Treatment strategies for locally advanced hepatocellular carcinoma
Introduction
Global estimates show that liver cancer ranks fifth in incidence and fourth in overall cancer-related mortality, with approximately 854,000 new cases and 810,000 deaths per year. These essentially overlapping incidence and mortality rates highlight the lethality of this neoplasm (1), with hepatocellular carcinoma (HCC) accounting for 90% of cases (2).
Most of these deaths probably happen in East Asia, as approximately 40% of all HCC cases occur in China. Over time, however, both the incidence and mortality of this cancer has been rising in several regions worldwide, including North America, Latin America, and Central Europe (1,3).
To facilitate proper assessment of the extent of the primary lesion and its remote spread, in addition to the aiding in the definition of treatment strategies and prognosis, several HCC staging systems have been proposed (4). In 1984, Okuda et al. (5) pioneered a staging system that combined anatomical tumor features and parameters related to the overlying liver disease.
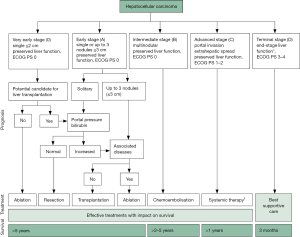
The Barcelona Classification (BCLC) is perhaps the most widely used staging scheme worldwide, particularly in the West (6). The BCLC integrates tumor-, patient-, and liver disease-related factors into an algorithm that yields four HCC stages, and proposes distinct treatment approaches for each.
According to the BCLC, the presence of multinodular disease, portal vein invasion, or performance status 1 or 2 is enough to classify the patient as having intermediate or advanced disease; palliative care is then indicated. The presence of portal hypertension rules out resection as a treatment alternative, directing patients to liver transplantation or ablation (4,6).
However, the Barcelona Classification has been the target of criticism. Some authors question the limit imposed by the Milan criteria for liver transplant selection, as satisfactory outcomes have been obtained with the San Francisco criteria (7). Likewise, until recently, the BCLC contraindicated transplantation in patients with advanced liver disease (Child C), even those with early-stage tumors. In 2018, the BCLC became more flexible and clear, stating that Child C patients should be transplanted if they meet the Milan criteria (8). This latest update notwithstanding, given its strict patient selection criteria, the BCLC is still difficult to follow in daily clinical practice.
Several Asian centers recommend more aggressive approaches to HCC, mainly aiming at surgical resection. Thus, they disregard many BCLC recommendations, pushing the boundaries of their treatment methods and achieving satisfactory outcomes (9).
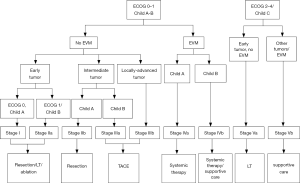
In 2014, Yau et al., published the experience of the University of Hong Kong in creating a model for classification and treatment of Asian patients with HCC, which became known as the Hong Kong Liver Cancer (HKLC) prognostic classification system (10). Analysis of their results revealed better stratification of patients at more advanced stages of the disease, culminating in a higher survival rate due to more aggressive treatment methods (10).
According to the HKLC classification, intrahepatic vascular invasion alone does not contraindicate surgical resection, nor does tumor multicentricity. In addition, the combination of advanced liver disease (Child C) and early tumor without extrahepatic vascular invasion or metastases still leaves patients eligible for liver transplantation (10).
Studies have already evaluated the outcomes of resection in patients with HCC and vascular invasion. Pawlik et al. (11), in a multicenter study, showed that, despite the poor prognosis associated with hepatic vascular invasion, surgical resection with removal of the affected vessel still confers greater survival than palliative care or watchful waiting. Likewise, Ikai et al. (12) demonstrated the superiority of surgical resection in this group of patients compared to palliative treatment.
Thus, several factors—related to the tumor, the patient, and the overlying liver disease—must be considered jointly when assessing prognosis. Treatment must be individualized, especially in those patients with intermediate-stage disease, for whom there is still no absolute truth. In this group, recent studies have called for a more aggressive treatment strategy, be it through resection, liver transplantation, locoregional therapies, or a combination thereof.
Resection
Liver resection is still the most effective treatment modality for HCC, with 5-year survival rates ranging from 50% to 70%, and is also a useful approach when waiting lists for liver transplantation are long. Underlying chronic liver disease or cirrhosis is present in 80% to 90% of patients who develop HCC. Thus, careful assessment of liver function is mandatory for correct decision-making.
The Child-Turcotte-Pugh score is a simple, easy-to-use, and straightforward method to evaluate liver function on the basis of clinical and laboratory data alone (13). Patients classified as Child A can potentially tolerate liver resection, but the score is not precise enough to predict postoperative liver failure (14). The MELD score, initially developed to predict survival in patients with portal hypertension undergoing transjugular intrahepatic portosystemic shunting, has become a popular method to determine liver resection risk worldwide; in patients with a MELD score <10, resection can be performed safely (15). The Child and MELD scores are useful tools; however, they lack precision to evaluate liver function. In Asian countries, the indocyanine green clearance (ICG) test is used routinely before liver resection and is considered most refined and precise method to evaluate liver function. Some centers have shown that ICG retention <14% within 15 minutes of IV injection allows major liver resection (16,17).
Evaluation of future liver remnant volume (FLRV%) is a very important test for patients who will undergo major liver resection. To avoid postoperative liver failure, the target FLRV% is 40% for patients with chronic liver disease or those with previous chemotherapy exposure and 30% for those without chronic liver disease (18). The presence of portal hypertension in cirrhotic livers is still controversial, but several centers are now willing to perform minor liver resections in Child A and more selective ones in Child B patients with MELD <11.
Anatomical resection, where entire segments, sectors, or a lobe of the corresponding portal pedicles are resected, has been proposed as the ideal treatment for HCC because tumor spread occurs principally through the portal vein; thus, en bloc resection of the tumor and its portal vein territory may lead to better oncologic outcomes (19,20). However, in patients with chronic liver disease and cirrhosis, parenchyma-sparing resections sometimes are necessary to avoid postoperative liver failure. In small peripheral and well-differentiated HCC, studies have shown similar results with anatomic and non-anatomic resections (20).
Of all the staging systems available to date, the BCLC is the most popular staging system worldwide for decision-making in HCC management (8) (Figure 1). However, as noted above, its applicability is being questioned, especially in patients from Asian nations. The HKLC staging system introduced in 2014 has become very attractive, especially for surgeons, as it provides for a wider range of therapies with curative intent. HKLC staging uses an approach similar to that of the BCLC system to classify patients into nine stages (five major stages), but recommends more aggressive treatment for stages I and II, particularly in those with preserved performance status (10) (Figure 2). In contrast, per BCLC staging, surgical resection is offered as a curative treatment option only for stage A patients.
Several authors have proposed that the BCLC options for intermediate/advanced HCC should be improved. Torzilli et al. (21), in a large, multicenter analysis of 2,046 resected HCC patients, reported that surgical resection is a potential tool for patients with multinodular, large, and macrovascular invasive HCC. Zhong et al. (22) reported a single-center experience with 1,259 consecutive resections for BCLC stage B/C patients, with similar findings.
Bhandare et al. (23) achieved long-term survival with liver resection in BCLC A and B patients, as well as in BCLC C if well selected (with good performance status and Child score). The median resected tumor size was 7 cm (range, 2–30 cm), and most of these patients would otherwise have fallen outside LT criteria. Three-year overall survival at stages A, B, and C was 55.2%, 62.6%, and 37.5% respectively.
