
Transplantation versus liver resection in patients with hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the top ten cancers in the world. It is ranked number 5 most common cancer in men and number 9th in women. Eastern and South-Eastern Asia are the two highest incidence of HCC in the world (1). HCC has quickly become the second most common cause of death from cancer worldwide, estimated to be responsible for nearly 746,000 deaths in 2012 (9.1% in total). The prognosis of HCC is very poor partly due to late detection of this malignancy, which in actual fact, has very effective treatment that can potentially provide a good curative long term outcome.
Treatments for HCC have evolved a great deal over the last 4 decades. For small tumours less than 5cm, surgery or transplant has been established as the first-line of treatment. However, the treatment of large HCCs (for example, those which are larger than 10 cm) is debatable with great variation in different modalities of treatment in different centres. HCC greater than 10 cm are often deem not resectable due to the unfavourable prognosis (2-4) with morbidity rate ranging from 25% to 50% and mortality rate from 0% to 8%. Variable studies suggested that tumour size is not the most critical parameters but rather liver remnant is the most determinant of treatment outcomes (5,6).
While a large majority of HCCs in hepatitis B patients develop in the background of cirrhosis, up to 20% to 30% of HCC can develop in patients with normal livers. However, it is rare to develop HCCs in chronic hepatitis C without chronic inflammation or fibrosis (7). Certainly, there are many other etiologies such as alcoholic liver disease, metabolic disease, non-alcoholic steatohepatitis (NASH), iron overload and other.
Surgical treatment remains the mainstay of curative treatment options. In the realm of surgical treatment for HCC, they include surgical resection, liver transplantation and possibly ablation of the tumors surgically. However, the best treatment option for patients with HCC are heavily influenced by the background of liver cirrhosis, presence of portal hypertension, tumor size and location and host condition such as patient fitness.
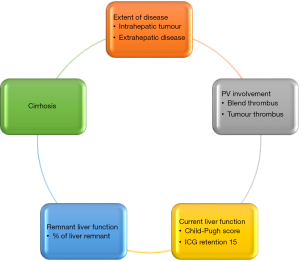
In patients with HCC that are suitable for curative treatment, there is often a tussle between surgical and liver transplantation. On the one hand, surgical resection is a treatment that involves individual patient’s decision that does not need to consider availability of organ. The existing remnant liver harbors a high risk of recurrence with some literature quoting intrahepatic HCC recurrence risk to be as high as 50% to 70%, especially in patients with chronic hepatitis (8). In addition, cirrhotic livers with borderline liver function will also pose risk of liver decompensation after surgical resection (Figure 1).
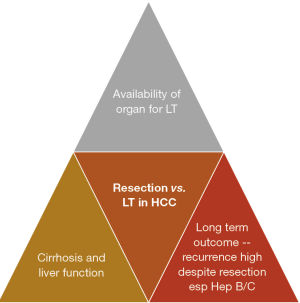
On the other hand, liver transplantation promises a solution that ‘kills two birds with one stone’ where cirrhotic liver and HCC are removed through the total hepatectomy and a new liver is replaced in the patients. However, recipient of liver transplantation requires a donor to donate the liver to him/her. While deceased donor liver organ availability is high in the West, the situation is very different in the East. Most of the gaps in organ shortage in the East is filled by the development of living donor liver transplantation (LDLT), which involves healthy donors undergoing surgery to have a part of their liver removed for transplantation purposes (Figure 1). While it has been shown to be a very safe procedure in the past 3 decades, the ethical considerations in this topic remain unabated. In addition, recipients of liver transplantation will need to commit to a lifetime of immunosuppressants in most circumstances, which is a huge lifestyle change (e.g., regular monitoring of drug levels, tedious and careful food preparations, avoidance of crowded places in the early period post-transplant), and the accompanying risks of infections.
How to select between surgical resection or liver transplantation as treatment for patients with HCC
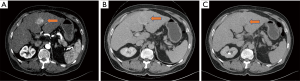
In patients with established risk factors for HCC, such as chronic hepatitis B/C, alcoholic liver disease, metabolic disease, NASH and others, the presence of liver lesions in the liver may indicate the presence of HCCs. In many countries, surveillance screening is the main source of early detection of HCCs. HCCs detected through serial imaging studies are often small, indicating early HCCs. Triphasic computer tomography (CT) scan of the liver shows arterial enhancing lesion with washout in the portovenous and delayed phase (Figure 2A,B,C) highly suggestive of HCC. Other additional information that may help the diagnosis include the serum alpha-fetoprotein (AFP) or PIVKA-II level. Large majority of patients with liver lesions fitting the criteria can almost certainly fit the diagnosis of HCC.
Before offering the appropriate treatments, evaluation of patient’s liver function is most essential. In the current clinical practice, the commonly employed methods of evaluation include Child-Pugh (CP), Model of End-Stage Liver Disease (MELD) and Albumin-Bilirubin (ALBI) scores. While most of the HCCs patients with Child’s A or low MELD scores may be suitable candidates for surgical resection, some of them may have significant portal hypertension (thrombocytopenia, ascites, bleeding varices) that make one think twice about offering resection. Additional tests such as indocyanine green (ICG) clearance test may be helpful in providing additional insight into the function of the liver parenchyma.
One key cornerstone in swaying the decision between surgical resection or liver transplantation is the presence of portal hypertension. As mentioned above, markers of portal hypertension in a cirrhotic liver include signs and symptoms of variceal bleeding, ascites, hepatic encephalopathy and thrombocytopenia due to hypersplenism. The current scoring systems that are widely used above (namely CP, MELD, ALBI scores) may or may not correlate with the portal pressure. Direct measurement of hepatic venous wedge pressure and gradient provides an accurate estimate of the portal pressure. It is recommended that only patients with hepatic venous wedge pressure of less than 10 mmHg be considered for hepatic resection (9,10). Understandably, the practice of measuring hepatic vein wedge pressure and the gradient is not common due to the invasive nature of this assessment.
As shown in Figure 1, the three aspects to consider when deciding for liver resection or transplantation for HCC include cirrhosis and liver function, availability of organ and long-term risk of recurrence of HCC in the native liver. The evaluation of liver function prior to liver resection has been illustrated in the previous paragraphs. When it comes to considering liver transplantation for HCC, it is important to consider the availability of organs in the country. Countries with very low deceased organ donation rate will have very limited organ sources for liver transplantation, and the allocation of organ will be dependent on severity of liver function (such as high MELD score). Low MELD early HCC patients will be very low in priority when it comes to organ waiting list. In this situation, if the liver function is fairly preserved, liver resection is often the primary treatment choice, although we know that liver transplantation in early HCC will probably provide the best long-term survival with low risk of HCC recurrence. However, in countries where the availability of organ seems sufficient, such as in the US, where fairly high MELD score of 22 is given to patients qualified to be put on the waiting list, liver transplant is certainly an attractive consideration. Moreover, there is progressive score of 3 points given to patients on the waiting list for every 3 months of waiting on the list till a maximum of 36. In this situation, where suitable live grafts can be available, the consideration may have to weigh the pros and cons of liver transplantation. LDLT is already an established way of performing liver transplantation. The safety profiles for LDLT have also been proven with a multitude of studies published over the past 3 decades. That being said, there are still risks involved in surgically removing partial liver grafts from healthy donors, whom do not require major surgery to be performed on them. The ethical debate in this area is still very heated.
