Teaching endoscopic retrograde cholangiopancreatography cannulation
Introduction
Teaching endoscopic retrograde cholangiopancreatography (ERCP) is the most difficult task in an endoscopic training program. As a consequence, attaining competence in ERCP necessitates extensive training (1). Selective cannulation of the common bile duct (CBD) and/or the main pancreatic duct (MPD) is fundamental to clinical success in the vast majority of the cases. ERCP is a complex procedure that is basically composed of three big parts. The first one is scope maneuvering and orientation, the second is cannulation of CBD and/or MPD and all operative maneuvers during ERCP, and the third one is the interpretation of X-rays. These three parts are intertwined during every ERCP procedure. In some cases, one must also deal with cannulation of the minor papilla, which is defined as the expert level. This chapter will focus only on teaching cannulation of the major papilla. ERCP is a skill demanding procedure that can lead to severe complications. This is the most important reason which justifies the increasing interest in performing the initial training on simulators.
Evaluation of the papilla and initial positioning
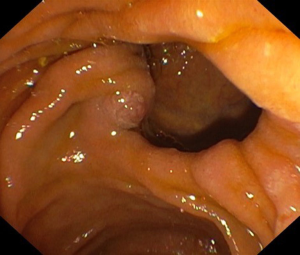
After reaching the second duodenal portion and following the standard shortening maneuver, the major papilla (if there are no anatomical abnormalities) is identified. An X-ray at this moment will show the shaft of the endoscope that flushes against the lesser curvature of the stomach (short position). At this point, the small wheel is locked, while the big wheel can be locked or left unlocked (operator dependent) and, if there are no contraindications, antispastic drugs should be given at this stage. Sometimes the papilla can be difficult to identify due to a diverticulum (Figure 1) or exuberant mucosal folds or can be distorted or infiltrated in patients with malignancy. In some cases, the papilla can be located more proximal, just beyond the apex of the duodenal bulb, or more distal, between the junction of the second and third duodenum. Once visualized, take your time to assess the type and size of the papilla (flat or protuberant), identify the orifice, evaluate the length of the infundibulum (which can predict difficulty due to a long syphon), the presence of ampulloma, impacted stone, diverticula, previous pre-cut or sphincterotomy, etc. Furthermore, if there is a single ampullary orifice it means that this provides access to a small common channel that further bifurcates via a septum into the CBD and the MPD. The papilla can also have two separate orifices (Figure 2) of which one is for the bile duct and is usually at 11 o’clock and one for the pancreatic duct at 1 o’clock, while a papillary ectropion (Figure 3) could make cannulation difficult. As it concerns the optimal distance from the tip of the endoscope to the papilla, generally 2 to 3 cm is appropriate especially when a sphincterotome is used because this distance will allow adequate space for proper bowing. Obviously, the distance should be selected accordingly to the site of the papilla and the duodenal wall in front of it.

Scope maneuvering and use of the elevator
There are several basic movements that a trainee must learn. The duodenoscope is a lateral viewing scope, and it works in a different way compared to standard endoscopes. Turning the big wheel up brings the tip of the scope close to the papilla while turning the big wheel down brings the scope far from the papilla. Turning to the left and to the right with the small wheel rotates the tip of the scope around the papilla for about 180°, while twisting the wrist joint (or body movement) to the left and to the right turns the tip of the scope left and right in the same plane that differs from the left and right of the small wheel (without rotation around the papilla). When the tip of the scope is up (close to the papilla) and the scope is pushed in, we just got far from the papilla, while pulling the scope out we will get us close to the papilla. These maneuvers are mostly used for the removal of biliary stones (pushing the scope in) and in combination with other maneuvers for placing biliary stents (pulling the scope out), and also for other operative procedures. The combination of all these maneuvers will lead us to the optimal position from which we can start cannulation.
