Endoscopic bariatric therapies for treating obesity: a learning curve for gastroenterologists
Introduction
Obesity represents a growing global public health threat. A 2014 report by the WHO estimated that 600 million adults have obesity in the world, and its prevalence has doubled since 1980 (1). In 2015–2016, the prevalence of obesity in the United States was 39.8% in adults and 18.5% in youths (2). Concomitantly, there has been a rise in the number of co-morbidities and complications associated with obesity. The cost of treating obesity-related illness in adults rose by 29% from 2001 to 2015 (3).
Bariatric surgery is an effective method for treating obesity that is resistant to lifestyle modifications and pharmacotherapy. Bariatric surgery results in improvement or remission of many obesity-related comorbid conditions, as well as sustained weight loss and improvement in quality of life (4-6). Available surgical procedures include laparoscopic and open Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, adjustable gastric band, vertical banded gastroplasty, duodenal switch, and biliopancreatic diversion. These methods have been effective in treating obesity and mortality rates associated with surgical intervention have progressively decreased. Chang et al. [2014] reported a meta-analysis which showed a mortality rate of 0.08% within 30 days and 0.31% after 30 days (7). Additionally, complication rates from bariatric procedures have progressively fallen from 10.5% in 1993 to 7.6% in 2006 (8).
More recently, endoscopic approaches using flexible gastrointestinal endoscopy have emerged as a reliable alternative to traditional surgical methods. Bariatric surgery offers a higher weight loss effect compared to endoscopic bariatric therapy (EBT), however, EBT is safer and more cost effective (9,10). Moreover, while surgery is indicated for patients with a BMI over 40 kg/m2 or a BMI of 35 with concomitant comorbidities, EBT can help fill the gap in patients with a BMI between 30 to 40 kg/m2 and serve as a safe alternative to patients who fail pharmacotherapy (11,12).
The history of EBT began with the advent and use of intragastric balloons (IGBs) in the 1980s (13). Since then, EBT has evolved into two major groups, namely, gastric and small bowel interventions. Gastric interventions work by stimulating gastric mechanical and chemical receptors, delaying gastric emptying, and modulating levels of gastric orexigenic hormones. Small bowel interventions work by bypassing the stomach to affect satiety and gastrointestinal motility (14,15). Overall, EBT can consist of any of the following endoluminal procedures: intragastric balloon (IGB) placement, endoscopic sleeve gastroplasty (ESG), gastric-bypass revision, and aspiration therapy (16). We review the design, clinically relevant evidence-based data, and learning curve associated with EBT.
Intragastric balloons
In 1985, the first intragastric balloon (IGB) was approved for use in the United States (Garren Edwards Gastric Bubble; American Edwards Laboratories, Mayaguez, PR). It had many adverse effects, including small bowel obstruction, and also failed to show efficacy in a sham-controlled trial and was thus pulled from the market (17). Since then, 3 gastric balloons have been FDA approved: the Orbera (Figure 1, Apollo Endosurgery, Austin, TX; 2015), the ReShape Duo (Figure 2, Reshape, San Clemente, CA; 2015) and the Obalon (Figure 3, Obalon Therapeutics, Carlsbad, CA; 2016). Two other balloons are currently under FDA investigation for approval: the Spatz Adjustable (Spatz Medical, Great Neck, NY) and the Elipse (Allurion, Natick, MA) (Table 1).

Full table
The Orbera balloon had begun development in the 1990s. The balloon was first introduced outside of the United States in 2004, with over 277,000 balloons placed worldwide. It was FDA approved on August 6th, 2015 for use in the United States. It is the most commonly used IGB, with over 230 peer-reviewed publications around the globe on over 8,000 patients (24). It is made of silicone, is inserted into the fundus and then inflated with 450–700 mL of saline (mixed with methylene blue, which will appear in the patient’s urine if the balloon ruptures). It is both placed endoscopically and removed endoscopically after 6 months. In the ORBERA pivotal study, 255 patients were studied, showing a TWL of 10.5%±6.6% after 6 months of balloon placement (18). A large meta-analysis of 3,698 patients showed EWL of 32.1% while a recent systematic review has noted the EWL to be 36.2%±6.3% after 6 months of balloon placement (25,26).
A long-term multi-center European study showed an EWL of 29.1%±60.3% 3 years after balloon placement. At the 3-year mark, they also noted complete resolution of the following: diabetes (in 33% of patients), hypertension (44%), hypercholesterolemia (34%), dyslipidemia (45%), osteoarthropathy (47%) and respiratory disorders (100%). They reported several minimal adverse effects, including nausea, vomiting, gastric perforation (0.1%) and bowel obstruction (0.8%). Early balloon removal was needed in 4.2% of patients due to those adverse effects (19). One Italian study demonstrated that the following diseases were better controlled or resolved 1 year from balloon placement: diabetes, metabolic disease, hyperlipidemia, hypertriglyceridemia, and hyperuricemia (27). Another Italian study demonstrated positive outcomes in obese women who were previously infertile (28).