The last aspect in the consideration is the long-term risk of HCC recurrence in a native liver that is cirrhotic. Particularly in chronic hepatitis B carrier where the viral DNA has been incorporated into the hepatocyte genome, the risk of new HCCs developing after resection of HCC is extremely high. On the other hand, liver transplantation in these patients will really help to remove the ‘fertile soil’ for subsequent HCC development. Moreover, lifelong antiviral therapy in this group of patients will reduce the risk of re-infection of hepatitis B in the new liver graft to very low level, around 3% (8). While liver transplantation may sound like an ideal treatment option for patients with HCC, the risk of recurrence in certain patients such as those with high AFP or PIVKA-II, or HCC with high risk features, will be extremely high, making LT an inappropriate option to consider. The predictive factors of recurrence in HCC after liver transplantation will be illustrated further.
Lastly, liver transplantation service is a complex tertiary healthcare service that requires a large team with multi-disciplinary specialists including liver transplant surgeons, transplant hepatologists, transplant coordinators, transplant pharmacists, specialist nurses and transplant infectious disease specialists etc. It also requires a lot of resources to set up such a complex service including isolation wards, radiological investigations, laboratories etc. While liver transplant services have taken flight in many countries in Europe, the Americas and some countries in Asia (such as South Korea, Japan, Taiwan, Hong Kong, Singapore and China), many countries in the region are still trying to start the liver transplant program. Therefore, when deciding on treatment for patients with HCC, this local factor must certainly be considered.
Liver resection for HCC
Liver resection has been one of the main treatments for HCC for many decades. While initial experience of liver resection for HCC was faced with high risk of morbidity and mortality, the safety profile for liver resection has improved significantly over the last 3 decades. The key challenge in this aspect is directly related to the high incidence of chronic liver disease and cirrhosis in patients with HCC, which contributes significantly to the risk of liver failure and mortality after liver resection. As such, the decision to undertake liver resection for patients with HCC with or without background liver cirrhosis is a perfect demonstration of arts and science of surgery.
When selecting patients with HCC for liver resection, there are three main areas for consideration (Figure 3), including oncologic appropriateness, technical resectability and host condition.
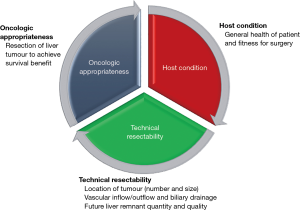
Oncology appropriateness
- Resection of liver tumour to achieve survival benefit
The very first consideration for liver resection for HCC is the presence of absence of extrahepatic disease or distant metastasis. The presence of distant metastasis is a clear contraindication for liver resection as most physicians will consider this an indication of poor tumour biology and poor survival outcome even if aggressive surgical therapy is instituted. Currently, a good quality triphasic CT scan of the liver together with CT thorax is considered adequate staging scans for HCC when considering therapeutic options.
The presence of portal vein tumour thrombosis (PVTT) in HCC patients is often associated with poor prognosis. PVTT represents intrinsic aggressiveness of tumor, reduced intolerance to anti-neoplastic treatment, impaired hepatic reserves and a high rate of developing complications related to portal hypertension. The HCCs with portal vein thrombosis is often large in size with multifocality, the tumours are often poorly differentiated and the remnant liver function on Child-Pugh class are poor and the serum alpha-fetoprotein (AFP) levels are usually very high (11,12). As a result, most centers will advise against offering aggressive surgical resection or liver transplantation to HCC patients with PVTT. However, selected patients with PVTT such as those with Type 1, 2 and 3 PVTT were able to enjoy survival benefits ranging from 54.8% to 25.4% at 1 year after surgical resection (13). Indeed, for young and fit patients with the unfortunate situation of having HCC with PVTT, aggressive surgical resection may still be considered as the last resort effort in curative attempt for treatment of the HCC. Realistically, the presence of PVTT may not be all that straightforward in some clinical settings as PVT can be present in patients with portal hypertension and cirrhosis and it may be just blend thrombus within the portal venous system. Clinicians and radiologists often will hunt for signs of vascular enhancement within the thrombus, whether the thrombus is in continuity with the primary tumour within then liver as well as correlating PVTT with the rise in AFP level to make a judgement.
Similarly, there remains debate regarding the appropriateness of aggressive surgical resection for patients with HCC that have hepatic vein tumor thrombosis (HVTT). Likely patients with PVTT, most will equate the presence of HVTT as evidence of macrovascular invasion and represents a predictor of poor prognosis and long-term survival. In one of the largest series of HCC patients with HVTT, Kokudo et al. reported equivalent survival of patients with HVTT compared with PVTT who underwent surgical resection. Patients with HVTT who had surgical resection had better survival in this group of patients who underwent R0 resection for HCC with HVTT as compared to those who received Sorafenib alone (14).
Size of tumour
Over the years, it has been established that small HCCs (<5 cm) can best be treated with surgical resection or liver transplant. However, for large HCCs, in particular the giant ones (more than 10 cm), there are many controversies in the treatment strategies. Giant HCCs ≥10 cm are often designated to palliative treatment as surgery is deemed unsuitable mainly due to poor prognosis (Figure 4). The morbidity rates range from 25% to 50% and mortality rates from 0% to 8% were reported in patients with giant HCCs (2-4,15). As mentioned before, a few studies now suggested that tumour size is not critical but the physiological parameters and the characteristics of the liver remnant are the main determinants of treatment outcomes (5,6).
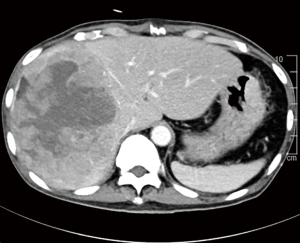
While the current BCLC criteria does not recommend surgical resection for giant HCCs, a study done in our centre shows that surgical resection of giant HCC has comparable outcomes with the smaller tumours. This was despite more major liver resections being performed in the giant HCC group (82.6% versus 31.7%; P<0.001) (15). We have also found that the presence of satellite lesions and perioperative blood transfusions had significant influence on the overall survival (OS) of our patients. Moreover, tumour size was not a determinant of OS in our study. These findings found significant concordance in other reported series (16,17). As such, curative surgery is certainly a viable consideration for patients with giant HCC. In our series, the median OS in patients with giant HCC after surgical resection was 39.0 months and it was clearly comparable to the median survival reported in other series, reported to be 30–32 months (18-21). These results suggest that resection should be considered as the primary treatment option for giant HCC whenever possible as long as it can be performed safely. Otherwise, many of these patients would be allocated to palliative treatment options, although some of them could excellent OS with aggressive surgical treatment instead.
Technical resectability (Figure 5):
- Location of tumour (number and size);
- Vascular inflow/outflow and biliary drainage;
- Future liver remnant (FLR) quantity and quality.
The liver surgeon needs to study the imaging studies of the liver lesion(s) to determine the location and size of the lesion. The relationship of the lesion(s) to critical inflow pedicular structures such as bile duct, portal vein and hepatic artery as well as outflow structures such as hepatic veins has significant influence on how the surgery will be conducted.