The elevator or bridge is of paramount importance and is continuously used in ERCP. The elevator is the “moving force” for all the accessories during every ERCP procedure. Trainees who are starting their training are usually already experts in upper and lower GI and are used to turning the big wheel with the thumb of the left hand. In ERCP this will result in “forgetting the elevator” and leads to unsuccessful cannulation. Another beginners’ mistake is to keep the right hand on the shaft of the scope: once the position in front of the papilla is stable, the right hand should move to the wheels of the scope or handle catheters. A nice tip for trainees who are starting ERCP is to suggest them to keep the thumb of the left hand continuously on the elevator, and turning the big and the small wheel to use only the right hand. In order to avoid duodenal perforations, it is mandatory to close the elevator every time we insert accessories in the operative channel and to open it only when resistance is felt at the distal outlet of the channel.
The art of cannulation
Once we are in a stable position in front of the papilla we are ready to cannulate. The first thing that almost all trainees will do in the attempt to cannulate is to push the sphincterotome or cannula to the papilla (Figure 4). This is done instinctively. In this way, the force that is applied for pushing the cannula (or sphincterotome) is transmitted directly from the hand through the catheter to the papilla, while the tip of the scope will stay firm. This type of cannulation is called “by hand” and most of the times is related to a failure in cannulation due to the wrong axis, it is not incorrect but rather improper. Proper cannulation is done with the scope: once we have decided the adequate distance between the scope and the papilla (2–3 cm), and the length of the sphincterotome that is out of the scope, the right hand should be moved from the sphincterotome to the big wheel. It is very important to keep the left thumb firmly on the elevator and the right hand on the big wheel (Figure 5). At this point cannulation is done by precise coordination between the elevator and the big wheel: gently closing the elevator brings the tip of the accessory to the papilla while gently turning the big wheel up brings the tip of the scope to the papilla. With this maneuver, the force of cannulation is transmitted with the scope directly to the papilla. This type of cannulation is called “by scope” and is the most appropriate for gaining correct access to both major and minor papilla.
Cannulation of the CBD
The first endoscopic cannulation of the ampulla of Vater was done in 1968 (2). Since then many things have changed and many catheters and techniques have become available for cannulation. Furthermore, ERCP has evolved from diagnostic to an almost exclusively therapeutic procedure and today cannulation of the CBD is done with the scope in the short position (if there are no anatomical problems) and below the papilla, while at the beginnings the long position was preferred (3).
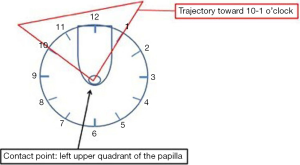
Cannulation can be done directly with a sphincterotome, can be contrast-assisted or can be performed with a wire or a combination of these three. Most of the times biliary cannulation is started with a sphincterotome directly. Triple lumen sphincterotomes can be preloaded with a wire and can carry out a contrast injection at the same time. Cannulation starts by looking at the biliary orifice that almost always will be located in the left upper quadrant, between the 10 o’clock and 1 o’clock position (Figure 6). Before approaching the papilla the angle and direction of the intraduodenal portion of the distal CBD can be evaluated most of the times. Putting together the location of the biliary orifice, the angle and direction of the intraduodenal portion of the distal CBD will help to guide the direction of cannulation. From this point on the endoscope and the sphincterotome should be manipulated with the big wheel and the elevator towards the presumed biliary orifice. Bowing the sphincterotome (if needed) will help to gain the correct axis of the CBD once the tip of it has partially engaged the orifice at its most superior point, above the septum of the common channel that separates the CBD and the MPD. At this point, a gentle movement up of the big wheel should be enough to cannulate the CBD.