Vauthey et al. (24), in an expert consensus meeting, suggested a few statements pertaining to the HCC staging debate:
- Based on current knowledge and experience, no single staging system is applicable to all patients with HCC.
- The use of regional staging systems is discouraged, because it precludes comparison between centers.
- In medical patients with advanced liver disease who are not candidates for liver transplantation or resection, the Barcelona Clinic Liver Cancer (BCLC) classification is appropriate.
- There is significant heterogeneity within stage B and C of the BCLC classification; thus, resection may be considered for some of these patients. Overall, BCLC criteria provide a reasonable guide for treatment, considering the caveat regarding stage B and C patients.
- The American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) classification is valid for HCC staging based on single and multicenter studies in the West and East, including Japan and China, for patients undergoing liver resection. It is useful in patients with a normal liver or chronic liver disease when coupled with the fibrosis score.
- Following resection or liver transplantation, report pathological outcomes using the AJCC/UICC system.
- In the future, incorporation of recently described biomarkers (VEGF plasma level and DNA index) may improve preoperative staging.
Portal vein tumor thrombus (PVTT), a complication of advanced HCC, is detected in 10–60% of patients with HCC at the time of diagnosis (25) and plays an important role in prognosis and clinical staging (26). Once PVTT has developed and progressed into the contralateral bifurcation or main trunk of the portal vein, obstruction by the tumor thrombus usually promotes disease progression, aggravates portal hypertension and its related complications, depletes liver function reserve, and induces tolerance to antitumor treatment. Moreover, when the primary tumor invades the portal venous system, HCC cells become distributed along the branches of the portal vein and spread to adjacent liver segments, leading to invisible intrahepatic metastasis, which is widely accepted as a major mechanism contributing to early intrahepatic recurrence (27). The prognosis of patients with HCC and PVTT is extremely poor, with a median survival period of only 2.7–4 months, versus 10–24 months in patients without PVTT (28,29). Cheng’s classification and the Japanese VP classification are widely used in clinical practice for patients with PVTT (12,30,31) (Table 1).
The 2016 edition of the Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (29) approves primary resectable HCC and Cheng’s I–III type PVTT as potential candidates for liver resection, preferentially with adjuvant therapies such as preoperative radiotherapy or postoperative TACE. The Hong Kong Consensus Recommendations on the Management of Hepatocellular Carcinoma (10), published in 2015, highlighted that intrahepatic vascular invasion is not an absolute contradiction for liver resection in selected patients with Child-Pugh A liver function and tumor size ≤5 cm. Similarly, the Liver Cancer Study Group of Japan 2014 Update JSH Consensus-based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma (32) encourage surgical resection as feasible for selected patients with type VP1–3 PVTT and Child-Pugh A liver function. Moreover, in the APPLE 2014 consensus statement (33), 10 top Asian experts from 10 institutions voted that portal venous invasion should not be defined as an absolute contraindication for surgical resection; the final vote was unanimous.
The controversy of liver transplantation with extended HCC criteria
Initial attempts at LT for HCC were disappointing due to high recurrence and poor survival rates (34,35). In 1985, the Starzl group (34) reported a recurrence rate of 75% in patients who had had LT for hepatic malignancies and lived for at least 2 months after LT. Bismuth et al. (35) reported a 3-year survival rate of 47% in 60 LTs for patients with HCC.
The Milan criteria were a watershed moment for LT in HCC. Since 1996, those criteria have been used by most centers worldwide. Patients who met the Milan criteria and underwent LT had comparable post-transplant survival rates to patients transplanted for non-tumor indications (36).
Many studies seeking to expand the Milan criteria are based on the idea that they are very restrictive, and thus exclude a significant number of patients with who might benefit from LT. Indeed, per the Milan criteria, only 6% of patients with HCC would be eligible for LT. Accordingly, many centers worldwide have attempted to expand the Milan criteria while maintaining similar post-transplant survival rates (37).
Various selection criteria with different concepts have been proposed to expand the Milan criteria (38-46). The first consistent expanded criteria was from the University of California San Francisco (UCSF), created by Yao et al. (47) in 2001. Based on pathologic data from 70 patients transplanted for HCC, they extended the selection criteria to: one tumor ≤6.5 cm in diameter, or two to three tumors each ≤4.5 cm in diameter and a total diameter of ≤8 cm (47). Patients within the UCSF criteria had 1-and 5-year survival rates of 90% and 75%. However, patients beyond the criteria had a 1-year survival of 50% (48). Such results were later validated in the same center prospectively, on the basis of pre-LT imaging. The 5-year recurrence rate was only 9.1%, and the recurrence-free survival rate was 80.7%. More recently, the United Network for Organ Sharing database was used to validate the UCSF criteria. In this large-scale analysis, survival of 59 patients beyond the Milan criteria but within the USCF was noninferior to that of 1,913 patients within the Milan criteria (1-, 2-, 3- and 4-year survival rates: 91%, 80%, 68%, and 51% versus 89%, 81%, 76%, and 72%, respectively, P=0.21) (49).
Mazzaferro et al. (50) have suggested a modified set, known as the “Up-to-seven” criteria, based on a web survey of patients beyond the Milan criteria transplanted for HCC. They extended the criteria up to seven tumors with a sum size of the largest tumor of 7 cm, using the so-called “Metroticket” concept. Patients within the Up-to-seven criteria without microvascular invasion had a 5-year overall survival rate of 71%, which was comparable to previous results based on the Milan criteria (50). Zou et al. (49), in 2008, analyzed 303 transplants and described three risk factors for fatal recurrence after LT for HCC:
- Macrovascular invasion;
- Tumor size >6.5 cm;
- Alpha-fetoprotein >1,000 mcg/dL.
The recurrence rate was 85.7% if all three risk factors were present, 37.84% if two risk factors were present, and 13.64% if only one risk factor was present. When any risk factor was involved, the recurrence rate was 6.71%.
Dendy et al. (51) were the first to report two successful cases of patients with HCC who underwent LT for PVTT after downstaging with yttrium-90 radioembolization, in 2017, Levi Sandri et al. (52) also reported four cases of PVTT who underwent LT after yttrium-90 radioembolization. Both reports (51,52) described LT in BCLC stage C (advanced) HCC.
In the author’s country, Brazil, the transplant law applies the Milan criteria to allocate special MELD points to patients, and cadaver grafts cannot be allocated to recipients outside the Milan criteria. Nevertheless, living-donor liver transplantation (LDLT) is not forbidden, and centers are free to perform LDLT in patients with beyond-criteria HCC. In this context, the author’s team has attempted to use biological markers, such as alpha-fetoprotein level <800 mg/dL, PIVKA below 400 mcg/dL, and tumor biopsy differentiation G1 or G2, to support such procedures. Using this strategy, more than 14 LDLTs have been performed in patients with extended-criteria HCC, with zero recurrences since 2010.
