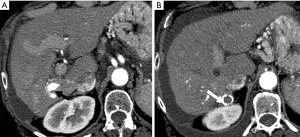
Many post-traumatic or iatrogenic HAPS may be asymptomatic and may be significant only in so far that the observed focal perfusion abnormalities may inaccurately be considered to represent a tumor (5,16). However, it is usually possible to distinguish an iatrogenic HAPS from a new tumor or marginal recurrence by evaluating the patient’s history with the radiologic characteristics regarding configuration and location (41).
The majority of iatrogenic HAPS should be monitored but will close spontaneously and generally within a short period of time of a few weeks (42). The frequency of HAPS following liver biopsy is initially as high as 50% during the first week but drops to 10% over subsequent weeks with many continuing to close with observation (42). Persistent fistulae especially those that re-high-flow arterioportal fistulae causing hyperkinetic portal hypertension are considered relatively rare (15).
Differential diagnosis
Because HCC remains amongst the most significant long-term sequelae of the cirrhotic liver, small HAPS when observed must carefully be distinguished from de novo HCC. The differentiation between HCC and HAPS is a critical clinical issue requiring a vigilant approach.
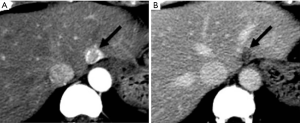
The major differentiating imaging features between HAPS and HCC are as follows: (I) a HAPS usually exhibits a peripheral location with a wedge-shaped appearance, straight margin, and cone-shape on three-dimensional reconstructed images. Normal vessels can be seen coursing through the area. (II) “Washout” is regarded as diagnostic for the differentiation of hypervascular HCC from non-tumorous HAPS. The equilibrium phase is also extremely useful in this differentiation as HCC usually arise as an area of heterogeneous low attenuation (or intensity), while the signal attenuation of a HAPS not arising with an HCC will not decrease below parenchymal background (Figure 16). (III) Repeat follow-up imaging procedures usually demonstrate the resolution or stability of a HAPS, as opposed to growth of an HCC.
Conclusions
HAPS is being observed with greater frequency with the greater utilization of temporal resolution imaging including dynamic CT and MRI. HAPS may be a signal of critical underlying hemodynamic alterations in various hepatic diseases as well as underlying hepatic malignancy (10). Clinicians should be aware of the mechanisms, causes, types, degrees, imaging features, and differential diagnoses of HAPS, so as to make a more sophisticated assessment of HAPS and any associated hepatic diseases to help better plan therapy for patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jackson FC, Perrin EB, Felix WR, et al. A clinical investigation of the portacaval shunt. V. Survival analysis of the therapeutic operation. Ann Surg 1971;174:672-701. [Crossref] [PubMed]
- Quiroga S, Sebastià C, Pallisa E, et al. Improved diagnosis of hepatic perfusion disorders: value of hepatic arterial phase imaging during helical CT. Radiographics 2001;21:65-81; questionnaire 288-94.
- Torabi M, Hosseinzadeh K, Federle MP. CT of nonneoplastic hepatic vascular and perfusion disorders. Radiographics 2008;28:1967-82. [Crossref] [PubMed]
- Bonaldi VM, Bret PM, Reinhold C, et al. Helical CT of the liver: value of an early hepatic arterial phase. Radiology 1995;197:357-63. [Crossref] [PubMed]
- Gryspeerdt S, Van Hoe L, Marchal G, et al. Evaluation of hepatic perfusion disorders with double-phase spiral CT. Radiographics 1997;17:337-48. [Crossref] [PubMed]
- Itai Y, Matsui O. 'Nonportal' splanchnic venous supply to the liver: abnormal findings on CT, US and MRI. Eur Radiol 1999;9:237-43. [Crossref] [PubMed]
- Oliver JH 3rd, Baron RL. Helical biphasic contrast-enhanced CT of the liver: technique, indications, interpretation, and pitfalls. Radiology 1996;201:1-14. [Crossref] [PubMed]
- Kim TK, Choi BI, Han JK, et al. Nontumorous arterioportal shunt mimicking hypervascular tumor in cirrhotic liver: two-phase spiral CT findings. Radiology 1998;208:597-603. [Crossref] [PubMed]
- McCuskey RS. A dynamic and static study of hepatic arterioles and hepatic sphincters. Am J Anat 1966;119:455-77. [Crossref] [PubMed]
- Choi BI, Lee KH, Han JK, et al. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol 2002;3:1-15. [Crossref] [PubMed]
- Bookstein JJ, Cho KJ, Davis GB, et al. Arterioportal communications: observations and hypotheses concerning transsinusoidal and transvasal types. Radiology 1982;142:581-90. [Crossref] [PubMed]
- Itai Y, Furui S, Ohtomo K, et al. Dynamic CT features of arterioportal shunts in hepatocellular carcinoma. AJR Am J Roentgenol 1986;146:723-7. [Crossref] [PubMed]
- Cho KJ, Lunderquist A. The peribiliary vascular plexus: the microvascular architecture of the bile duct in the rabbit and in clinical cases. Radiology 1983;147:357-64. [Crossref] [PubMed]
- Itai Y, Matsui O. Blood flow and liver imaging. Radiology 1997;202:306-14. [Crossref] [PubMed]