Peripherally located tumours can be easily resected if the quality of the liver parenchyma allows so. In most circumstances, resection will be safer in patients with Child’s A liver as compared to Child’s B and C. Small wedge resection should be reasonably safe in patients with Child’s B liver function. If the tumours are located deep within the parenchyma of the liver and near to major hepatic veins, portal veins or biliary pedicles, major liver resection will be necessary in order to achieve R0 resection. In this circumstance, careful consideration must be given to the size of the FLR and the adequacy of liver function post resection.
As a general guide, in a patient with non-cirrhotic liver, up to 70% to 75% of liver could be resected with the remnant liver volume contributing to 25% to 30% of the total liver volume (22,23). Therefore, the safety margin increases significantly in these patients with non-cirrhotic liver if a smaller resection is required. However, if there is significant cirrhosis but the patients’ liver function is Child’s A without portal hypertension, the remnant liver volume must certainly be more. The general rule of thumb is at least 40% to 50% of the liver volume left behind with 3 to 4 contiguous segments of liver functioning to sustain the patients’ need post resection (24). In patients with Child’s B or C liver function or patients with significant portal hypertension (as stated above), major liver resection is certainly very risky and not recommended.
Additional information regarding the quality and function of the hepatocytes in cirrhotic livers can be obtained using the ICG clearance test (25). The ICG dye is exclusively cleared by the hepatocytes and excreted into the biliary system, the amount of ICG retained in the blood at a certain duration after injection can be used to stratify the risk of major liver resection. Imamura et al. proposed the use of Makuuchi decisional algorithm using ICG retention at 15 minutes as follows (26):
- <10% at 15 min for trisectionectomy or bisectorectomy of liver;
- 10% to 19% for hemihepatectomy, right sided sectorectomy;
- 20% to 29% for segmentectomy;
- 30% to 39% for limited resection (e.g., wedge resection);
- >40% for enucleation.
It is also important to actively look out for evidence of portal hypertension when deciding on major liver resection for patients with HCC. When there is radiological, endoscopic and/or clinical evidence of splenomegaly, thrombocytopenia, varices, the surgeons must think carefully to offer surgical resection. If the HCC is within criteria, liver transplant would certainly be a much better option to help solve the problem of liver tumour in a suboptimal quality liver parenchyma, reducing the risk of liver failure post-operatively.
In the situation where resection is suitable and oncologically feasible but the remnant liver volume is deemed inadequate (i.e., <25% of the total liver volume), methods to grow the FLR have been actively explored by the hepatobiliary surgical communities around the world. Options to grow FLR can be largely grouped into:
- Portal vein embolization and staged hepatectomy;
- Portal vein ligation (PVL) and staged hepatectomy;
- Associating liver partition with portal vein ligation for staged hepatectomy (ALPPS).
To harness the regenerative potential of the liver to grow the FLR, the concepts of PVL was explored by the Japanese in the 1975. Honjo et al. introduced the technique of PVL (27). However, the concepts of inducing liver hypertrophy by manipulating the portal blood flow was first emphasized by Cantlie in 1897 and later by Rous in 1920 (28). PVL is used routinely in two-stage procedures, where sometimes a ‘cleansing’ of the FLR from tumour is performed along with PVL. After reaching adequate hypertrophy of the FLR, resection of the diseased liver part is undertaken during a second stage (29).
Kinoshita et al. (30) and Makuuchi and co-workers (31) in the late 1980s introduced the techniques of portal vein embolization (PVE) by injecting embolizing agents into one of the portal branches. Over the past decades, this approach has gained wide acceptance in the field of liver surgery. Direct comparisons between PVL and PVE regarding the hypertrophy of the FLR were reported with controversial results (29,32-34). While these techniques are popular, it is plagued with a high drop-out rate. The drop-out rate was reported to be up to 35% of patients due to either insufficient liver hypertrophy of the FLR or tumour progression (35,36).
Recently, a new technique of ALPPS has been introduced. Several reports suggested that by combining PVL and partitioning the liver parenchymal in the same setting, greater hypertrophy of FLR could be achieved as compared to PVE or PVL alone, with almost 96–99% of patients undergoing definitive hepatectomy (29,37-39). However, the issues of higher mortality and morbidity (as high as 12% and 40% respectively) associated with ALPPS has dampened the initial enthusiasm with this promising technique (40).
In a recent study done by our center, ALPPS induced a superior volumetric response when compared to PVE/PVL (Figure 6A,B,C,D,E) (41). Our study showed that the FLR in ALPPS patients grew by 163.0±90.5 mL representing a 48% increase in size over a median duration of 7 days between both stages. In contrast, the FLR in Conventional Staged Hepatectomy (CSH) (PVL or PVE) patients grew by 57.0±80.8 mL or 12% over a median interval of 20 days. This finding was consistent with a recent meta-analysis by Eshmuminov et al., showing that ALPPS induced 81% FLR increase compared to 35–38% in the PVE/PVL group (29). However, further study demonstrated that, if the underlying disease was HCC requiring ALPPS, the FLR grew significantly less after ALPPS-Stage 1 compared to non-HCC patients. We have found that the presence of hepatic fibrosis on the final histopathology was associated with negative impact on the FLR growth. When considering suitability for ALPPS, patients with HCC may benefit from additional pre-operative assessment of fibrosis (42).
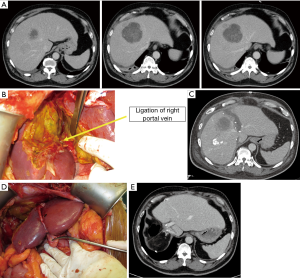
It is clear based on current evidence that volume growth in ALPPS is not directly reflective of the functional status of the liver parenchyma. Even when evidence clearly showed that ALPPS rapidly increases FLR volume, Matsuo et al. demonstrated that the hepatocytes seen on light and electron microscopy were immature after ALPPS when compared to portal vein embolization (43). In addition, Sparrelid et al. showed that found that using scintigraphy and single-photon emission computed tomography (SPECT), the magnitude of increase in FLR function was 50% of the magnitude of increase in FLR volume (44). As such, there is a new direction recently to better conduct cross-sectional assessment of FLR function after ALPPS-Stage 1 with the deployment of liver specific tracers (e.g., 99m Tc-galactosyl, 99m Tc-mebrofenin) and magnetic resonance imaging contrast agents (e.g., gadolinium ethoxybenzyl, gadobenate dimeglumine). These modalities may reflect FLR function more accurately compared to volumetry alone (25,44,45).
While ALPPS may be able to provide the surgeons with a better chance of securing an R0 resection for HCC, the selection of patients remains the key consideration. In the first international expert meeting on ALPPS, HCC was listed as one of the pathologies where ALPPS procedure should be used with caution due to higher morbidity and mortality rate (46).
Host condition
- General health of patient and fitness for surgery.
Regardless of the most ideal treatment options available, the patients must be fit to receive them. In this circumstance, even if the HCC is suitable for resection or transplantation but the patient is of poor health with multiple comorbidities and the risk of general anesthesia for the liver surgery is prohibitive, we must consider alternative treatment options. Non-operative treatments such as ablative therapies with radiofrequency ablation or microwave ablation, transarterial chemoembolization, selective internal radiation therapy such as Yttrium-90 treatment, systemic therapy options such as Sorafenib, Lenvatinib, Regorafenib or immunotherapy such as Nivolumab can be offered to the patients.