The ReShape balloon is composed of 2 silicone balloons connected by a single flexible tube. The device was approved by the FDA on July 29th, 2015 for patients with a BMI between 30–40 kg/m2. The dual balloon design was intended to increase satiety and reduce the risk of migration. This balloon system is also inserted and removed endoscopically at the end of 6 months’ time. It is filled with a total of 900 mL of saline mixed with methylene blue. Each of the two balloon chambers holds 450 mL. In the REDUCE trial with 326 patients, 25.1% EWL was shown. Its adverse event profile is low; during the trial, there were no deaths, device migrations, gastric perforations or intestinal obstructions. During development, the device caused ulcers in 39.6% of patients. After modifications were made to the device, the rate and size of ulceration decreased to 10.3%. They reported 3 serious adverse effects: an esophageal perforation that was managed conservatively, an esophageal tear which required clipping and one post-retrieval aspiration pneumonitis. It is worth noting that 9.1% of patients had an early-retrieval for non-ulcer related intolerance of the balloon. This was reduced to 7.7% with the reduction of fill volumes in short-statured patients (20). A smaller, previous study of 30 patients demonstrated 31.8% EWL (29).
The Obalon balloon is a 250 mL gas filled balloon and does not need to be inserted endoscopically. It does, however, require endoscopic removal at the end of 12 or 24 weeks. It is a gelatin capsule swallowed under fluoroscopic guidance, and once in place, it is inflated with a nitrogen-sulfur hexafluoride gas. A second balloon can be placed at the end of 4 weeks, and a third at the end of 8 weeks. The SMART trial, a multi-center U.S. pivotal trial of 387 patients, showed TWL of 6.81%±5.1% with just one episode of gastric ulcer as an adverse effect. They also noted significant improvement in fasting glucose, low-density lipoprotein cholesterol, systolic blood pressure and triglycerides at the 24-week mark (21). A smaller, previous study with 17 patients showed approximately 98% of patients had swallowed the balloon correctly and also showed an average of 36.2% EWL at 12 weeks (30).
The Spatz balloon is made of silicone, placed endoscopically, and has an inflation tube through which more or less saline can be added or removed to help reduce adverse symptoms or increase weight loss. This in-situ adjustment makes it unique amongst the balloons. It is designed to remain in place for one year. The device has had modifications made to its inflation tube system and is currently in trials in the United States. Outside of the U.S. it is approved for 12-month use. One small study showed EWL to be 26.4% at 6 months and 38.8% at 12 months. However, 39% of the patients had early balloon removal due to catheter shear, perforating gastric ulcer, deflation, gastritis and Mallory-Weiss tear (31). Another trial in the United Kingdom showed EWL at 12 months to be 45.7% in 49 patients. Twenty-one patients had the balloon in place for less than 12 months due to early removal. They noted a 30% early removal rate due to intolerance, adjustment refusal, and premature satisfaction (22). In an Italian study directly comparing the Spatz to the Orbera, the Spatz was noted to have similar weight loss outcomes, but with a higher adverse outcome rate (32).
The Elipse balloon is not placed or retrieved endoscopically. The balloon is swallowed, inflated with 550 mL of liquid and has an internal valve that catastrophically fails after 4 months. It is covered in a vegetarian shell. The device is then completely deflated and the balloon is passed in the stool (33). One small study in the Czech Republic showed an EWL of 12.4% at 6 weeks, with no adverse outcomes reported (34). Another study showed improvements in LDL, triglycerides and HbA1c at 6 months. At 4 months, EWL was noted to be 37.2%. At 6 months, 93% of patients had maintained that weight loss. This study noted nausea, vomiting and abdominal cramps as the major side effects; all were self-limiting or resolved with medication, which is to be expected with balloon therapy (23). In theory, because the Elipse does not require endoscopy, hospitalization for placement and retrieval will not be required, decreasing its overall cost. Due to this, wider patient population may be reached who would not be able to afford the other balloons.
It is important to note that, as practitioners gain more experience in placing and managing these balloons, lower rates of adverse events and early balloon retrievals have been noted (35). To our knowledge, while no formal studies have been done on the learning curve for IGB placement learning curves, Thompson et al. noted that IGBs are extremely easy to place with minimal training and a minimal learning curve in the hands of an experienced gastroenterologist (36). That being said, the FDA has issued a statement in regards to the risk of acute pancreatitis associated with over-inflation of the fluid-filled balloons. Also, the FDA recently a statement regarding the deaths of 12 patients using the Orbera and ReShape balloons. Labeling changes were approved to reflect the risk of death possibly associated with these devices (37).