- Sato M, Ishida H, Konno K, et al. Longstanding arterioportal fistula after laparoscopic liver biopsy. Abdom Imaging 1999;24:383-5. [Crossref] [PubMed]
- Choi BI, Chung JW, Itai Y, et al. Hepatic abnormalities related to blood flow: evaluation with dual-phase helical CT. Abdom Imaging 1999;24:340-56. [Crossref] [PubMed]
- Itai Y, Murata S, Kurosaki Y. Straight border sign of the liver: spectrum of CT appearances and causes. Radiographics 1995;15:1089-102. [Crossref] [PubMed]
- Yu JS, Kim KW, Sung KB, et al. Small arterial-portal venous shunts: a cause of pseudolesions at hepatic imaging. Radiology 1997;203:737-42. [Crossref] [PubMed]
- Quiroga S, Sebastia MC, Moreiras M, et al. Intrahepatic arterioportal shunt: helical CT findings. Eur Radiol 1999;9:1126-30. [Crossref] [PubMed]
- McKillop IH, Moran DM, Jin X, et al. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res 2006;136:125-35. [Crossref] [PubMed]
- Okuda K, Musha H, Yoshida T, et al. Demonstration of growing casts of hepatocellular carcinoma in the portal vein by celiac angiography: The thread and streaks sign. Radiology 1975;117:303-9. [Crossref] [PubMed]
- Dipasco PJ, Misra S, Koniaris LG. Conformational technique for non-anatomic resection of liver lesions. J Gastrointest Surg 2012;16:1972-5. [Crossref] [PubMed]
- Kim KW, Kim TK, Han JK, et al. Hepatic hemangiomas with arterioportal shunt: findings at two-phase CT. Radiology 2001;219:707-11. [Crossref] [PubMed]
- Yamashita Y, Ogata I, Urata J, et al. Cavernous hemangioma of the liver: pathologic correlation with dynamic CT findings. Radiology 1997;203:121-5. [Crossref] [PubMed]
- Brown JJ, Naylor MJ, Yagan N. Imaging of hepatic cirrhosis. Radiology 1997;202:1-16. [Crossref] [PubMed]
- Koniaris LG, McKillop IH, Schwartz SI, et al. Liver regeneration. J Am Coll Surg 2003;197:634-59. [Crossref] [PubMed]
- Haratake J, Hisaoka M, Yamamoto O, et al. Morphological changes of hepatic microcirculation in experimental rat cirrhosis: a scanning electron microscopic study. Hepatology 1991;13:952-6. [Crossref] [PubMed]
- Villeneuve JP, Dagenais M, Huet PM, et al. The hepatic microcirculation in the isolated perfused human liver. Hepatology 1996;23:24-31. [Crossref] [PubMed]
- Ahn JH, Yu JS, Hwang SH, et al. Nontumorous arterioportal shunts in the liver: CT and MRI findings considering mechanisms and fate. Eur Radiol 2010;20:385-94. [Crossref] [PubMed]
- Murata S, Itai Y, Asato M, et al. Effect of temporary occlusion of the hepatic vein on dual blood in the liver: evaluation with spiral CT. Radiology 1995;197:351-6. [Crossref] [PubMed]
- Chen WP, Chen JH, Hwang JI, et al. Spectrum of transient hepatic attenuation differences in biphasic helical CT. AJR Am J Roentgenol 1999;172:419-24. [Crossref] [PubMed]
- Gabata T, Kadoya M, Matsui O, et al. Dynamic CT of hepatic abscesses: significance of transient segmental enhancement. AJR Am J Roentgenol 2001;176:675-9. [Crossref] [PubMed]
- Balci NC, Semelka RC, Noone TC, et al. Pyogenic hepatic abscesses: MRI findings on T1- and T2-weighted and serial gadolinium-enhanced gradient-echo images. J Magn Reson Imaging 1999;9:285-90. [Crossref] [PubMed]
- Yamashita K, Jin MJ, Hirose Y, et al. CT finding of transient focal increased attenuation of the liver adjacent to the gallbladder in acute cholecystitis. AJR Am J Roentgenol 1995;164:343-6. [Crossref] [PubMed]
- Dakeishi M, Shioya T, Wada Y, et al. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum Mutat 2002;19:140-8. [Crossref] [PubMed]
- Kjeldsen AD, Vase P, Green A. Hereditary hemorrhagic telangiectasia. A population-based study on prevalence and mortality among Danish HHT patients. Ugeskr Laeger 2000;162:3597-601. [PubMed]
- Ianora AA, Memeo M, Sabba C, et al. Hereditary hemorrhagic telangiectasia: multi-detector row helical CT assessment of hepatic involvement. Radiology 2004;230:250-9. [Crossref] [PubMed]
- Reilly PJ, Nostrant TT. Clinical manifestations of hereditary hemorrhagic telangiectasia. Am J Gastroenterol 1984;79:363-7. [PubMed]
- Plauchu H, de Chadarevian JP, Bideau A, et al. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989;32:291-7. [Crossref] [PubMed]
- Bernard G, Mion F, Henry L, et al. Hepatic involvement in hereditary hemorrhagic telangiectasia: clinical, radiological, and hemodynamic studies of 11 cases. Gastroenterology 1993;105:482-7. [Crossref] [PubMed]
- Fujita T, Honjo K, Ito K, et al. Dynamic MR follow-up of small hepatocellular carcinoma after percutaneous ethanol injection therapy. J Comput Assist Tomogr 1998;22:379-86. [Crossref] [PubMed]
- Lee SJ, Lim JH, Lee WJ, et al. Transient subsegmental hepatic parenchymal enhancement on dynamic CT: a sign of postbiopsy arterioportal shunt. J Comput Assist Tomogr 1997;21:355-60. [Crossref] [PubMed]
Cite this article as: Wang Q, Koniaris LG, Milgrom DP, Patel A, Hu M, Cui E, Deng Y, Akisik F. CT and MRI imaging and interpretation of hepatic arterioportal shunts. Transl Gastroenterol Hepatol 2019;4:34.