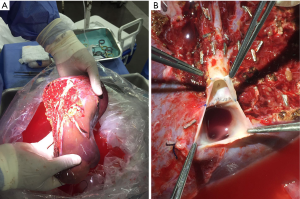
In July 2017, the authors performed rescue LDLT using a right lobe (Figure 3A) with middle hepatic vein (MHV) (Figure 3B) graft in a cirrhotic Child A patient who had undergone associated liver partition and portal vein ligation for staged hepatectomy (ALPPS) for a 13-cm HCC and developed liver failure 3 weeks later (Figure 4). The alpha-fetoprotein level was 8.5 mg/dL, and biopsy showed a G1 tumor. As of August 2018, the patient is doing well, with no signs of recurrence.
In summary, the surgical treatment of advanced HCC has a pivotal role to play in patient survival. Use of the BCLC staging system, with its highly restrictive rules, tends to limit treatment options for more advanced tumors. The HKLC staging system is more flexible and evaluates a broader range of parameters, but is still not very practical, and may exclude good potential candidates for LT or liver resection. Individualization of treatment approaches driven by the latest evidence, with usage of multidisciplinary teams (comprising hepatologists, HPB surgeons, oncologists, anesthetists) and locoregional liver donor offers, is the best strategy for patients with advanced HCC. Yttrium-90 radioembolization may also have an important place in downstaging advanced HCC before liver resection and transplantation.
Resection of large HCCs in the non-cirrhotic liver
A long-outdated concept, recently endorsed by current EASL guidelines, is that liver resection is the treatment of choice for HCC in non-cirrhotic patients. Survival in this group of patients after hepatic resection ranges from 35.9% to 86.6% (53-73). Many series about this subject are retrospective, and differences in survival outcomes between them have mostly been due to inclusion of non-comparable populations (different etiologies of liver disease, wide range of fibrosis severity, year of publication, etc.). The prevalence of hepatitis B (HBV) and C (HCV) virus varies between regions (with B-virus infection more common in Eastern countries and the C virus in Western ones), as are alcohol-related and non-alcoholic liver disease; this may play a role in outcomes after liver surgery. Some studies have shown that HBV carriers appear to have better survival than those with HCV (64,74), and that presence of underlying viral hepatitis is associated with worse overall survival (59,67) compared to non-carrier status. Other main prognostic factors associated with worse overall survival after hepatic resection in major series have been: advanced age (57,58,68), low albumin level (<3.5 g/dL) (53,64), large lesions (>5, >7, or >8 cm) (53,69,71,73), absence of capsule (66,71), presence of satellite lesions (53,66,71), vascular invasion (53,56,59,63,67,69,71,73), blood transfusion (57,64,66,68), high-grade tumors (60,68), positive resection margins (63,67,70), multiple tumors (64,68), and presence of fibrosis (73,75).
Studies addressing the association between HCC and absence of cirrhosis have demonstrated that such tumors are often larger than those arising in cirrhotic livers. The average lesion size in this group of patients ranges from 5 to 10.3 cm (53,54,56-58,60-62,65,66,70,71,73,74,76,77). Some explanations have been postulated. First, most cirrhotic patients are under active screening for HCC, while in non-cirrhotic patients, diagnosis is often made only after symptoms arise due to mass effect (abdominal pain, palpable mass, weight loss, etc.) or incidentally (53,76). Consequently, many of these patients are no longer eligible for LT at the time of diagnosis, leaving resection as the sole procedure with curative intent.
Second, as non-cirrhotic patients have better liver function, they can be offered major procedures with a greater assurance of safety. The consequence of the disparities in pathological characteristics and liver function between cirrhotic vs. non-cirrhotic patients is that some publications actually demonstrate comparable survival outcomes in both groups. However, when comparing groups using comparable tumor characteristics (for example, patients within the Milan criteria), survival outcomes are in favor of non-cirrhotic patients (54,55). This is corroborated by a systematic review and meta-analysis published by Zhou et al. (78). Multicentric de novo carcinogenesis due to cirrhosis seems to be the main cause of recurrence, and thus, poorer survival outcomes in the cirrhotic group (55).
Postoperative outcomes demonstrate that surgery in non-cirrhotic patients is usually safe, with a mortality rate of 0.7–7.9% (53,54,57,58,60,62-70,71,73,79); the major causes of death are post-hepatectomy liver failure (PHLF), sepsis, and cardiorespiratory problems (myocardial infarction, pulmonary embolism, etc.) (53,60,66,67,69,70,73). Conversely, the complication rate is often high, ranging from 22% to 43% (57,58,60,62,63,65-68,70,71,73). This may be explained by the high rate of major liver resections in non-cirrhotic patients, which exceeds 50% in some series (67,68,70,72). The main complications reported were PHLF, sepsis (pneumonia, abdominal collections), bile leakage, and pleural effusion (57,63,65-67,70,71).
Although outcomes in terms of overall survival are better in patients with preserved liver function, recurrence rates are still high, ranging from 35.9% to 59.5% (53,54,56-58,60,61,63,64,66,68-71,73,79). Most recurrences arise in the first 2 years after resection (54,73), but can occur up to 5 years later, which makes prolonged postoperative surveillance essential (69). Prognostic factors associated solely with recurrence in some studies were: multiple tumors (53,68,69), tumor size >5 cm (69), satellite nodules (69), HCV infection, and vascular invasion (63). Most recurrences are intrahepatic (53,58,60,63,66,68-71,79). Some authors have demonstrated that potentially curative treatments can be pursued in these patients, with around 20% to 40% being amenable to consecutive procedures (53,58,63,69,73), and that good outcomes can be achieved with such aggressive management. Shrager et al. (53) achieved a median survival of 50 months after resection/ablation of intrahepatic recurrences, and Chiche et al. (69), 104 months after surgical therapy of intra- and extrahepatic metastases. Extrahepatic recurrences are most commonly observed in the lungs, bones, adrenal glands, peritoneum, lymph nodes, and brain (53,58,60,67,69-71).
Whether the size of the lesion itself is a prognostic factor for survival has been a matter of debate. Tumor size seems to be a surrogate of more aggressive disease, as it represents a higher prevalence of vascular invasion (80-84). As noted by Vauthey et al. (82) and corroborated by others (83), when selecting only patients with single tumors without vascular invasion on anatomopathologic analysis, lesion size itself was not a prognostic factor. Lim et al. (83), in their series of more than 600 resections for single HCC, showed that, above 5 cm, there was no impact of lesion size on overall survival or recurrence-free survival; specifically, patients with lesions >10 cm had a 5-year OS of 53%. Similar results were found by Kluger et al. (84). In large HCCs, imaging patterns may have a role in defining prognosis. Yang et al. (85) demonstrated that patients with HCCs >5 cm with an intact capsule or pseudocapsule and no identifiable satellite nodules have the same long-term outcomes as patients with tumors ≤5 cm. Lu et al. also reported that, for large, solitary, HCCs with an identifiable capsule on magnetic resonance imaging, survival and response to ablation therapies were higher than in unencapsulated tumors (86).
Specifically regarding huge HCCs (those measuring >10 cm), 5-year survival ranges from 18.2% to 51.6% (87-96), and recurrence rates are as high as 76% (95) after resection. Postoperative 90-day mortality is extremely variable, ranging from 2.5% to 18.2% (88-90,92,94). As noted before, in huge HCCs the presence of cirrhosis is independently associated with worse survival outcomes (87,88,90,91). Ng et al. (88) identified no 5-year survivors in cirrhotic patients with huge HCCs; thus, this subgroup should be evaluated cautiously before resection. On the other hand, 40% of non-cirrhotic patients achieved long-term survival. Other independent prognostic factors associated with impaired outcomes in this group are vascular invasion (87,91,94,95), multiple tumors/satellite nodules (87,91,94,95), and poor differentiation (87,88). Despite worse long-term outcomes overall, the group of patients with huge HCCs is very heterogeneous. Lim et al. (89) demonstrated that the BCLC correlates well with long-term results in these patients, as BCLC A patients (those with solitary tumors) demonstrated much better OS than BCLC C patients (those with PVTT): 81.7 vs. 4.8 months, respectively.