Liver transplantation for HCC
When liver transplantation was being explored as treatment for liver diseases, liver malignancies were the few indications. In 1991, Penn reported the long-term results of transplantation for primary and metastatic hepatic malignancies which was performed on 637 patients. The best results were obtained with uncommon tumors: incidental hepatomas (13% recurrence; 57% 2- and 5-year follow-up); epithelioid hemangioendotheliomas (33% recurrence; 82% and 43% 2- and 5-year survival); hepatoblastomas (33% recurrence; 50% 2- and 5-year survival); and fibrolamellar hepatomas (39% recurrence; 60% and 55% 2- and 5-year survival). Hemangiosarcomas had 64% recurrence, and all patients died within 27.5 months. Transplantation for hepatomas was only reserved for patients with favourable risk factors as hepatomas had 39% recurrence with 2- and 5-year survival rates of 30% and 18%, respectively after liver transplantation (47).
With that early experience showing that selected patients with HCC could enjoy good long term survival, the transplant community next saw a big paradigm shift when Mazzaferro et al. introduced the Milan’s criteria in 1996 (48). In this landmark paper, patients with small HCCs (single tumour less than 5 cm in diameter or multiple tumours no more than 3 nodules and largest nodule less than 3 cm in diameter) was shown to enjoy actuarial survival of 75% at 4 years with recurrence-free survival rate at 83% after liver transplantation. This marks the beginning of the era where HCC becomes widely accepted as indication for liver transplantation. Liver transplantation is now considered the primary treatment for patients with early HCC and poor liver function. On the other hand, for patients with preserved hepatic function, surgical liver resection is performed. A 5-year survival of 50% to 70% has been reported in series in cirrhotic patients treated with resection for HCC within Milan Criteria (9,49-52). As a result, the current benchmark for liver transplantation for malignancy is 70% overall survival at 5 years (52).
Yao et al. from the University College of San Francisco (UCFS) proposed an expanded criterion in 2001 as Milan’s criteria was rather restrictive. In the study, patients with HCC meeting the following criteria: solitary tumor <6.5 cm, or <3 nodules with the largest lesion <4.5 cm and total tumor diameter <8 cm, had survival rates of 90% and 75.2%, at 1 and 5 years, respectively, after Orthotopic LT versus a 50% 1-year survival for patients with tumors exceeding these limits (P=0.0005) (53). The UCSF group went on to publish a validation study in 2007 showing consistently excellent survival outcomes using these expanded criteria (54). That same year in 2007, Singapore has adopted the UCSF criteria as the listing criteria for patients with HCC needing liver transplantation.
The advantage of liver transplantation for HCC was clear. Firstly, total hepatectomy allows elimination of the entire liver, together with that, the occult intrahepatic metastatic disease as well (55). Secondly, the risk of more HCCs developing in a liver that is chronically inflamed and cirrhotic is reported to be as high as 50% to 70% after liver resection (49). The presence of advanced cirrhosis with associated portal hypertension remains one of the biggest challenges in liver resections, albeit the safety of major hepatectomies that has improved tremendously over the past three decades.
However, there remain many challenges for liver transplantation in HCC.
Organ allocation and priority in listing for HCC patients
One of the major constraints to implement liver transplant widely for HCC is the shortage of donor organs (56). Progression of either the cancer or the underlying cirrhosis has led to substantial dropout rate while on the waiting list. Given the limited availability of donor organs and poor outcomes of patients dropping off the waiting list, judicious organ allocation has become extremely crucial. While many countries have adopted using the Modified End-stage Liver Disease (MELD) score as the system to stratify the priority for listing liver patients on waitlist, there is a huge variation in how much MELD scores are allocate to HCC patients as compared to non-HCC patients. The organ allocation policy must appear to be fair to both HCC and non-HCC patients, such that HCC patients don’t unnecessary be conferred additional advantage in getting the organ allocation due to the malignancy due to higher MELD score. On the other hand, the MELD score for HCC patients must not be too low that they don’t get the organ allocation and risks progression of the malignancy and dropping off the waitlist. There is no idea organ allocation system at this point in time that can cater to all these considerations.
As discussed above, a patient with HCC in Singapore (must be a citizen or permanent resident) must fulfil the UCSF criteria before he/she can be put on the waitlist for deceased donor liver organ allocation. In the presence of the HCC, the patients are given a MELD score of 15 upon listing. This is the HCC MELD exception score that is given. A unique circumstance arises here as there will be two sets of MELD scores for a patient with HCC and with or without cirrhosis. A non-cirrhotic patient with HCC will get a permanent 15 points on the MELD score, while a cirrhotic patients can have a MELD score that is higher than MELD 15 due to the natural cirrhotic and decompensated state of his/her liver functions. We call this the natural MELD score. Whichever the score is higher, it is taken as the score to give priority on the waitlist. As previously alluded to, low MELD early HCC patients will be very low in priority when it comes to organ waiting list. To overcome the challenge of shortage of organ in a low deceased donation rate country, LDLT becomes an extremely crucial strategy to harness. All patients in our center is offered the option to consider LDLT upfront on the first consult, with comprehensive information being provided to both the potential recipients and his/her family members. Quite often, there will be interested family members who will step forward to be considered as potential living liver donors.
In countries where deceased donor organ donation rates are higher, such as in the US, a fairly high MELD score of 22 is given to patients who fulfilled the listing criteria within Milan’s criteria. Moreover, there is progressive score of 3 points given to patients on the waiting list for every 3 months of waiting on the list till a maximum of 36 (57). In contrast, in Asian countries such as Hong Kong, a MELD score of 20 is given to patients with HCC being placed on the waitlist and there is progressive score of additional 3 points being allocated for every 6 months waiting on the list (58). In many European centers except in the UK, an initial MELD score of 22 is given to patients with HCC (57,59). Vitale et al. performed a nice study to evaluate the impact of MELD score on the survival of patients with HCC who underwent liver resection or liver transplantation, combing data from an Italian center and two Taiwanese liver transplant centers. Survival after liver resection was compared to that predicted after LT by the Metroticket calculator in relationship with staging, MVI, and MELD score using Monte Carlo simulation. They found that LT conferred less survival benefit in patients with resectable HCC with a low MELD score (<10) or with aggressive tumors (with MVI). As a result of a shortage of donors, only selected resectable tumors with a MELD score of ≥10 should be considered for transplantation (60).
High costs of liver transplantation
Liver transplantation for patients with HCC is often described as providing second chance in life in well-selected patients who will have a very high chance of long term survival with low risk of tumour recurrence. While this is true, transplantation is a costly treatment. Its hefty price tag does not only come from the transplant operation, but the long term need for immunosuppressants requires a heavy financial commitment from transplant recipients. Its consumption of healthcare cost cannot be underestimated. In fact, liver transplant treatment is often viewed as highly expensive therapy that many underdeveloped countries with limited healthcare resources would not fund the development of transplant program and provision of transplant care using taxpayers money. As a results, patients from these countries with the financial means will travel abroad to seek transplant treatments.