Sleeve gastroplasty and stapling devices
The laparoscopic gastric plication (LGP), which is also known as gastric imbrication, was first done by Dr. Kirk in 1968 (38). The procedure involves folding the stomach over and stitching it to itself. Stomach stapling, also known as vertical banded gastroplasty (VBG), was first done by Dr. Edward E. Mason in 1980 (39). In VBG, a small pouch was created along the lesser curve of the stomach by vertically stapling the upper stomach while also banding the bottom of the pouch to create a 1 cm opening to the remainder of the stomach. Due to a high failure rate of the procedure, as well as the amount of long-term complications, this procedure is no longer performed.
Two endoscopic methods attempted to recreate the VBG: the EndoCinch Endoluminal Vertical Gastroplasty (EVG) (C.R. Bard, Inc, Murray Hill, NJ) and the TransOral GAstroplasty system (TOGA) (Satiety Inc, Palo Alto, CA) (14). EVG using the EndoCinch suturing device performs the same suturing as VBG, but excludes the banding portion of the procedure. In 2008, Fogel et al. were the first to demonstrate that EVG could be successfully used to achieve gastric volume reduction using the EndoCinch device. One study of 64 patients showed no serious adverse effects with a one-year excess weight loss of 58.1%±19.9% (EWL) (40).
The TOGA suturing device was first introduced in 2008. It was used to create a restricted outlet by stapling a sleeve along the lesser curvature of the stomach. A multi-center study of 67 patients with an average BMI of 41.5 kg/m2 demonstrated EWL of 29.3%±11.6%, 36.8%±15.7%, and 38.7%±17.1% at 3, 6 and 12 months respectively. Furthermore, the study reported that hemoglobin A1c and triglyceride levels decreased while high density lipoprotein levels increased. Reported complications included one case of respiratory distress associated with anesthesia in a patient with COPD and one case of pneumoperitoneum without perforation, which was managed conservatively (41). Devière et al. [2008] reported a multicenter TOGA study involving 21 patients with an average BMI of 43.3 kg/m2. Patients experienced an average weight loss of 17.6 pounds at 1 month, 24.5 pounds at three months, and 26.5 pounds at 6 months post-treatment with EWL of 16.2%, 22.6%, and 24.4%, respectively (42). The TOGA system was not approved by the United States Food and Drug Administration (FDA) and Satiety Inc. halted operations as of 2011.
Three endoscopic procedures attempt to recreate LGP: RESTORe suturing system (Bard/Davol, Warwick, RI), Overstitch for Endoscopic Sleeve Gastroplasty (Apollo Endosurgery, Austin, TX) and the Incisionless Operating Platform for Primary Obesity Surgery Endolumenal (USGI Medical, San Clemente, CA).
ESG endoscopically reduces gastric capacity and volume by way of creating a sleeve (43-50). The RESTORe system is essentially a newer version of the EndoCinch system, which can reload in vivo and is also able to perform deeper tissue acquisition. In addition to creating the pouch along the lesser curve, the RESTORe system also creates a pouch along the greater curve. The TRIM trial of 18 patients demonstrated 27.7%±21.9% EWL in one year with no significant adverse events (51,52).
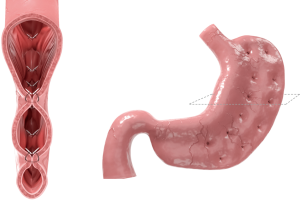
The Apollo Overstitch is capable of full-thickness suturing in a variety of patterns, using a curved needle driver. The device is fitted to a dual channel endoscope. The device is FDA-approved to place endoscopic sutures. It was used to perform ESG in India for the first time in 2012; in 2013, it was first done in the United States by Abu Dayyeh et al. The procedure has undergone technical refinement over time. Initially, interrupted reinforcing sutures were used for sleeve creation but the technique evolved into a novel, double row plication (Figure 4). This created a small diameter sleeve and reduced the volume of the stomach from the prepyloric antrum to the GE junction. Suturing technique was also adjusted in order to improve gastric fundus closure, a previously problematic portion of the procedure. However, the technique eventually progressed to not closing the fundus at all (43,53,54).
Thoughts on marking for the procedure have changed. Argon plasma coagulation is used to create a dotted target line for the sutures, but is no longer deemed necessary since the targets move during the procedure. Initially there was concern on whether the sutures were fully penetrating the tissue. This was remedied by using a tissue helix for tissue acquisition to achieve full thickness sutures. This led to expected post procedure free air therefore it is recommended to perform the procedure using carbon dioxide, to allow for more efficient reabsorption. Patients are typically noted to regain full functionality in 1–3 days after the procedure. They follow a clear liquid diet for 24–48 hours post-procedure then transition to a full liquid diet for one to two weeks, then a soft diet for the next 2–4 weeks, after which they may eat a solid diet (43,53,54).