As previously mentioned, major procedures are often needed in non-cirrhotic patients due to their large lesions. However, surgeons often face the problem of insufficient FLRV% at preoperative evaluation. There is a consensus that, to avoid PHLF, the most important complication after liver surgery, FLRV% should be at least 20% in healthy livers (97). In those with parenchymal damage, things are less clear. The minimum acceptable FLRV% ranges from 30% to 35% in early cirrhosis and mild steatosis to at least 50% in cirrhotic patients without functional impairment (measured in some studies by ICG retention at 15 min) or portal hypertension (98). Efforts should be made to ensure that these patients, deemed unresectable due to insufficient FLRV%, can still be considered for major liver procedures. The prognosis in non-cirrhotic patients receiving palliative care due to advanced disease not amenable to potentially curative procedures is dismal (median survival, 7 to 22 months) (56,61,76,99).
Several strategies to improve FLRV% and make liver resection safer have been proposed in the literature. Portal vein embolization (PVE) was one of the first and most widely used strategies for this purpose. Farges et al., in the first prospective trial addressing this issue, demonstrated that performing PVE before right hepatectomy for HCC with chronic liver disease was associated with fewer complications and shorter hospital stays (100). A systematic review by Glantzounis et al. (101) showed a median excision rate of 90% after PVE in included studies, and another review by Tustumi et al. (102) demonstrated that mean FLR hypertrophy was 31%. One important point about this strategy is that failure to achieve adequate post-PVE hypertrophy predicts a high risk of PHLF and death, as it indicates inability of the liver parenchyma to regenerate, therefore contraindicating major resection (101). Ribeiro et al. (103) showed that <5% hypertrophy after PVE is associated with a high risk of liver-related complications and 90-day mortality.
Transarterial chemoembolization (TACE) has been proposed as a strategy to improve FLRV% and control possible HCC growth in those undergoing PVE. As is widely known, HCC lesions have their blood supply maintained almost exclusively by arteries, and obliteration of the ipsilateral portal vein could increase arterial flow and lead to tumor growth (104). Again, a systematic review and meta-analysis by Tustumi et al. (50) demonstrated superiority of TACE + PVE over PVE/PVL by allowing a higher resection rate (14% higher) and increasing overall survival after HCC resection.
Another option that has been proposed in recent cohorts to overcome this problem is the ALPPS procedure. Publications about this new strategy are scarce, cohorts have been small—as of the time of writing, the largest series had 45 patients, reported by Wang et al. (105)—and results have been conflicting as to postoperative outcomes. Mortality ranges from 11.1% to 50% (105-110), while the rate of PHLF after the second stage ranges from 25% to 58.5% (105-107,109). Some centers (105,107,109) demonstrated that FLR hypertrophy correlated negatively with severity of fibrosis; lower rates were found in patients with cirrhosis (105,109). Indeed, some series show that, compared to the results achieved in patients with liver metastases from colorectal cancer, ALPPS for HCC provides worse outcomes (109). Although it seems reasonable to propose this approach in patients not amenable to other strategies, due to the increase in FLR achievable in a short time even in diseased livers (111,112), long-term outcome data are lacking, and this strategy should be approached with caution. Encouraging results have been reported by Wang et al. (105), with 3-year OS and DFS rates of 60.2% and 43.9% respectively, despite still-high postoperative mortality (11.1% in the overall cohort) and PHLF rates (58.5% after the second stage). Also, a propensity score-matched analysis was conducted to compare the results with those achieved after TACE and single-stage hepatectomy. Overall survival was much better compared to TACE (7.1% at 3 years), and comparable to that of a one-stage procedure.
Last but not least, another surgical approach for non-cirrhotic patients that is rarely investigated in the literature due to controversial outcomes is LT. Publications in the 1990s reported very poor transplantation outcomes in non-cirrhotic patients, with 5-year OS ranging from 11% to 27.1% (113-115). One important finding in these publications is that more than half of patients were considered to have advanced tumors (multiple bilobar lesions or major vascular invasion), which may have biased outcomes unfavorably. Much later, in 2012, Mergental et al. (116) showed better results after LT in patients with non-resectable HCC and no underlying liver disease; the 5-year survival rate was 43%, although data on recurrence was not clear. A subgroup of patients undergoing salvage LT for recurrence after resection achieved comparable results (58% 5-year OS). Most importantly, selected patients without macrovascular invasion or hilar lymph node metastasis achieved a 5-year survival rate of 59%; in those selected for salvage LT who had recurrence at least 12 months after resection, 5-year survival was 71%, making this the ideal setting in which to propose transplantation.
Systemic therapy
As HCC usually occurs in the cirrhotic liver, it combines two serious clinical conditions in the same patient: a malignant tumor and significant hepatic impairment. Thus, especially in cases of advanced disease, antitumor treatment must be not only effective but also safe, as reduced liver reserve can be a determinant of its failure.
Sorafenib, a multikinase inhibitor with antiproliferative and antiangiogenic activity, was the first drug approved by the Food and Drug Administration (FDA) for patients diagnosed with advanced (BCLC C) HCC. The SHARP trial (117), conducted in the West, included 602 patients randomized to receive sorafenib or placebo and reported longer median survival with sorafenib (10.7 vs. 7.9 months; P<0.001). This increase in survival was confirmed in an East Asian study (118) of 226 patients, which reported a median survival of 6.5 months with sorafenib vs. 4.2 months with placebo (P=0.014). Together, these two studies revolutionized treatment strategies for HCC. Patients with compensated cirrhosis and metastatic tumor and/or PVTT, for whom no therapeutic options were previously available, have since become candidates for sorafenib therapy.
Over time, clinical experience with sorafenib has consolidated and the medical community has learned to control its adverse effects and expand the range of patients eligible for its use. This experience was best translated into the GIDEON real-life study (119), which found sorafenib to be safe for use in patients with advanced (Child-Pugh B) HCC. In clinical practice, this drug has been used in BCLC B and/or Child-Pugh B patients for years. A recent Brazilian study (120) evaluated the real-life use of sorafenib in real life and reported excellent outcomes. In a general sample of 572 patients with HCC, the authors found that, among patients with indications for sorafenib who received the drug, 1-year survival was significantly greater than in those who did not receive it (88.7% vs. 44.4%, P<0.001). There was no difference in survival between Child-Pugh A vs. B or between BCLC C vs. B patients.
On the other hand, as sorafenib therapy requires moderately preserved hepatic function and may be limited by adverse effects, several attempts have been made in recent years to develop a new option for the first-line treatment of advanced HCC. Sunitinib, brivanib, linifanib, and erlotinib, among other targeted agents, were unsuccessful (121). The first drug to be effective in this setting was lenvatinib. Recently, a randomized noninferiority trial comparing lenvatinib to sorafenib in a sample of 954 Child-Pugh A patients with advanced HCC was published (122). Treatment was continued until disease progression, toxicity, or withdrawal of consent. Median OS was 13.6 months with lenvatinib and 12.3 months with sorafenib, reaching the established noninferiority margin.