Another technique is the shoehorn maneuver. After engaging the papillary orifice, a gentle relaxation of the elevator is done followed by a subtle withdrawal of the device and/or the endoscope, and un-bowing of the sphincterotome to follow the horizontal portion of the intramural CBD. At this point, the small wheel is turned left and the elevator is slightly closed and the small wheel is turned slightly to the right simultaneously turning up the big wheel to follow the vertical portion of the intramural CBD (4). This technique most of the times will allow for deep entry into the CBD. During the above-mentioned maneuvers, a small amount of contrast can be injected, and this helps just to “see the way”. In the past three decades, there have been many clinical trials on the type of cannulation technique comparing guidewire versus conventional contrast cannulation of the CBD in terms of prevention of post-ERCP pancreatitis (5,6). The general conclusion of these trials is that the contrast-assisted cannulation technique is more frequently linked to post-ERCP pancreatitis. However, the most important bias here is the quantity of injected contrast, and this, being assistant-dependent, is a parameter that cannot be measured. Furthermore, a non-ionic contrast should be preferred. There are two different techniques for direct guidewire cannulation of the CBD, one is “touch” and the other one is “no touch”. The touch technique consists in engaging the sphincterotome into the papillary orifice (after evaluation of the direction to the CBD), and advancing the guidewire into the papilla. The no-touch technique consists in engaging the papilla only with the guidewire. These two techniques have been compared in a recent trial and it resulted that the touch technique is superior for deep biliary cannulation (7). The choice of the guidewire is highly dependent on local expertise. J-shaped guidewires with a soft hydrophilic tip or fully hydrophilic wires are most widely used. A good tip for choosing the best type of wire is to inject a small amount of contrast, just to see the first part of the CBD, and then to advance the wire in the correct direction. In case the cephalic MPD is injected, this will help to avoid that direction and to direct the tip of the accessory more tangentially towards the CBD. Concerning the diameter of the wire, these are generally from 0.025- to 0.035-inch, but it seems that the diameter does not affect neither the success rate of selective bile duct cannulation nor the incidence of acute pancreatitis (8). Visibility of 0.035-inches guidewire is better especially when good quality fluoroscopy is not available. To facilitate biliary cannulation, the double wire technique is very useful. This consists of leaving one wire in the MPD that will straighten the siphon and then carry out the cannulation with another wire. A small and flapless (i.e., 5 French, 3 to 4 cm long) pancreatic stent is usually placed to prevent pancreatitis, but can also be used as an alternative in case of difficult cannulation. This technique has several advantages: the pancreatic stent blocks the pancreatic duct orifice and prevents further cannulations, facilitating cannulation of the biliary orifice, and, at the same time pancreatic duct stenting reduces the risk of post-ERCP pancreatitis by ensuring pancreatic ductal decompression and drainage (9). The pancreatic stent also gives important information on the direction of the MPD and helps to better orientate the tip of the accessory to the CBD. Flapless pancreatic stents will typically fall out spontaneously after several days and if this does not occur, upper GI endoscopy should be carried out to remove the prophylactic stent. Furthermore, pancreatic stenting can help to perform pre-cut when cannulation fails in expert hands.
Cannulation of the MPD
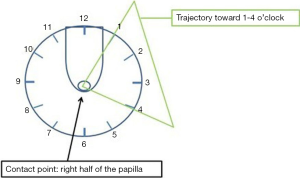
Once in front of the papilla, the pancreatic orifice should located at a 1 o’clock and 4 o’clock position (Figure 7). Unlike the cannulation of the CBD, cannulation of the MPD is done with the sphincterotome relaxed and straight. The same technique can be used for cannulation of the MPD as for biliary cannulation: contrast, wire or both. Cannulation of the MPD from the Major Papilla is generally done with the scope in the short position and with the scope slightly above the level of the papilla (unlike the cannulation of the CBD) and by turning the small wheel slightly left. In this case as well the distance from the tip of the scope and the papilla should be 2–3 cm.