An international, multi-center study with 77 patients showed 17.4%±1.2% TWL at the 1-year mark (53). Also, the PROMISE trial, with 22 patients, showed 48.2%±9.9% EWL at the 1 one-year mark, with no serious adverse effects (50). Another study of 248 patients showed that ESG could sustain better than 10% weight loss in approximately 75% of patients who had mild-moderate obesity over a 24-month period (55). In one study, significant reductions were noted at 12 months in HbA1c, systolic blood pressure, serum triglycerides and alanine aminotransferase. In the same study, 6-month TBWL was 14.4% (with an 80% follow up rate), 12-month TBWL was 17.6% and a 24-month TBWL was 20.9% (56).
Another study showed that the physiologic changes that occur in ESG parallel those in bariatric surgery, as well as noting a decrease in ghrelin (an appetite stimulating hormone), increase in insulin sensitivity and delay in gastric emptying. Three months after ESG, there was a 90-minute increase in time it took to empty 50% of the ingested meal. It was also noted that 4 hours after ingestion, 32.25% of the meal was retained in the stomach after ESG as compared to only 5.25% before ESG. There was no delay in gastric emptying of liquids after ESG. Satiation was significantly increased after ESG, decreasing caloric intake by 59% and leading to earlier termination of meals. Meals were completed by 11.5±2.3 minutes post-ESG as compared to 35.2±9.9 minutes pre-ESG. Post-prandial ghrelin levels were noted to be decreased by 29.4% 3 months post-ESG. Using the fasting homeostatic model for insulin resistance, it was noted that the patients’ insulin resistance score improved. Their area under the curve for post-prandial glucose and insulin decreased by 36% and 34% as well. There was no significant change in the levels of leptin, GLP-1 and PYY (47).
The Incisionless Operating Platform for Primary Obesity Surgery Endolumenal works by creating full-thickness plications and is still under FDA review. It is a 54-Fr flexible tube, has a control handle and 4 working channels (33). The Primary Obesity Surgery Endolumenal procedure (POSE) was first reported in 2013, using 8–9 plications in the fundus, followed by 3–4 plications in the distal end of the body. A study of 45 patients showed 49.4%±21.5% EWL at 6 months (57). The ESSENTIAL trial, with 332 patients, showed patients in the study arm lost 4.95%±7.04% EWL while the patients in the sham arm lost 1.38%±5.58% EWL in 1 year (58). Two other studies showed a one-year EWL of 44.9%±24.4% and 45%, with no serious short- or long-term side effects. Mean procedures time was noted to be 51.8±14.5 minutes (46,59). Recently, physiological changes in regards to POSE were studied. They noted a significant reduction in intake capacity, delayed gastric emptying, improved glucose homeostasis, and a decrease in post-prandial ghrelin (60).
A number of studies have been conducted regarding the learning curve of ESG (Table 2). A study of 55 patients showed that ESG using the OverStitch was a safe and efficacious procedure when done by expert endoscopists in a controlled hospital setting with appropriate inpatient postoperative surveillance. In fact, they reported no major complications. They did not define what was meant by an “expert endoscopist” (49). Kumbhari et al. performed a study using the OverStitch device showing that total length of procedure, number of plications placed during the procedure and the amount of time spent per placing each plication dropped significantly with the number of performed procedures. They also noted that the decrease in total number of plications per procedure were a demonstration of “mastery.” Efficiency was defined as a decrease in time spent per plication as well a decrease in total length of procedure. They determined that 7 procedures were sufficient to develop 90% potential efficiency after a 1-day training session. It is worth noting that the endoscopist in this study was already familiar and proficient with the OverStitch device and was only learning the ESG procedure (61).

Full table
In 2017, Kahaleh et al. conducted a study using the OverStitch device (56). Again, it was a single-endoscopist study who already had proficiency with the suturing device. They demonstrated that efficiency was achieved after 35 cases; there was a significant decrease in total procedure time and that number essentially plateaued after 35 cases (mean procedure time for first 35 cases was 144.9±39.4 minutes while the mean procedure time for subsequent cases was 74.32±18.7 minutes).
In 2018, Sharaiha et al. also conducted another study to define the learning curve for ESG (62). In their study, the primary outcome was procedure time, with a secondary outcome of efficiency and mastery. The study was done with the OverStitch platform. They defined efficiency “as the point on the learning curve where the operator was able to make procedural improvements to decrease procedure time”. Mastery was defined “as the point at which the procedure time became consistent by eliminating outliers in terms of operating time”. Based on their analysis, efficiency was achieved after 29–38 procedures, while mastery was achieved after 55 procedures. They also noted that improvement in procedure time did not adversely affect the overall outcome of weight loss.