Options for second-line treatment after sorafenib, both for intolerant patients and for patients with tumor progression, were also nonexistent until recently. The first agent approved by the FDA for salvage use was regorafenib. The RESORCE study (123) randomized 573 Child-Pugh A patients who tolerated but progressed on sorafenib to receive oral regorafenib or placebo. Median survival was 10.6 months in the regorafenib group vs. 7.8 months in the placebo group (P<0.001). It was recently estimated that 21.6% of patients who fail sorafenib therapy may be good candidates for regorafenib salvage (120).
Some other second-line options for advanced HCC in Child-Pugh A patients have also been evaluated with positive results: nivolumab, cabozantinib, and ramucirumab. On the basis of a phase-II study, nivolumab, an intravenous checkpoint inhibitor, has received FDA approval for use in sorafenib-tolerant or non-tolerant Child-Pugh A patients with advanced HCC. This approval is provisional, however, pending the results of phase-III trials. The results of the CELESTIAL study, a phase-III clinical trial of the cMET inhibitor cabozantinib, were recently presented. The authors evaluated 760 patients (124), randomized to receive either cabozantinib or placebo. Median survival was approximately 10.2 vs. 8 months (P=0.0049). The REACH phase-III trial (125) evaluated ramucirumab as an option for salvage therapy after sorafenib failure. Efficacy was demonstrated in patients with an alpha-fetoprotein level ≥400 ng/mL, which may represent the first-ever successful personalized treatment for patients with advanced HCC. To date, there are no options for second-line treatment in patients who have failed lenvatinib.
In short, systemic therapy can provide good outcomes, which justifies its indication in the treatment of advanced HCC. Furthermore, recent studies have expanded the armamentarium beyond sorafenib. In fact, the latest version of the BCLC classification (8) replaced its recommendation of “sorafenib” with the broader term “systemic therapy”, further consolidating evidence that survival in this group of patients can now exceed 1 year. It is expected that better-designed studies, with patient stratification based on individual characteristics and combinations of agents, may make systemic therapy even more successful in future (126).
Locoregional therapies in advanced HCC
HCC corresponds to more than 90% of primary liver cancers. As noted above, the BCLC classification is widely accepted for tumor characterization and definition of therapeutic approaches (127). Nevertheless, there is significant heterogeneity among HCC patients, especially at the intermediate and advanced stages. Optimal management of HCC requires a multidisciplinary approach that combines expertise in liver surgery, hepatology, interventional radiology, and medical oncology.
Locoregional therapies (LRT)—transarterial, percutaneous, or both—are currently the first-line treatment of choice for intermediate (BCLC B) tumors, producing survival benefits and favorable response rates without significant complications (117,127,128). LRT is considered the standard of care in BCLC B patients who have preserved liver function and large or multinodular disease without portal vein thrombosis or extrahepatic metastasis.
On the other hand, advanced disease (BCLC C) is generally considered a contraindication to transarterial approaches. Currently, sorafenib and other targeted agents are the standard treatment for advanced HCC, especially in cases with microvascular invasion (MVI) or extrahepatic disease, or even in refractory disease after LRT; it is recommended by the National Comprehensive Cancer Network (NCCN) guidelines for HCC (version 2.2016), depending on the patient’s overall functional status and liver function (117,127,128).
On the other hand, the recently updated AASLD guidelines stress that treatment selection may vary depending on the extent of MVI, although no recommendation can be made for systemic therapy over LRT or any one type of LRT over other modalities (128).
Two sorafenib registration trials have demonstrated improved survival with active intervention compared to best supportive care in advanced HCC, including patients with or without MVI (118,127,128).
However, poor outcomes have been reported with systemic therapies for BCLC C patients.
Therefore, LRT techniques have been increasingly studied and refined in this patient population, with encouraging results; highlights will be reviewed below.
Technical considerations
LRT encompasses at least six distinct modalities:
- Conventional transarterial chemoembolization (TACE). Conventional transarterial chemoembolization (TACE) involves the catheter-based delivery of chemotherapeutic agents to tumor-supplying arteries, combined with embolization to reduce arterial inflow, thus prolonging the chemotherapeutic effect. Ethiodol is usually used as an emulsifying agent due to its preferential ability to reach tumor cells, delivering chemotherapeutic agents and inducing ischemia through vascular occlusion. Other embolic materials are also used in TACE, including Gelfoam, microspheres (Embozene® and Embospheres®), and polyvinyl alcohol (PVA) particles (118,129). Gelfoam is indicated for temporary vascular occlusion where recanalization is desired after a short duration. Microspheres and PVA particles are indicated for more permanent vascular occlusion (129).
- Bland embolization (TAE). Bland embolization, known as TAE, relies solely on induction of ischemia within the tumor. However, the ischemia caused by TAE without tumoricidal agents may theoretically trigger peritumoral angiogenesis and paradoxical tumor growth with metastatic spread.
- Drug-eluting beads transarterial chemoembolization (DEB-TACE) was developed to increase levels of chemotherapy concentrated within the tumor. Embolic particles which interact ionically with doxorubicin can gradually release the drug over time when administered (drug-eluting beads). The kinetics of drug elution from the beads after delivery vary depending on the osmolarity of the tumor bed, drug concentration, and bead size (127,129).
- Radioembolization (RAE) is a catheter-based approach for delivery of beads radiolabeled with yttrium-90 (90Y) directly into the tumor bed. However, there are no studies demonstrating a significant impact on survival. Also, there is no consensus as to the optimal use of this therapy, particularly when and if it should be chosen over TACE for treatment of unresectable HCC. RAE may be preferred over TACE is in the setting of an HCC complicated by malignant main or lobar-branch PVTT. Theoretically, RAE induces less arterial ischemia than TACE because of its smaller particle size (32 versus 70–300 microns), which suggests it should be safer in the setting of portal vein thrombosis. Compared to TACE, rates of severe adverse effects with RAE appear to be low (130).
- Radiofrequency ablation (RFA). This technique induces coagulation necrosis by tumor-directed puncture with an 18-gauge needle. It is more effective in lesions <5 cm, but also can be used in combination with other techniques for larger lesions.
- Hepatic arterial infusion chemotherapy (HAIC). HAIC involves repeated arterial infusions of chemotherapeutic agents through a port attached to tumor-feeding arteries. Currently, two regimens are available: intra-arterial low-dose cisplatin combined with 5-fluorouracil with or without subcutaneous interferon, usually recombinant interferon alfa-2b (131,132).
As systemic therapies have long shown little survival benefit and considerable side effects in advanced HCC, many authors have studied LRT in this setting.
Kirstein et al. showed that TACE is noninferior to sorafenib in patients with advanced disease. They compared 98 patients receiving sorafenib to 74 undergoing TACE, and found similar median overall survival (132).
A retrospective, observational study compared TACE alone (n=295), TACE with radiation (n=196), and sorafenib alone (n=66) in advanced HCC with portal vein thrombosis. The TACE-alone group had a longer median time to progression (TTP) (3.4 vs. 1.8 months; P<0.001) and OS (5.9 vs. 4.4 months; P=0.003) (133).