Training ERCP
One of the major issues with ERCP training is that, because of the great difficulty of the procedure, a lot of practice is required for achieving sufficient competence. It is well known that ERCP may result in serious short and long-term complications compared to other endoscopic procedures (10). The current guideline of the American Society for Gastrointestinal Endoscopy (ASGE) recommends mastery of at least 200 ERCP procedures on real patients in order to gain competence (11). Some authors have even reported that in order to achieve a successful selective cannulation rate of 80% (of the duct of interest) it is necessary to perform 350–400 procedures (12). Recently, these numbers have been reconsidered to 250 cases as minimum ERCP volumes that should be offered per trainee in “high quality” advanced endoscopy training programs (13). Furthermore, high cognitive and procedural in upper endoscopy and colonoscopy are required to achieve competence in any advanced endoscopic procedure including ERCP. The training in ERCP on patients in advanced endoscopy structured training programs should typically last 12 months (13,14).
In the past years training on patients has become a serious problem in terms of informed consent, legal issues, complication rates, etc. However, there are many ways of involving trainees in real procedures. Ideally, training should start from the day before the procedure by talking to the patient and if necessary the relatives, checking for indication reviewing all the clinical history, blood tests, and imaging. The day of the procedure the trainee should be involved in whole or at least in a part of the procedure: from manipulating the accessories to “trying to cannulate” or just “being in the room” (that is also useful). The day after the procedure the trainee should check how the patient is doing, if there have been any complications and if so how these should be treated. Being in the ERCP room should not be reduced to “simply watching a procedure”. Active presence is intended as making reasonable questions, watching the hands of the operator, not only the screen, learning how to use the accessories, reviewing ERCP imaging and much more. In particular, reviewing X-rays done during ERCP is of extreme importance in order to learn the radiological part of the procedure. In fact, an expert in ERCP should understand what is on an ERCP X-ray with a blink of eyes.
ERCP simulators bypass the problem of training on patients and can be digital, ex vivo or mechanical. The Simbionix GI-MentorTM and its most current version, the GI-Mentor IITM and main rival, the CAE Healthcare AccuTouchTM are the existing digital simulators. These are computer-based simulators housed within a manikin that permits to carry out every endoscopic procedure by exchanging case-based modules. These simulators have a definite role in learning upper and lower endoscopy, but the main problem is the lack of haptic feedback for ERCP, costs and the size (15,16).
Biological models are usually made of a combination of plastic material and biological material (ex. pig stomach, liver, chicken heart, etc.). For instance, a combination of a vacuum cleaner tube and a chicken heart has also been used as an ERCP training model, where the chicken heart is the ‘papilla’ (17). Models like this allow to develop skills in scope and accessories handling and can allow for sphincterotomy. A chicken heart can be combined also with pigs stomach and can perform as in the previous case.
In our institution we have developed a simplified mechanical ERCP model (The Boskoski-Costamagna ERCP trainer, Cook Medical, Limerick, Ireland), for training endoscopists in scope handling, wheel maneuvers, cannulation, stones extraction, plastic, and metal stent insertion, electrocautery, etc. (Figure 8). This model has also been recently validated (18). The ERCP trainer is small in size, easily transportable and can be used in every type of endoscopy unit with normal endoscopes.
Competence development in ERCP is a process that goes through structured training programs that should be supervised by experienced endoscopists. ASGE suggests that 200 ERCP’s on patients is the target number for gaining competence, but if we also add simulators in these structured training programs than evaluation of competence should be based on learning curves rather than threshold numbers alone (1).
Structured training programs should be provided in every center where ERCP is done. Unfortunately, these programs are lacking on a global level and especially in developing countries. These programs should be promoted by scientific societies, not only for ERCP but for all types of GI endoscopy.
Conclusions
Deep cannulation of the CBD and/or MPD through the major duodenal papilla is mandatory for a successful ERCP.
ERCP is a skill demanding procedure, therefore trainees who will train in ERCP should be selected between those who are likely to achieve competence and will make good use of the valuable skills.
Even if ERCP training has improved in the past decade, structured ERCP training programs are still not widely available, especially in developing countries. Scientific societies should promote training in endoscopy on all levels especially through scholarships for doctors from developing countries.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Ivo Boškoski is consultant for Cook Medical, Apollo Endosurgery, Boston Scientific and has a research grant from Apollo Endosurgery. Professor Guido Costamagna is consultant for Cook Medical and Boston Scientific. Dr. Andrea Tringali is consultant for Boston Scientific.