Recently, Fayad et al. conducted a case-matched study comparing ESG with laparoscopic sleeve gastrectomy (LSG) (63) (Figure 5). They noted that at one month follow up, ESG had a significantly greater %TBWL than LSG (9.8%±2.5% vs. 6.6%±2.4%). However, they noted that at the six-month follow up, LSG had significantly greater %TBWL than ESG (23.6%±7.6% vs. 17.1%±6.5%). A further subgroup analysis demonstrated that in patients with a BMI over 40, LSG remained superior to ESG at the 6-month mark. However, in patients with BMI under 40, ESG and LSG were comparable in regard to %TBWL at the 6-month mark. The ESG group demonstrated significantly less adverse effects than the LSG group (5.2% vs. 16.9%). Also, new onset GERD was significantly lower in the ESG group as compared to the LSG group (1.9% vs. 14.5%). Given these findings, ESG was a good option for patients, especially in the subgroup of patients with a BMI under 40, since most would not even qualify for LSG.
Transpyloric shuttle
The transpyloric shuttle is used in the treatment of obesity by delaying gastric emptying (64). It consists of two bulbs joined by a flexible catheter which facilitates partial gastric obstruction (Figure 6). The ENDObesity 1 study was conducted in Australia with 20 patients and a mean BMI of 36 (65). All patients had the transpyloric shuttle inserted and follow up at 3 and 6 months. The study reported progressive weight loss at 3 months (31.3%±15.7% EWL) and 6 months (50%±26.4% EWL). All patients also tolerated the device while 15 patients had evidence of mucosal erosion. Two patients had the device removed due to persistent ulceration. A follow-up trial (ENDObesity II) has completed its recruitment phases and is being studied (https://clinicaltrials.gov/ct2/show/NCT02518685).
Incisionless Anastomosis System (IAS)
Another novel technique called the incisionless magnetic anastomosis system, utilizes two self-forming magnets. The magnets are endoscopically deposited which eventually join and compress two regions of small bowel. Stomach contents are then diverted into the new pathway, and eventually the magnets are passed in the stool. A prospective study involving 10 patients delivered the magnets through the working channel of a colonoscope, with laparoscopic supervision (31). Patients were not required to participate in an intensive lifestyle/diet management program. Endoscopic visualization of the anastomosis was obtained at 2, 6, and 12 months. Patient weight, glycemic profile, and metabolic panels were acquired at 0.5, 1, 2, 3, 6, 9, and 12 months. The study reported an average total weight loss of 14.6% (40.2% excess weight loss) at 12 months. Larger studies are needed to validate these initial results before IAS can be implemented in practice.
Aspiration therapy
The aspiration therapy system has also been used to treat obesity which consists of an endoscopically placed gastrostomy tube and siphon assembly (66). Patients can aspirate gastric contents about 20 min after eating. This process takes 10 mins to perform and can remove up to 30% of gastric contents. Noren and Forssel [2016] performed a prospective study involving 25 patients with a mean BMI of 39.8 kg/m2 and excess weight loss was 54.4% (SD 28.8, P<0.01) (67). At 2 years BMI was 31.0 kg/m2 (5.1, P<0.01), and excess weight loss was 61.5% (28.5, P<0.01). No serious adverse effects including electrolyte disturbance was reported. The rate of compliance was 80% after 1 year and 60% after 2 years. The study showed that weight was halved in a year and that aspiration therapy was a safe treatment for obesity. Additionally, Thompson et al. reported the results of a clinical trial involving 111 patients treated with AspireAssist and life-style counseling (AALC) versus life-style counseling alone (LCA) (68). The study reported that at 52 weeks, the mean %EWL of the AALC group was 31.5%±26.7%, on a modified Intent to Treat basis (mITT) basis, and 37.2%±27.5%, on per-protocol (PP) basis, versus 9.8%±15.5% of the LCA group on a mITT basis and 13.0%±17.6%, on a PP basis. The difference in mean %EWL achieved between groups was 21.7% (95% CI: 15.3, 28.1) on a mITT basis and 24.2% (95% CI: 15.5, 32.9). The average difference in both study arms was 21.7% (95% CI: 15.3, 28.1, P=0.0083) on a mITT basis and 24.2% (95% CI: 15.5, 32.9, P=0.0038) on a PP basis. Adverse effects related to the AspireAssist device include occasional indigestion, nausea, vomiting, constipation and diarrhea. The AspireAssist device was approved for use by the FDA in June of 2016 for patients with a BMI of 35–55 kg/m2. Although there are no studies looking at the learning curve for placing the AspireAssist device, proficiency can be achieved after one has mastered percutaneous endoscopic gastrostomy.
Botulinum toxin injection
Botulinum toxin A (BTX-A) has been hypothesized to lead to weight loss by selectively inhibiting smooth and striated muscle contraction. By injecting the stomach antrum with BTX-A, food contents may be slowed down by increasing transit time and inducing satiation. Elshakh et al. conducted a review of 60 articles of which only 11 were relevant for examining the efficacy of BTX-A injections on obesity (69). The study concluded that current data does not demonstrate sustained weight loss through the use of endoscopic BTX-A injection. de Moura et al. reported a recent randomized double blinded control trial involving 32 super-obese patients to study the effect of endoscopic guided BTX-A injections as a bridge to bariatric surgery (70). Patients were grouped into one of two groups: BTX-A, in which 200 units of BTX-A were injected into the gastric antrum and body; and control, in which the same injections were performed with 0.9% saline. Both study arms were followed for 6 months. The study reported that both groups showed similar weight loss (P<0.001). No statistical significance was observed between groups regarding weight loss, excess weight, total loss of excess weight, total weight loss, or change in BMI. Furthermore, due to variability in dosages, poor sample sizes, site of injection, and methods used to measure weight reduction, meta-analyses are also unreliable.