Attempts to put a value to the price of liver transplantation have been published (61). In general, cost calculations will consider an intervention cost-effective if it is US $50,000 per quality-adjusted life years saved after a certain intervention (62). Majno et al. showed that the gain in life expectancy with primary LT was almost always achieved at an acceptable cost. Nevertheless, their study did not incorporate a quality-of-life analysis but rather relied on data for ‘‘organs saved’’ for an argument in favor of primary resection and salvage LT. A limitation of the report is that the impact of each ‘‘organ saved’’ on life expectancy and on cost to the medical care system has not been assessed (63). In another paper published by Lim et al., they compared the cost effectiveness of liver resection versus cadaveric liver transplantation across different geographical cost settings: the USA, Switzerland and Singapore. Liver resection produced 3.9 quality-adjusted life years (QALYs) while cadaveric liver transplant (CLT) had an additional 1.4 QALYs. The incremental cost-effectiveness ratio (ICER) of CLT versus liver resection ranged from $111,821/QALY in Singapore to $156,300/QALY in Switzerland, and was above thresholds for cost-effectiveness in all three countries. They concluded that, in patients with HCC within the Milan criteria and Child-Pugh A/B cirrhosis, LR is more cost-effective than CLT across three different costing scenarios: the USA, Switzerland, Singapore (64).
Long term immunosuppressants
In the early days of liver transplantation following successful experimentations by the pioneer Professor Thomas Starzl, liver transplantation was often challenged with high rejection rate with graft and life loss (65). Not until the discovery of effective immunosuppressants that liver transplantation entered a new era of stability and slowly becoming the standard of care today (66). In order to preserve the graft survival and functions, patients will require mandatory immunosuppressants every day.
Therefore, patients with HCC who underwent liver transplantation must be prepared to adhere to a new lifestyle. The lifestyle that requires daily dosing of immunosuppressants (often multiple agents at the beginning before tailing down to single agent in stable state), high risk of infections including opportunistic infections in immunosuppressed situation, as well as the long term side effects of the immunosuppressants. It is a complete change of one chronic state of disease in cirrhosis with HCC to another different chronic state of health and strong mindset change is often required to help them adapt to this change.
Technical demands of liver transplantation and availability of skilled surgeons and specialised transplant team
As alluded to previously, liver transplantation is one of the most challenging operative procedures in the abdomen. Highly skilled surgical team is essential to ensure good outcomes for the patients. Extra resources are needed to help train and develop the liver transplant surgery training, especially in countries where such expertise is unavailable. Structured approach to develop a comprehensive multidisciplinary program comprising liver transplant surgeons, transplant hepatologists, transplant anaesthetists and intensivists, transplant infectious disease physicians, transplant pharmacists and specialized transplant coordinators is essential to provide the best outcomes for transplant patients. Such programs require large amount of funding, resources and commitments from the governmental and healthcare regulatory bodies as well as the hospitals/institutions.
Selection criteria for HCC for liver transplantation
The most widely accepted selection criteria for HCC for liver transplantation is the Milan’s criteria (48). However, the expanded criteria such as UCSF criteria is also generally accepted as suitable to yield comparably similar survival outcomes for liver transplant patients with HCC (53,54). These selection criteria are important particularly when fair allocation of organ is essential in deceased donor liver transplantation as mentioned above. However, in countries where deceased organ donation rates are low and living organ donation activities are high to compensate for the organ shortage, these selection criteria have been challenged by more expanded criteria. Table 1 summarizes the published selection criteria for HCC for liver transplantation with the recent addition of the Japanese National Expanded Criteria for LDLT in HCC (67-75).
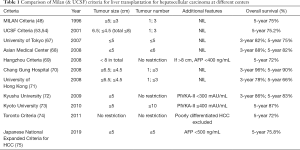
Full table
In order to investigate proposals to expand the Milan’s criteria, a multicenter study was conducted in 24 European centers to collect data transplanted for HCC patients whose tumors exceeded the CMC at post-transplant pathologic assessment (http://www.hcc-olt-metroticket.org) (76,77). The results were plotted in a tumor size-and number Cartesian contour plot showing the 5-year survival probability according to the size and number of HCC nodules detected in the explanted liver. Based on a preliminary analysis, a ‘‘HCC forecast chart’’ was been developed, which can predict 5-year post-transplant survival rates on the basis of morphological tumor characteristics.
It is clear that most of the accepted selection criteria for HCC in liver transplantation, Milan or outside of Milan’s criteria, are based on preoperative imaging. However, we also recognized that understaging using the current imaging modalities remains one of the key archille’s heel in developing ideal selection criteria for HCC patients needing liver transplantation. In a few published literature, the limitations of understaging of the HCC on pre-transplant imaging studies was estimated to be at least 20% to 30% (54,76).
Recently, further refinement to the criteria to include biomarkers and other parameters to better select patients with HCC for liver transplantation have been proposed. One of the most popular biomarkers to be included was alpha-fetoprotein (AFP) level prior to transplantation. Hameed et al. reported that pre-transplant AFP levels of >500 and >1,000 ng/mL were associated with a hazard ration of HCC recurrence of 3.1 and 4.5 respectively. AFP level of >1,000 ng/mL was the only significant predictor of HCC recurrence in this study and using it as a cutoff point, 4.7% of the patients would be excluded from LT and a 20% reduction in HCC recurrence post-transplant could be expected (78). This proposal was further expended into using rate of rise of AFP in predicting the presence of microvascular invasion (MVI) which is the single most important prognostic factor in HCC recurrence. Lai et al. suggested that AFP slope >15 ng/mL per month was predictive of survival with a HR of 5.4 while Giard et al. studied the effect of AFP slope >7.5 ng/mL per month. It was suggested that LT should be placed on hold until an observed decrease in AFP over time with additional locoregional therapies (LRT) was observed (79,80).
In some Asian liver transplant centers, other biomarkers such as des-carboxy prothrombin (or PIVKA-II) are commonly used in the management of patients with HCC. Ryu et al. from Japan found that using the three biomarkers including AFP, AFP-L3 (Lens culinaris agglutinin-reactive fraction of AFP) and DCP (des-carboxy prothrombin), both double- and triple-positive tumor markers prior to liver transplantation were associated with early recurrence and poor survival in HCC within Milan criteria (81). Concurrently, a Metroticket 2.0 model was created by the Mazzaferro group to enhance the selection criteria using serum AFP prior to liver transplantation. They found that, for patients with HCC to have a 70% chance of HCC-specific survival 5 years after transplantation, their level of AFP should be <200 ng/mL and the sum of number and size of tumors (in centimeters) should not exceed 7; if the level of AFP was 200–400 ng/mL, the sum of the number and size of tumors should be ≤5; if their level of AFP was 400–1,000 ng/mL, the sum of the number and size of tumors should be ≤4. They concluded that this new model, based on patients’ level of AFP and HCC number and size, outperformed the Milan; University of California, San Francisco; Shanghai-Fudan; Up-to-7 criteria (P<0.001); and AFP French model (P=0.044) to predict which patients will survive for 5 years after liver transplantation (82).
Role of bridging therapy while on LT Waitlist
While patients with active HCC are being put on the waitlist, there is reasonable concern that the tumour might progress leading to untransplantable state. Most centers would consider treating these patients with LRT with the aims to prevent progression of the HCC and reduce the measurable disease burden of HCC prior to transplantation (83). In a systematic review and meta-analysis done by Kulik et al., they reported that, for adults with T2 HCC awaiting LT, transplant with any bridging therapy showed a nonsignificant reduction in the risk of waitlist dropout due to progression [relative risk (RR), 0.32; 95% confidence interval (CI), 0.06–1.85; I2 50%) and of waitlist dropout from all causes (RR, 0.38; 95% CI, 0.060–2.370; I2 85.7%) compared to no therapy (84-91).