Choi et al. compared TTP and OS in patients with advanced HCC who received sorafenib plus TACE vs. sorafenib monotherapy. Conventional ethiodol-based TACE plus sorafenib was performed in 164 patients; 191 received sorafenib alone. In the combined and monotherapy groups, respectively, 64.6% and 49.2% of patients had MVI, 87.8% and 91.1% had extrahepatic metastasis, and 54.3% and 47.1% had both. The median TTP and OS in the combined group were longer than in the monotherapy group. At univariate and subsequent multivariate analyses, additional TACE was an independent predictor of better TTP and OS (134).
In a meta-analysis of five comparative studies including 899 patients, Wang et al. showed that TACE plus sorafenib can improve TTP, but does not appear to prolong OS (135).
In another meta-analysis, Cai et al. assessed OS, objective response, disease control rate, and adverse reactions in 14 studies including 1,670 patients with advanced HCC. Compared with the TACE-alone treatment group, better prognosis and fewer adverse reactions were found with the combination of sorafenib plus TACE (136).
TACE plus sorafenib
Two studies provide further evidence for the combination of TACE plus sorafenib in patients with advanced HCC and PVTT. Zhang et al. retrospectively analyzed 45 patients treated with combination therapy and 45 treated with sorafenib alone. Median OS was equivalent (7.0 and 6.0 months, respectively; P=0.544) (137).
Ha et al., in a retrospective study including 658 patients with advanced HCC, showed that, among 257 patients with portal vein invasion, survival was significantly longer with combination therapy (TACE plus sorafenib; 25.7 months) or TACE followed by sorafenib (14.0 months) than with sorafenib monotherapy (5.5 months) (138).
RAE
Safety and efficacy of RAE in patients with HCC, with or without MVI, has been reported in some studies. Research restricted to patients with HCC and PVTT reported direct comparisons of RAE vs. sorafenib (128,139).
Cho et al. showed similar OS results in 32 patients with PVTT without extrahepatic spread. They were treated with RAE and compared to 31 consecutively enrolled patients, also with PVTT without extrahepatic spread, who received sorafenib. However, the sorafenib group showed significantly more grade 3–4 adverse effects than the RAE group (140).
De la Torre et al. compared 26 patients with PVTT treated with RAE and 47 treated with sorafenib, with comparable baseline characteristics, also with similar OS results. Median survival was 8.8 months in the RAE group and 5.4 months in the sorafenib group (139).
TACE plus RFA
Peng et al. studied the synergistic cytotoxic effects of TACE combined with RFA. In a retrospective multicenter study, they found better overall survival rates, response rates, and TTP with combination therapy (TACE plus RFA and sorafenib) than with sorafenib alone in advanced HCC. The rationale for concurrent use of TACE plus RFA and sorafenib is based on inhibiting hypoxia-induced angiogenesis after TACE or RFA. However, patients with tumors >7 cm or more than five lesions were excluded from this study (141).
RFA plus sorafenib
A randomized controlled trial compared HCC and PVTT patients treated with sorafenib plus percutaneous RFA of both intraparenchymal HCC and PVTT versus sorafenib alone. Giorgio et al. analyzed 99 patients with Child A cirrhosis (49 in the combination group and 50 in the sorafenib monotherapy group). Survival rates at 1, 2, and 3 years were 60%, 35%, and 26%, respectively, in the combination group and 37% and 0% at 1 and 2 years, respectively, in the sorafenib monotherapy group. At multivariate analysis, combination treatment was the only factor predicting survival (142).
HAIC plus sorafenib
Another randomized phase-II trial comparing sorafenib alone versus sorafenib plus LRT therapies was published by Ikeda et al. One hundred and eight patients with advanced HCC with or without MVI were randomized to receive sorafenib (n=42) or sorafenib plus HAIC with cisplatin (n=66). Median survival was 8.7 months in the sorafenib monotherapy group vs. 10.6 months in the combination group (P=0.031). In a subgroup analysis of patients with PVTT, combination treatment did not prolong OS (9.1 months) compared to sorafenib alone (7.1 months) (131).
Multimodal treatment including radiotherapy
In a retrospective study with propensity-score analysis comparing TACE plus radiotherapy (n=27) versus sorafenib (n=27) in patients with HCC and MVI, OS in the TACE-plus-radiotherapy group was significantly prolonged compared to OS with sorafenib alone (143).
Another retrospective, observational, single-center study compared TACE alone (n=295), TACE plus radiation (n=196), and sorafenib alone (n=66) in patients with PVTT. Median TTP was longer in the TACE-plus-radiation group (5.1 vs. 1.6 months; P<0.001), as was overall survival (8.2 vs. 3.2 months; P<0.001) (140).
TACE plus radiotherapy
Yoon et al. randomized 90 treatment-naive patients with liver-confined HCC and evidence of macroscopic vascular invasion to receive sorafenib (400 mg twice daily) or TACE (every 6 weeks) plus radiotherapy (within 3 weeks after the first TACE; maximum 45 Gy, fraction size 2.5–3 Gy). TACE plus radiotherapy was well tolerated and improved progression-free survival, ORR, TTP, and OS compared with sorafenib alone (143).
Critical considerations
Although LRT (alone or combined with RFA) is the standard protocol for patients with intermediate HCC, the heterogeneity of patients with this condition and the lack of standardization among TACE protocols means decision-making is highly complex. Refinements in technique now allow treatment of patients with advanced HCC, which was formerly considered an absolute contraindication to LRT. Several studies have demonstrated the safety of TACE in PVTT.
However, LRT protocols in these studies have varied widely, even regarding inclusion of antiangiogenic therapies. As mentioned above, Peng et al. showed survival benefits involving TACE plus RFA and sorafenib compared with sorafenib alone in advanced HCC. In their protocol, TACE was performed with epirubicin, Lipiodol®, and absorbable gelatin sponge particles (141). Calibrated microspheres or even DEB-TACE might be used instead, perhaps to better effect.
Kirstein et al. reported similar outcomes with sorafenib vs. LRT in HCC with extrahepatic disease, but TACE modalities differed in the cohort; most patients were treated with TACE (n=49; 73.1%), followed by DEB-TACE (n=16; 23.9%) (132).
Wang et al. did not report which type of TACE procedure was performed in the studies included in their meta-analysis (136), nor did Wang et al. in theirs (135).
Feng et al. evaluated just how different chemoembolization protocols can be. In a systematic review, they found reports of 5-fluorouracil, Adriamycin, platinum, mitomycin C, hydroxycamptothecin, and combinations thereof in various studies of TACE (144).
Another systematic review and meta-analysis of 14 studies (3 RCTs and 11 observational studies) was performed by Finn et al. Only three of the included studies adequately characterized TACE techniques, each involving a different protocol (cisplatin infusion, epirubicin and Lipiodol®, and TAE with 150–500 micron PVA particles, respectively) (145).