References
- Ekkelenkamp VE, Koch AD, Rauws EA, et al. Competence development in ERCP: the learning curve of novice trainees. Endoscopy 2014;46:949-55. [Crossref] [PubMed]
- McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg 1968;167:752-6. [Crossref] [PubMed]
- Cotton PB. Fifty years of ERCP: a personal review. Gastrointest Endosc 2018;88:393-6. [Crossref] [PubMed]
- Hawes RH, Deviere J. How I cannulate the bile duct. Endoscopy 2018;50:75-7. [PubMed]
- de Moura ET, de Moura EG, Bernardo W, et al. Guide wire-a sisted cannulation versus conventional contrast to prevent pancreatitis. A systematic review and meta-analysis based on randomized control trials. Rev Gastroenterol Peru 2016;36:308-19. [PubMed]
- Tse F, Yuan Y, Moayyedi P, et al. Guide wire-assisted cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy 2013;45:605-18. [Crossref] [PubMed]
- Bassi M, Luigiano C, Ghersi S, et al. A multicenter randomized trial comparing the use of touch versus no-touch guidewire technique for deep biliary cannulation: the TNT study. Gastrointest Endosc 2018;87:196-201. [Crossref] [PubMed]
- Kitamura K, Yamamiya A, Ishii Y, et al. 0.025-inch vs 0.035-inch guide wires for wire-guided cannulation during endoscopic retrograde cholangiopancreatography: A randomized study. World J Gastroenterol 2015;21:9182-8. [Crossref] [PubMed]
- Hakuta R, Hamada T, Nakai Y, et al. Early pancreatic stent placement in wire-guided biliary cannulation: A multicenter retrospective study. J Gastroenterol Hepatol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007;102:1781-8. [Crossref] [PubMed]
- Faulx AL, Lightdale JR, Acosta RD, et al. Guidelines for privileging, credentialing, and proctoring to perform GI endoscopy. Gastrointest Endosc 2017;85:273-81. [Crossref] [PubMed]
- Verma D, Gostout CJ, Petersen BT, et al. Establishing a true assessment of endoscopic competence in ERCP during training and beyond: a single-operator learning curve for deep biliary cannulation in patients with native papillary anatomy. Gastrointest Endosc 2007;65:394-400. [Crossref] [PubMed]
- Wani S, Han S, Simon V, et al. Setting minimum standards for training in EUS and ERCP: results from a prospective multicenter study evaluating learning curves and competence among advanced endoscopy trainees. Gastrointest Endosc 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Wani S, Keswani RN, Han S, et al. Competence in Endoscopic Ultrasound and Endoscopic Retrograde Cholangiopancreatography, From Training Through Independent Practice. Gastroenterology 2018;155:1483-94.e7. [Crossref] [PubMed]
- Bar-Meir S. Simbionix simulator. Gastrointest Endosc Clin N Am 2006;16:471-8. vii. [Crossref] [PubMed]
- Gerson LB, Van DJ. Technology review: the use of simulators for training in GI endoscopy. Gastrointest Endosc 2004;60:992-1001. [Crossref] [PubMed]
- Rustemovic N, D'Assuncao M, Bilic B, et al. A simple ex vivo, biologic ERCP training model for sphincterotomy. Endoscopy 2015;47 Suppl 1 UCTN:E401-3.
- van der Wiel SE, Koch AD, Bruno MJ. Face and construct validity of a novel mechanical ERCP simulator. Endosc Int Open 2018;6:E758-65. [Crossref] [PubMed]
Cite this article as: Boškoski I, Tringali A, Costamagna G. Teaching endoscopic retrograde cholangiopancreatography cannulation. Transl Gastroenterol Hepatol 2019;4:30.