Similar study was also performed in our centre and the modes of LRT as bridging therapy (BT) was compared in liver transplant patients with HCC. The overall dropout rate was 44.4% and 31.0% in the BT and non-BT groups, respectively (P=0.269). There was no difference in survival or recurrence between the BT and non-BT groups (P=0.862). BT does not influence the dropout rate or survival after LT but it should be considered in patients who are on the waitlist for more than 6 months (92). On a different note, if the patient is within UCSF criteria and there is a living donor liver transplant option available and the LDLT can take place within 1 to 2 months, we often choose not to perform the bridging therapy as the ultimate therapy that will solve the issue is the transplantation.
Role of downstaging of HCC for LT
Whether patients with cirrhosis and HCC beyond Milan criteria (T3) should be transplanted if they are successfully down-staged using LRT to within Milan criteria (T2) is a topic that is hotly debated currently. As the long-term outcomes of patients beyond Milan criteria are demonstrably poorer, most waitlist system would not accept these cases as potential transplant recipients. However, in a recent prospective trial published by the UCSF group led by Yao et al. on down-staging of HCC with LRT was shown to have comparable over survival and recurrence rates after liver transplantation compared to those with T2 HCC within Milan criteria (93). But the dropout rate (35% at 8.2 months) was significantly higher in the downstaged group (P=0.02). The Metroticket devised the “up-to-seven” criteria based on the explant pathology including the size of the largest tumor nodules, number of tumor nodules, and presence or absence of microvascular invasion (94). Among patients exceeding Milan who met the up-to-seven criteria without microvascular invasion, 5-year overall survival was excellent at 71.2%. Lower overall survival was noted in those meeting the expanded criteria with microvascular.
Once a tumour has been successfully downstaged to within acceptable criteria, a minimum observation period of 3 months is recommended before considering LT. Liver transplantation should not be performed if the patient fail to meet the downstaging criteria as follows (83):
- Failure to achieve listing criteria;
- Tumor progression with development of vascular invasion;
- Extrahepatic spread;
- Tumour size and number remaining beyond inclusion criteria;
- After listing: tumor progression requiring delisting and recurrence of HCC after LT.
Is LDLT for HCC associated with poorer outcomes? (52)
As LDLT becomes a well-accepted alternative to deceased DDLT in countries with low organ donation rate, the criteria for selection of HCC for LDLT is also widened significantly. This has led to some concerns regarding the potential higher risk of recurrence in HCC patients. Some studies have suggested that higher risk of tumor recurrence with the use of partial graft in LDLT for HCC. As the HCC patients undergoing LDLT often do not have to wait for availability of organs, the fast-tracked procedure with short delay between diagnosis and liver transplantation might not allow the biological behavior of the tumor to manifest (52). But the collective experiences reported by recent studies did not show convincing data to suggest higher risk of recurrence of HCC and poorer prognosis (95-100). Despite that, the international consensus conference meeting which was held in 2012 in Zurich, Switzerland has recommended an observation period of 3 months before offering LDLT in recipients with HCC.
While LDLT removes the issue of organ allocation as there is no concern of ‘public’ organ allocation in LDLT, the ethical dimension of subjecting healthy donors to surgery for organ donation surfaces. When the HCC is out of criteria, should LDLT be offered to patients with tumour stages beyond accepted criteria? The key consideration is the risk to the donor (52). In order for the risk of the donor to be worthwhile, the recipient expected survival must be above a certain threshold. The tricky situation may arise if the transplant team is met with graft failure after LDLT for out of criteria HCC, should deceased donor graft be used in that situation? As this is against the principle of utility, justice and equity, it would have been fair to use the deceased donor organ in such cases. Luckily, this situation is very rare.
Long term outcomes of HCC for resection vs. LT
Ultimately, the best treatment strategy for HCC, be it liver resection or transplantation, should be determined by the long-term outcome. Bigourdan et al. demonstrated that overall survival and freedom from recurrence for liver transplantation was generally better than liver resection for HCC patients with Child A cirrhosis (101). However, it is extremely important to consider the limited availability of organs for transplantation. Likewise, a French study demonstrated excellent results when salvage LT was performed for HCC recurrence after primary resection. But in a cirrhotic patient with HCC, even when the tumour is resectable, primary LT should be considered as the ideal choice of treatment (102). Majno et al. showed that life expectancy was better for primary transplantation as compared to salvage transplantation for HCC (8.8 years for primary transplantation versus 7.8 years for resection eventually followed by salvage transplantation). They demonstrated that the calculated use of grafts at 5 years for primary transplantation was 52% versus 23% for salvage transplantation. As such, they suggested that this strategy may be a rational way to cope with lengthening of the waiting list (103).
Baccarani et al. demonstrated superiority of liver transplantation versus resection for HCC. They reported only two dropouts due to tumour recurrence while on the waiting list. In their series, all HCCs listed for transplantation were treated with TACE and they were transplanted within a mean of <4 months from listing, which compares favourably with waiting time reported in the literature for HCC (104). According to Sarasin et al., when compared to liver resection, transplantation in an otherwise resectable HCC offers substantial survival benefit among well-targeted subgroups of patients as long as an organ donor is available within a maximal 6–10 months time delay (105).
Conclusions
While liver resection for HCC has been the mainstay of treatment for good long-term survival, increasingly liver transplantation has emerged as a better option in selection situation. The key in the selection process in finding a sweet spot to switch from resection to transplant, taking into account the stages of the HCC, be in early or intermediate to advanced. Clearly advanced HCC has limited treatment options and surgical resection or liver transplantation is often not suitable. It is important to consider oncological suitability, availability of organ as well as ability to achieve good long-term outcomes in patients with HCC with or without cirrhosis. There is no ‘One size fits all’ approach in the treatment of HCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Globoscan 2012. Epidemiology and Statistics of Hepatocellular Carcinoma. Parkin DM, Whelan SL, Ferlay J, et al. Cancer incidence in five continents, vol. VII. IARC Science Publications No. 143. Lyon, France: IARC, 1997.