There is a clear need for additional studies designed to provide higher levels of evidence and, mainly, greater standardization of the chemotherapeutic and embolic agents used before LRT can be said to have a definitive positive impact on survival rates in HCC.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683-91. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016;122:1312-37. [Crossref] [PubMed]
- Maida M, Orlando E, Camma C, et al. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol 2014;20:4141-50. [Crossref] [PubMed]
- Okuda K, Obata H, Nakajima Y, et al. Prognosis of primary hepatocellular carcinoma. Hepatology 1984;4:3S-6S. [Crossref] [PubMed]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587-96. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Zhong JH, Ke Y, Wang YY, et al. Liver resection for patients with hepatocellular carcinoma and macrovascular invasion, multiple tumours, or portal hypertension. Gut 2015;64:520-1. [Crossref] [PubMed]
- Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-700.e3. [Crossref] [PubMed]
- Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery 2005;137:403-10. [Crossref] [PubMed]
- Ikai I, Yamamoto Y, Yamamoto N, et al. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am 2003;12:65-75. ix. [Crossref] [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 1999;229:210-5. [Crossref] [PubMed]
- Teh SH, Christein J, Donohue J, et al. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg 2005;9:1207-15; discussion 15. [Crossref] [PubMed]
- Fan ST, Lai EC, Lo CM, et al. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg 1995;130:198-203. [Crossref] [PubMed]
- Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011;29:339-64. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Conci S, et al. How much remnant is enough in liver resection? Dig Surg 2012;29:6-17. [Crossref] [PubMed]
- Cucchetti A, Cescon M, Ercolani G, et al. A comprehensive meta-regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol 2012;19:3697-705. [Crossref] [PubMed]
- Zhou Y, Xu D, Wu L, et al. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbecks Arch Surg 2011;396:1109-17. [Crossref] [PubMed]
- Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? an observational study of the HCC East-West study group. Ann Surg 2013;257:929-37. [Crossref] [PubMed]
- Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329-40. [Crossref] [PubMed]
- Bhandare MS, Patkar S, Shetty N, et al. Liver resection for HCC outside the BCLC criteria. Langenbecks Arch Surg 2018;403:37-44. [Crossref] [PubMed]
- Vauthey JN, Dixon E, Abdalla EK, et al. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010;12:289-99. [Crossref] [PubMed]
- Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62-7. [Crossref] [PubMed]
- Li SH, Wei W, Guo RP, et al. Long-term outcomes after curative resection for patients with macroscopically solitary hepatocellular carcinoma without macrovascular invasion and an analysis of prognostic factors. Med Oncol 2013;30:696. [Crossref] [PubMed]
- Hao S, Fan P, Chen S, et al. Distinct recurrence risk factors for intrahepatic metastasis and multicenter occurrence after surgery in patients with hepatocellular carcinoma. J Gastrointest Surg 2017;21:312-20. [Crossref] [PubMed]
- Li DH, Sun B. Research progress in intrahepatic metastasis and multiple centre carcinogenesis of hepatocellular carcinoma. Int J Surg 2006;33:28-31.
- Cheng S, Chen M, Cai J, et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. Oncotarget 2017;8:8867-76. [PubMed]
- Shuqun C, Mengchao W, Han C, et al. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology 2007;54:499-502. [PubMed]
- Shi J, Lai EC, Li N, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci 2011;18:74-80. [Crossref] [PubMed]
- Kudo M, Matsui O, Izumi N, et al. JSH Consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer 2014;3:458-68. [Crossref] [PubMed]
- Ho MC, Hasegawa K, Chen XP, et al. Surgery for intermediate and advanced hepatocellular carcinoma: a consensus report from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014). Liver Cancer 2016;5:245-56. [Crossref] [PubMed]
- Iwatsuki S, Gordon RD, Shaw BW Jr, et al. Role of liver transplantation in cancer therapy. Ann Surg 1985;202:401-7. [Crossref] [PubMed]
- Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993;218:145-51. [Crossref] [PubMed]
- Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl 2011;17 Suppl 2:S44-57. [Crossref] [PubMed]
- Guerrero-Misas M, Rodriguez-Peralvarez M, De la Mata M. Strategies to improve outcome of patients with hepatocellular carcinoma receiving a liver transplantation. World J Hepatol 2015;7:649-61. [Crossref] [PubMed]
- Silva M, Moya A, Berenguer M, et al. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl 2008;14:1449-60. [Crossref] [PubMed]
- Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-45. [Crossref] [PubMed]
- Toso C, Trotter J, Wei A, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2008;14:1107-15. [Crossref] [PubMed]
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32. [Crossref] [PubMed]
- DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-72. [Crossref] [PubMed]
- Shirabe K, Taketomi A, Morita K, et al. Comparative evaluation of expanded criteria for patients with hepatocellular carcinoma beyond the Milan criteria undergoing living-related donor liver transplantation. Clin Transplant 2011;25:E491-8. [Crossref] [PubMed]
- Kaido T, Ogawa K, Mori A, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013;154:1053-60. [Crossref] [PubMed]
- Akamatsu N, Sugawara Y, Kokudo N. Living donor liver transplantation for patients with hepatocellular carcinoma. Liver Cancer 2014;3:108-18. [Crossref] [PubMed]
- Kim JM, Kwon CH, Joh JW, et al. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma. Transplant Proc 2014;46:726-9. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Patel SS, Arrington AK, McKenzie S, et al. Milan Criteria and UCSF Criteria: a preliminary comparative study of liver transplantation outcomes in the United States. Int J Hepatol 2012;2012:253517. [Crossref] [PubMed]
- Zou WL, Zang YJ, Chen XG, et al. Risk factors for fatal recurrence of hepatocellular carcinoma and their role in selecting candidates for liver transplantation. Hepatobiliary Pancreat Dis Int 2008;7:145-51. [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Dendy MS, Camacho JC, Ludwig JM, et al. Infiltrative hepatocellular carcinoma with portal vein tumor thrombosis treated with a single high-dose y90 radioembolization and subsequent liver transplantation without a recurrence. Transplant Direct 2017;3:e206. [Crossref] [PubMed]
- Levi Sandri GB, Ettorre GM, Colasanti M, et al. Hepatocellular carcinoma with macrovascular invasion treated with yttrium-90 radioembolization prior to transplantation. Hepatobiliary Surg Nutr 2017;6:44-8. [Crossref] [PubMed]
- Shrager B, Jibara G, Schwartz M, et al. Resection of hepatocellular carcinoma without cirrhosis. Ann Surg 2012;255:1135-43. [Crossref] [PubMed]
- Chang CH, Chau GY, Lui WY, et al. Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg 2004;139:320-5; discussion 26. [Crossref] [PubMed]
- Taura K, Ikai I, Hatano E, et al. Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery 2007;142:685-94. [Crossref] [PubMed]
- Wörns MA, Bosslet T, Victor A, et al. Prognostic factors and outcomes of patients with hepatocellular carcinoma in non-cirrhotic liver. Scand J Gastroenterol 2012;47:718-28. [Crossref] [PubMed]