Conclusions
Endoscopic bariatric and metabolic therapies are safe and effective strategies for weight loss, especially when used in conjunction with diet and lifestyle modification. As more practitioners gain proficiency, we can expect concomitant expansion and widespread application of these procedures. Moving forward, it will be important to keep in mind that these therapies should be tailored to each patient based on their clinical phenotype and physiology. While a lot of information abounds on the clinical effects of EBT, technical information related to training in this subspecialized area is sparse. Trainees are expected to understand the limitations of each technique and its application in the right clinical setting. For instance, not all balloons are equal, choosing between the Orbera and the ReShape balloon may be a matter of clinical context, user experience, or preference. Furthermore, training in this area may be limited to large University training centers. As a result, trainees at smaller community centers may not have access to this form of training. To address this dilemma, national organizations such as the ACG, AGA, ASGE should consider advance fellowship training in EBT for those willing to focus their future practice in this area. Other trainees can undergo elective rotations to gain more experience and exposure. Despite these shortcomings, the learning curve associated with EBT requires dedicated practice. EBT is a relatively new field and will help bridge the gap in patients who fail medical therapy and are not candidates for traditional surgery. To this end, algorithms and guidelines are needed to further delineate the appropriate application of EBT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. Obesity and overweight fact sheet. WHO. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief 2017.1-8. [PubMed]
- Biener A, Cawley J, Meyerhoefer C. The impact of obesity on medical care costs and labor market outcomes in the US. Clin Chem 2018;64:108-17. [Crossref] [PubMed]
- Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964-73. [Crossref] [PubMed]
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753-61. [Crossref] [PubMed]
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017;14:160-9. [Crossref] [PubMed]
- Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg 2014;149:275-87. [Crossref] [PubMed]
- Flum DR, Belle SH, King WC, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445-54. [Crossref] [PubMed]
- Stimac D, Majanović SK. Endoscopic approaches to obesity. Dig Dis 2012;30:187-95. [Crossref] [PubMed]
- Choi HS, Chun HJ. Recent Trends in Endoscopic Bariatric Therapies. Clin Endosc 2017;50:11-6. [Crossref] [PubMed]
- Ali MR, Moustarah F, Kim JJ. American Society for Metabolic and Bariatric Surgery position statement on intragastric balloon therapy endorsed by the Society of American Gastrointestinal and Endoscopic Surgeons. Surg Obes Relat Dis 2016;12:462-7. [Crossref] [PubMed]
- Goyal D, Watson RR. Endoscopic bariatric therapies. Curr Gastroenterol Rep 2016;18:26. [Crossref] [PubMed]
- Gleysteen JJ. A history of intragastric balloons. Surg Obes Relat Dis 2016;12:430-5. [Crossref] [PubMed]
- Jirapinyo P, Thompson CC. Endoscopic bariatric and metabolic therapies: surgical analogues and mechanisms of action. Clin Gastroenterol Hepatol 2017;15:619-30. [Crossref] [PubMed]
- Gómez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: results of a prospective study. Obesity 2016;24:1849-53. [Crossref] [PubMed]
- Kim SH, Chun HJ, Choi HS, et al. Current status of intragastric balloon for obesity treatment. World J Gastroenterol 2016;22:5495-504. [Crossref] [PubMed]
- Hogan RB, Johnston JH, Long BW, et al. A double-blind, randomized, sham-controlled trial of the gastric bubble for obesity. Gastrointest Endosc 1989;35:381-5. [Crossref] [PubMed]
- Dayyeh BKA, Eaton LL, Woodman G, et al. A randomized, multi-center study to evaluate the safety and effectiveness of an intragastric balloon as an adjunct to a behavioral modification program, in comparison with a behavioral modification program alone in the weight management of obese subjects. Gastrointest Endosc 2015;81:AB147. [Crossref]
- Genco A. Adjustable intragastric balloon vs non-adjustable intragastric balloon: case–control study on complications, tolerance, and efficacy. Obes Surg 2013;23:953-8. [Crossref] [PubMed]