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002;35:519-24. [Crossref] [PubMed]
- Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 2002;122:1609-19. [Crossref] [PubMed]
- Hassoun Z, Gores GJ. Treatment of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2003;1:10-8. [Crossref] [PubMed]
- Uchiyama K, Mori K, Tabuse K, et al. Assessment of liver function for successful hepatectomy in patients with hepatocellular carcinoma with impaired hepatic function. J Hepatobiliary Pancreat Surg 2008;15:596-602. [Crossref] [PubMed]
- Lau H, Man K, Fan ST, et al. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg 1997;84:1255-9. [Crossref] [PubMed]
- Akoad ME, Pomfret EA. Surgical resection and liver transplantation for Hepatocellular carcinoma. Clin Liver Dis 2015;19:381-99. [Crossref] [PubMed]
- Shindoh J, Hasegawa K, Inoue Y, et al. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford) 2013;15:31-9. [Crossref] [PubMed]
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection vs transplantation. Hepatology 1999;30:1434-40. [Crossref] [PubMed]
- Bruix J, Castellas A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996;111:1018-22. [Crossref] [PubMed]
- Connolly GC, Chen R, Hyrien O, et al. Incidence, risk factors and consequences of portal vein and systemic thromboses in hepatocellular carcinoma. Thromb Res 2008;122:299-306. [Crossref] [PubMed]
- Chan SL, Chong CCN, Chan AWH, et al. Management of hepatocellular carcinoma with portal vein tumour thrombosis: Review and update at 2016. World J Gastroenterol 2016;22:7289-300. [Crossref] [PubMed]
- Shi J, Lai EC, Li N, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci 2011;18:74-80. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Yamamoto S, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol 2014;61:583-8. [Crossref] [PubMed]
- Thng Y, Tan JK, Iyer SG, et al. Outcome of resection of giant hepatocellular carcinoma in a tertiary institution: does size matter? HPB 2015;17:988-93. [Crossref] [PubMed]
- Ng KK, Vauthey JN, Pawlik TM, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol 2005;12:364-73. [Crossref] [PubMed]
- Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg 2002;194:592-602. [Crossref] [PubMed]
- Lee SG, Hwang S, Jung JP, et al. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg 2007;94:320-6. [Crossref] [PubMed]
- Yamashita Y, Taketomi A, Shirabe K, et al. Outcomes of hepatic resection for huge hepatocellular carcinoma (>10 cm in diameter). J Surg Oncol 2011;104:292-8. [Crossref] [PubMed]
- Liau KH, Ruo L, Shia J, et al. Outcome of partial hepatectomy for large (> 10 cm) hepatocellular carcinoma. Cancer 2005;104:1948-55. [Crossref] [PubMed]
- Pawlik TM, Poon RT, Abdalla EK, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg 2005;140:450-7; discussion 457-8. [Crossref] [PubMed]
- Shoup M, Gonen M, D’Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 2003;7:325-330. [Crossref] [PubMed]
- Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000;127:512-9. [Crossref] [PubMed]
- Clavien PA, Emond J, Vauthey JN, et al. Protection of the liver during hepatic surgery. J Gastrointest Surg 2004;8:313-27. [Crossref] [PubMed]
- Hoekstra LT, de Graaf W, Nibourg GAA, et al. Physiological and biochemical basis of clinical liver function tests: A review. Ann Surg 2013;257:27-36. [Crossref] [PubMed]
- Imamura H, Sano K, Sugawara Y, et al. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg 2005;12:16-22. [Crossref] [PubMed]
- Honjo I, Suzuki T, Ozawa K, et al. Ligation of a branch of the portal vein for carcinoma of the liver. Am J Surg 1975;130:296-302. [Crossref] [PubMed]
- van Gulik TM, van den Esschert JW. James Cantlie’s early messages for hepatic surgeons: how the concept of pre-operative portal vein occlusion was defined. HPB (Oxford) 2010;12:81-3. [Crossref] [PubMed]
- Eshmuminov D, Raptis DA, Linecker M, et al. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg 2016;103:1768-82. [Crossref] [PubMed]
- Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986;10:803-8. [Crossref] [PubMed]
- Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521-7. [PubMed]
- Aussilhou B, Lesurtel M, Sauvanet A, et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg 2008;12:297-303. [Crossref] [PubMed]
- Robles R, Marin C, Lopez-Conesa A, et al. Comparative study of right portal vein ligation versus embolisation for induction of hypertrophy in two-stage hepatectomy for multiple bilateral colorectal liver metastases. Eur J Surg Oncol 2012;38:586-93. [Crossref] [PubMed]
- van Lienden KP, Hoekstra LT, Bennink RJ, et al. Intrahepatic left to right portoportal venous collateral vascular formation in patients undergoing right portal vein ligation. Cardiovasc Intervent Radiol 2013;36:1572-9. [Crossref] [PubMed]
- Broering DC, Hillert C, Krupski G, et al. Portal vein embolization versus portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg 2002;6:905-13. [Crossref] [PubMed]
- Capussotti L, Muratore A, Baracchi F, et al. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg 2008;143:978-82. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Schadde E, Ardiles V, Slankamenac K, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 2014;38:1510-9. [Crossref] [PubMed]
- Alvarez FA, Ardiles V, de Santibanes M, et al. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg 2015;261:723-32. [Crossref] [PubMed]
- Shindoh J, Vauthey JN, Zimmitti G, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 2013;217:126-33. [Crossref] [PubMed]
- Chia DKA, Yeo Z, Loh S, et al. Greater hypertrophy can be achieved with Associating Liver Partition with Portal vein ligation for Staged hepatectomy compared to Conventional Staged Hepatectomy, but with a higher price to pay? Am J Surg 2018;215:131-7. [Crossref] [PubMed]
- Chia DKA, Yeo Z, Loh SEK, et al. ALPPS for hepatocellular carcinoma is associated with decreased liver remnant growth. J Gastrointest Surg 2018;22:973-80. [Crossref] [PubMed]
- Matsuo K, Murakami T, Kawaguchi D, et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery 2016;159:1289-98. [Crossref] [PubMed]
- Sparrelid E, Jonas E, Tzortzakakis A, et al. Dynamic Evaluation of Liver Volume and Function in Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. J Gastrointest Surg 2017;21:967-74. [Crossref] [PubMed]
- Geisel D, Ludemann L, Hamm B, et al. Imaging-Based Liver Function Tests—Past, Present and Future. Rofo 2015;187:863-71. [Crossref] [PubMed]
- Oldhafer KJ, Stavrou GA, van Gulik TM, et al. ALPPS-Where Do We Stand, Where Do We Go? Eight Recommendations From the First International Expert Meeting. Ann Surg 2016;263:839-41. [Crossref] [PubMed]
- Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery 1991;110:726-34; discussion 734-5. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002;235:373-82. [Crossref] [PubMed]
- Cha CH, Ruo L, Fong Y, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg 2003;238:315-21. [PubMed]
- Giuliante F, Ardito F, Pinna AD, et al. Liver resection for hepatocellular carcinoma</ = 3 cm: results of an Italian multicenter study on 588 patients. J Am Coll Surg 2012;215:244-54. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-e22. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587-96. [Crossref] [PubMed]
- Turcotte S, DeMatteo R. Resection versus Transplantation for Early Hepatocellular Carcinoma. More Art than Science. Annals of Surgery 2012;256:892-3. Editorial. [Crossref] [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Elwir S, Lake J. Current status of liver allocation in the United States. Gastroenterol Hepatol (N Y) 2016;12:166-70. [PubMed]
- Chan SC, Cheung TT, Chan ACY, et al. New insights after the first 1000 liver transplantations at the University of Hong Kong. Asian J Surg 2016;39:202-10. [Crossref] [PubMed]
- Menon KV, Hakeem AR, Heaton ND. Review article: liver transplantation for hepatocellular carcinoma-a critical appraisal of the current worldwide listing criteria. Aliment Pharmacol Ther 2014;40:893-902. [Crossref] [PubMed]
- Vitale A, Huo TL, Cucchetti A, et al. Survival benefits of liver transplantation versus resection for hepatocellular carcinoma: Impact of MELD score. Ann Surg Oncol 2015;22:1901-7. [Crossref] [PubMed]
- Llovet JM, Bruix J, Gores GJ. Surgical resection versus transplantation for early hepatiocellular carcinoma: Clues for the best strategy. Hepatology 2000;31:1019-21. [Crossref] [PubMed]
- Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473-81. [PubMed]
- Majno PE, Adam R, Mazzaferro V, et al. Liver transplantation for recurrence after resection of solitary hepatocellular carcinoma Liver Transpl Surg 1999;5:S18-S67. [Abstract].