- Ponce J, Woodman G, Swain K, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis 2015;11:874-81. [Crossref] [PubMed]
- Sullivan S, Swain JM, Woodman G, et al. The Obalon swallowable 6-month balloon system is more effective than moderate intensity lifestyle therapy alone: results from a 6-month randomized sham controlled trial. Gastroenterology 2016;150:S1267. [Crossref]
- Brooks J, Srivastava ED, Mathus-Vliegen EM. One-year adjustable intragastric balloons: results in 73 consecutive patients in the U.K. Obes Surg 2014;24:813-9. [Crossref] [PubMed]
- Chuttani R, Machytka E, Raftopoulos I, et al. The first procedureless gastric balloon for weight loss: final results from a multi-center, prospective study evaluating safety, efficacy, metabolic parameters, quality of life, and 6-month follow-up. Gastroenterology 2016;150:S26. [Crossref]
- Behary J, Kumbhari V. Advances in the endoscopic management of obesity. Gastroenterol. Res. Pract. 2015;2015:757821. [Crossref] [PubMed]
- Imaz I, Martinez-Cervell C, Garcia-Alvarez EE, et al. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg 2008;18:841-6. [Crossref] [PubMed]
- Yorke E, Switzer NJ, Reso A, et al. Intragastric balloon for management of severe obesity: a systematic review. Obes Surg 2016;26:2248-54. [Crossref] [PubMed]
- Crea N, Pata G, Della Casa D, et al. Improvement of metabolic syndrome following intragastric balloon: 1 year follow-up analysis. Obes Surg 2009;19:1084-8. [Crossref] [PubMed]
- Musella M, Milone M, Bellini M, et al. The potential role of intragastric balloon in the treatment of obese-related infertility: personal experience. Obes Surg 2011;21:426-30. [Crossref] [PubMed]
- Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Relat Dis 2013;9:290-5. [Crossref] [PubMed]
- Mion F, Ibrahim M, Marjoux S, et al. Swallowable Obalon gastric balloons as an aid for weight loss: a pilot feasibility study. Obes Surg 2013;23:730-3. [Crossref] [PubMed]
- Machytka E, Bužga M, Zonca P, et al. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc 2017;86:904-12. [Crossref] [PubMed]
- Genco A, Lopez-Nava G, Wahlen C, et al. Multi-centre European experience with intragastric balloon in overweight populations: 13 years of experience. Obes Surg 2013;23:515-21. [Crossref] [PubMed]
- Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic bariatric and metabolic therapies: new and emerging techniques. Gastroenterology 2017;152:1791-801. [Crossref] [PubMed]
- Machytka E, Chuttani R, Bojkova M, et al. Elipse, a procedureless gastric balloon for weight loss: a proof-of- concept pilot study. Obes Surg 2016;26:512-6. [Crossref] [PubMed]
- Abu Dayyeh BK, Kumar N, Edmundowicz SA, et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc 2015;82:425-38.e5. [Crossref] [PubMed]
- Thompson CC. Evolving techniques for bariatric surgery and endoscopy. Gastroenterol Hepatol (N Y) 2015;11:830-2. [PubMed]
- FDA. Available online: https://www.fda.gov/NewsEvents/Newsroom/FDAInBrief/ucm609757.htm
- Kirk RM. Gastric imbrication as a method of weight control. Brit J Surg 1968;55:867. [PubMed]
- Mason EE. Vertical banded gastroplasty for obesity. Arch Surg 1982;117:701-6. [Crossref] [PubMed]
- Fogel R, De Fogel J, Bonilla Y, et al. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc 2008;68:51-8. [Crossref] [PubMed]
- Familiari P, Costamagna G, Blero D, et al. Transoral gastroplasty for morbid obesity: a multicenter trial with a 1-year outcome. Gastrointest Endosc 2011;74:1248-58. [Crossref] [PubMed]
- Devière J, Ojeda Valdes G, Cuevas Herrera L, et al. Safety, feasibility and weight loss after transoral gastroplasty: First human multicenter study. Surg Endosc 2008;22:589-98. [Crossref] [PubMed]
- Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc 2013;78:530-5. [Crossref] [PubMed]
- Lopez-Nava G, Galvao MP, da Bautista-Castano I, et al. Endoscopic sleeve gastroplasty for the treatment of obesity. Endoscopy 2015;47:449-52. [PubMed]
- Sharaiha RZ, Kedia P, Kumta N, et al. Initial experience with endoscopic sleeve gastroplasty: technical success and reproducibility in the bariatric population. Endoscopy 2015;47:164-6. [PubMed]
- Lopez-Nava G, Galvao MP, Bautista-Castano I, et al. Endoscopic sleeve gastroplasty: how do I do it? Obes Surg 2015;25:1534-8. [Crossref] [PubMed]
- Abu Dayyeh BK, Acosta A, Camilleri M, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol 2017;15:37-43.e1. [Crossref] [PubMed]
- Lopez-Nava G, Galvao M, Bautista-Castano I, et al. Endoscopic sleeve gastroplasty with 1-year follow-up: factors predictive of success. Endosc Int Open 2016;4:E222-7. [Crossref] [PubMed]
- López-Nava Breviere G, Bautista-Castano I, Fernandez-Corbelle JP, et al. Endoscopic sleeve gastroplasty (the Apollo method): a new approach to obesity management. Rev Esp Enferm Dig 2016;108:201-6. [Crossref] [PubMed]
- Kumar N, Lopez-Nava G, Sahdala HNP, et al. Endoscopic sleeve gastroplasty: multicenter weight loss results. Gastroenterology 2015;148:S179. [Crossref]
- Brethauer SA, Chand B, Shauer PR, et al. Transoral gastric volume reduction for weight management: technique and feasibility in 18 patients. Surg Obes Relat Dis 2010;6:689-94. [Crossref] [PubMed]
- Brethauer SA, Chand B, Shauer PR, et al. Transoral gastric volume reduction as intervention for weight management: 12-month follow-up of TRIM trial. Surg Obes Relat Dis 2012;8:296-303. [Crossref] [PubMed]
- Kumar N, Sahdala HNP, Shaikh S, et al. Endoscopic sleeve gastroplasty for primary therapy of obesity: initial human cases. Gastroenterology 2014;146:S571-2. [Crossref]
- Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, et al. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc 2018;32:2159-64. [Crossref] [PubMed]
- Lopez-Nava G, Sharaiha RZ, Vargas EJ, et al. Endoscopic sleeve gastroplasty for obesity: a multicenter study of 248 patients with 24 months follow-up. Obes Surg 2017;27:2649-55. [Crossref] [PubMed]
- Sharaiha RZ, Kumta NA, Saumoy M, et al. Endoscopic sleeve gastroplasty significantly reduces body mass index and metabolic complications in obese patients. Clin Gastroenterol Hepatol 2017;15:504-10. [Crossref] [PubMed]
- Espinós JC, Turro R, Mata A, et al. Early experience with the Incisionless Operating Platform (IOP) for the treatment of obesity: the Primary Obesity Surgery Endolumenal (POSE) procedure. Obes Surg 2013;23:1375-83. [Crossref] [PubMed]
- Sullivan S, Swain JM, Woodman G, et al. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: The ESSENTIAL trial. Obesity 2017;25:294-301. [Crossref] [PubMed]
- Miller K, Turro R, Greve JW, et al. MILEPOST multicenter randomized controlled trial: 12-month weight loss and satiety outcomes after pose SM vs. medical therapy. Obes Surg 2017;27:310-22. [Crossref] [PubMed]
- Espinós JC, Turro R, Moragas G, et al. Gastrointestinal physiological changes and their relationship to weight loss following the POSE procedure. Obes Surg 2016;26:1081-9. [Crossref] [PubMed]
- Hill C, El Zein M, Agnihotri A, et al. Endoscopic sleeve gastroplasty: the learning curve. Endosc Int Open 2017;5:E900-4. [Crossref] [PubMed]
- Saumoy M, Schneider Y, Zhou XK, et al. A single-operator learning curse analysis for the endoscopic sleeve gastroplasty. Gastrointest Endosc 2018;87:442-7. [Crossref] [PubMed]
- Fayad L, Adam A, Dunlap M, Badurdeen DS, et al. Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: a case-matched study. Gastrointest Endosc 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Sampath K, Dinani AM, Rothstein RI. Endoscopic devices for obesity. Curr Obes Rep 2016;5:251-61. [Crossref] [PubMed]
- Marinos G, Eliades C, Raman Muthusamy V, et al. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: clinical results from a 3- and 6-month study. Surg Obes Relat Dis 2014;10:929-34. [Crossref] [PubMed]
- Sullivan S, Stein R, Jonnalagadda S, et al. Aspiration therapy leads to weight loss in obese subjects: a pilot study. Gastroenterology 2013;145:1245-52. [Crossref] [PubMed]
- Norén E, Forssell H. Aspiration therapy for obesity; a safe and effective treatment. BMC Obes 2016;3:56. [Crossref] [PubMed]
- Thompson CC, Dayyeh BK, Kushner R, et al. The AspireAssist is an effective tool in the treatment of class II and class III obesity: results of a one-year clinical trial. Gastroenterology 2016;150:S86. [Crossref]
- Elshakh H, El-Ejji K, Taheri S. The role of endoscopic intra-gastric botulinum toxin-A for obesity treatment. Obes Surg 2017;27:2471-8. [Crossref] [PubMed]
- de Moura EGH, Ribeiro IB, Frazão MS, et al. EUS-Guided Intragastric Injection of Botulinum Toxin A in the Preoperative Treatment of Super-Obese Patients: a Randomized Clinical Trial. Obes Surg 2019;29:32-9. [Crossref] [PubMed]
Cite this article as: Shahnazarian V, Ramai D, Sarkar A. Endoscopic bariatric therapies for treating obesity: a learning curve for gastroenterologists. Transl Gastroenterol Hepatol 2019;4:16.