- Lim KC, Wang VW, Siddiqui FJ, et al. Cost-effectiveness analysis of liver resection versus transplantation in early hepatocellular carcinoma within Milan’s criteria. Hepatology 2015;61:227-37. [Crossref] [PubMed]
- Starzl TE, Marchioro TL, Vonkaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659-76. [PubMed]
- Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979;2:1033-6. [Crossref] [PubMed]
- Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis 2007;25:310-2. [Crossref] [PubMed]
- Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-45. [Crossref] [PubMed]
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32. [Crossref] [PubMed]
- Concejero A, Chen CL, Wang CC, et al. Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation 2008;85:398-406. [PubMed]
- Chan SC, Fan ST, Lo CM, et al. A decade of right liver adult-to-adult living donor liver transplantation: the recipient mid-term outcomes. Ann Surg 2008;248:411-9. [PubMed]
- Taketomi A, Sanefuji K, Soejima Y, et al. Impact of des-gamma-carboxy prothrombin and tumour size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation 2009;87:531-7. [Crossref] [PubMed]
- Takada Y, Uomoto S. Liver transplantation for hepatocellular carcinoma: the Kyoto experience. J Hepatobiliary Pancreat Sci 2010;17:527-32. [Crossref] [PubMed]
- DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumour differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-72. [Crossref] [PubMed]
- Shimamura T, Akamatsu N, Fujiyoshi M, et al. Expanded living-donor liver transplantation criteria for patients with hepatocellular carcinoma based on the Japanese nationwide survey: the 5-5-500 rule - a retrospective study. Transpl Int 2019;32:356-68. [Crossref] [PubMed]
- Bruix J, Fuster J, Llovet JM. Liver transplantation for hepatocellular carcinoma: Foucault pendulum versus evidence-based decision. Liver Transpl 2003;9:700-2. [Crossref] [PubMed]
- Majno P, Mazzaferro V. Living donor liver transplantation for hepatocellular carcinoma exceeding conventional criteria: questions, answers and demands for a common language. Liver Transpl 2006;12:896-8. [Crossref] [PubMed]
- Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level >1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945-51. [Crossref] [PubMed]
- Lai Q, Inostroza M, Rico Juri JM, et al. Delta-slope of alpha-fetoprotein improves the ability to select liver transplant patients with hepatocellular cancer. HPB (Oxford) 2015;17:1085-95. [Crossref] [PubMed]
- Giard JM, Mehta N, Dodge JL, et al. Alpha-fetoprotein slope >7.5ng/mL per month predicts microvascular invasion and tumour recurrence after liver transplantation for hepatocellular carcinoma. Transplantation 2018;102:816-22. [Crossref] [PubMed]
- Ryu T, Takami Y, Wada Y, et al. Double- and Triple-positive tumour markers predicts early recurrence and poor survival in patients with hepatocellular carcinoma within the Milan criteria and Child-Pugh class. J Gastrointest Surg 2017;21:957-66. [Crossref] [PubMed]
- Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018;154:128-39. [Crossref] [PubMed]
- Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology 2018;67:381-400. [Crossref] [PubMed]
- Andorno E, Bottino G, Morelli N, et al. Preliminary results of liver transplantation for hepatocellular carcinoma among allocation organ policy strategies, neoadjuvant treatments, and intention-to-treat analysis. Transplant Proc 2008;40:1972-3. [Crossref] [PubMed]
- Cabrera R, Dhanasekaran R, Caridi J, et al. Impact of transarterial therapy in hepatitis C-related hepatocellular carcinoma on long-term outcomes after liver transplantation. Am J Clin Oncol 2012;35:345-50. [Crossref] [PubMed]
- DuBay DA, Sandroussi C, Kachura JR, et al. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. HPB (Oxford) 2011;13:24-32. [Crossref] [PubMed]
- Eguchi S, Hidaka M, Tomonaga T, et al. Actual therapeutic efficacy of pre-transplant treatment on hepatocellular carcinoma and its impact on survival after salvage living donor liver transplantation. J Gastroenterol 2009;44:624-9. [Crossref] [PubMed]
- Eswaran SL, Pierce K, Weaver F, et al. Transarterial chemoembolization for HCC in patients with extensive liver transplantation waiting times. Angiology 2012;63:206-12. [Crossref] [PubMed]
- Frangakis C, Geschwind JF, Kim D, et al. Chemoembolization decreases drop-off risk of hepatocellular carcinoma patients on the liver transplant list. Cardiovasc Intervent Radiol 2011;34:1254-61. [Crossref] [PubMed]
- Heinzow HS, Meister T, Nass D, et al. Outcome of supraselective transarterial chemoembolization in patients with hepatocellular carcinoma. Scand J Gastroenterol 2011;46:201-10. [Crossref] [PubMed]
- Hołówko W, Wroblewski T, Wojtaszek M, et al. Transarterial chemoembolization prior to liver transplantation in patients with hepatocellular carcinoma. Ann Transplant 2015;20:764-8. [Crossref] [PubMed]
- Tan CHN, Yu Y, Tan YRN, et al. Bridging therapies to liver transplantation for hepatocellular carcinoma: A bridge to nowhere? Ann Hepatobiliary Pancreat Surg 2018;22:27-35. [Crossref] [PubMed]
- Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968-77. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Di Sandro S, Slim AO, Giacomoni A, et al. Living donor liver transplantation for hepatocellular carcinoma: long-term results compared with deceased donor liver transplantation. Transplant Proc 2009;41:1283-85. [Crossref] [PubMed]
- Fisher RA, Kulik LM, Freise CE, et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant 2007;7:1601-8. [Crossref] [PubMed]
- Hwang S, Lee SG, Joh JW, et al. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl 2005;11:1265-72. [Crossref] [PubMed]
- Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology 2004;127:S277-82. [Crossref] [PubMed]
- Lo CM, Fan ST, Liu CL, et al. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg 2007;94:78-86. [Crossref] [PubMed]
- Vakili K, Pomposelli JJ, Cheah YL, et al. Living donor liver transplantation for hepatocellular carcinoma: increased recurrence but improved survival. Liver Transpl 2009;15:1861-6. [Crossref] [PubMed]
- Bigourdan JM, Jaeck D, Meyer N, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl 2003;9:513. [Crossref] [PubMed]
- Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg 2003;238:508. [PubMed]
- Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology 2000;31:899. [Crossref] [PubMed]
- Baccarani U, Adanin GL, Benzoni E, et al. Superiority of transplantation versus resection for the treatment of small hepatocellular carcinoma. Transplant International 2008;21:247-54. [Crossref] [PubMed]
- Sarasin FP, Giostra E, Mentha G, et al. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology 1998;28:436. [Crossref] [PubMed]
Cite this article as: Kow AW. Transplantation versus liver resection in patients with hepatocellular carcinoma. Transl Gastroenterol Hepatol 2019;4:33.