- Young AL, Adair R, Prasad KR, et al. Hepatocellular carcinoma within a noncirrhotic, nonfibrotic, seronegative liver: surgical approaches and outcomes. J Am Coll Surg 2012;214:174-83. [Crossref] [PubMed]
- Faber W, Sharafi S, Stockmann M, et al. Long-term results of liver resection for hepatocellular carcinoma in noncirrhotic liver. Surgery 2013;153:510-7. [Crossref] [PubMed]
- Zhou YM, Zhang XF, Li B, et al. Prognosis after resection of hepatitis B virus-related hepatocellular carcinoma originating from non-cirrhotic liver. Ann Surg Oncol 2014;21:2406-12. [Crossref] [PubMed]
- Yip VS, Gomez D, Tan CY, et al. Tumour size and differentiation predict survival after liver resection for hepatocellular carcinoma arising from non-cirrhotic and non-fibrotic liver: a case-controlled study. Int J Surg 2013;11:1078-82. [Crossref] [PubMed]
- Verhoef C, de Man RA, Zondervan PE, et al. Good outcomes after resection of large hepatocellular carcinoma in the non-cirrhotic liver. Dig Surg 2004;21:380-6. [Crossref] [PubMed]
- Shimada M, Rikimaru T, Sugimachi K, et al. The importance of hepatic resection for hepatocellular carcinoma originating from nonfibrotic liver. J Am Coll Surg 2000;191:531-7. [Crossref] [PubMed]
- Nagasue N, Ono T, Yamanoi A, et al. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg 2001;88:515-22. [Crossref] [PubMed]
- Chen MF, Tsai HP, Jeng LB, et al. Prognostic factors after resection for hepatocellular carcinoma in noncirrhotic livers: univariate and multivariate analysis. World J Surg 2003;27:443-7. [Crossref] [PubMed]
- Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999;229:790-9; discussion 99-800. [Crossref] [PubMed]
- Laurent C, Blanc JF, Nobili S, et al. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg 2005;201:656-62. [Crossref] [PubMed]
- Bège T, Le Treut YP, Hardwigsen J, et al. Prognostic factors after resection for hepatocellular carcinoma in nonfibrotic or moderately fibrotic liver. A 116-case European series. J Gastrointest Surg 2007;11:619-25. [Crossref] [PubMed]
- Smoot RL, Nagorney DM, Chandan VS, et al. Resection of hepatocellular carcinoma in patients without cirrhosis. Br J Surg 2011;98:697-703. [Crossref] [PubMed]
- Chiche L, Menahem B, Bazille C, et al. Recurrence of hepatocellular carcinoma in noncirrhotic liver after hepatectomy. World J Surg 2013;37:2410-8. [Crossref] [PubMed]
- Thelen A, Benckert C, Tautenhahn HM, et al. Liver resection for hepatocellular carcinoma in patients without cirrhosis. Br J Surg 2013;100:130-7. [Crossref] [PubMed]
- Arnaoutakis DJ, Mavros MN, Shen F, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol 2014;21:147-54. [Crossref] [PubMed]
- Lee HW, Choi GH, Kim DY, et al. Less fibrotic burden differently affects the long-term outcomes of hepatocellular carcinoma after curative Resection. Oncology 2017;93:224-32. [Crossref] [PubMed]
- Truant S, Boleslawski E, Duhamel A, et al. Tumor size of hepatocellular carcinoma in noncirrhotic liver: a controversial predictive factor for outcome after resection. Eur J Surg Oncol 2012;38:1189-96. [Crossref] [PubMed]
- van Meer S, van Erpecum KJ, Sprengers D, et al. Hepatocellular carcinoma in cirrhotic versus noncirrhotic livers: results from a large cohort in the Netherlands. Eur J Gastroenterol Hepatol 2016;28:352-9. [Crossref] [PubMed]
- Bilimoria MM, Lauwers GY, Doherty DA, et al. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Arch Surg 2001;136:528-35. [Crossref] [PubMed]
- Giannini EG, Marenco S, Bruzzone L, et al. Hepatocellular carcinoma in patients without cirrhosis in Italy. Dig Liver Dis 2013;45:164-9. [Crossref] [PubMed]
- Schütte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol 2014;14:117. [Crossref] [PubMed]
- Zhou Y, Lei X, Wu L, et al. Outcomes of hepatectomy for noncirrhotic hepatocellular carcinoma: a systematic review. Surg Oncol 2014;23:236-42. [Crossref] [PubMed]
- Kim JM, Kwon CH, Joh JW, et al. Differences between hepatocellular carcinoma and hepatitis B virus infection in patients with and without cirrhosis. Ann Surg Oncol 2014;21:458-65. [Crossref] [PubMed]
- Tsai TJ, Chau GY, Lui WY, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery 2000;127:603-8. [Crossref] [PubMed]
- el-Assal ON, Yamanoi A, Soda Y, et al. Proposal of invasiveness score to predict recurrence and survival after curative hepatic resection for hepatocellular carcinoma. Surgery 1997;122:571-7. [Crossref] [PubMed]
- Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol 2002;20:1527-36. [Crossref] [PubMed]
- Lim C, Mise Y, Sakamoto Y, et al. Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World J Surg 2014;38:2910-8. [Crossref] [PubMed]
- Kluger MD, Salceda JA, Laurent A, et al. Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol 2015;62:1131-40. [Crossref] [PubMed]
- Yang LY, Fang F, Ou DP, et al. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg 2009;249:118-23. [Crossref] [PubMed]
- Lu DS, Siripongsakun S, Kyong Lee J, et al. Complete tumor encapsulation on magnetic resonance imaging: a potentially useful imaging biomarker for better survival in solitary large hepatocellular carcinoma. Liver Transpl 2013;19:283-91. [Crossref] [PubMed]
- Chang YJ, Chung KP, Chang YJ, et al. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg 2016;103:1513-20. [Crossref] [PubMed]
- Ng KM, Yan TD, Black D, et al. Prognostic determinants for survival after resection/ablation of a large hepatocellular carcinoma. HPB (Oxford) 2009;11:311-20. [Crossref] [PubMed]
- Lim C, Compagnon P, Sebagh M, et al. Hepatectomy for hepatocellular carcinoma larger than 10 cm: preoperative risk stratification to prevent futile surgery. HPB (Oxford) 2015;17:611-23. [Crossref] [PubMed]
- Yang J, Li C, Wen TF, et al. Is hepatectomy for huge hepatocellular carcinoma (>/= 10 cm in diameter) safe and effective? A single-center experience. Asian Pac J Cancer Prev 2014;15:7069-77. [Crossref] [PubMed]
- Pawlik TM, Poon RT, Abdalla EK, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg 2005;140:450-7; discussion 457-8. [Crossref] [PubMed]
- Zhu SL, Zhong JH, Ke Y, et al. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J Gastroenterol 2015;21:9630-7. [Crossref] [PubMed]
- Choi GH, Han DH, Kim DH, et al. Outcome after curative resection for a huge (>or=10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg 2009;198:693-701. [Crossref] [PubMed]
- Hwang S, Lee YJ, Kim KH, et al. Long-term outcome after resection of huge hepatocellular carcinoma >/= 10 cm: single-institution experience with 471 patients. World J Surg 2015;39:2519-28. [Crossref] [PubMed]
- Shrager B, Jibara GA, Tabrizian P, et al. Resection of large hepatocellular carcinoma (>/=10 cm): a unique western perspective. J Surg Oncol 2013;107:111-7. [Crossref] [PubMed]
- Chan YC, Kabiling CS, Pillai VG, et al. Survival outcome between hepatic resection and transarterial embolization for hepatocellular carcinoma more than 10 cm: a propensity score model. World J Surg 2015;39:1510-8. [Crossref] [PubMed]
- Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1271-80. [Crossref] [PubMed]
- Khan AS, Garcia-Aroz S, Ansari MA, et al. Assessment and optimization of liver volume before major hepatic resection: Current guidelines and a narrative review. Int J Surg 2018;52:74-81. [Crossref] [PubMed]
- Edeline J, Raoul JL, Vauleon E, et al. Systemic chemotherapy for hepatocellular carcinoma in non-cirrhotic liver: a retrospective study. World J Gastroenterol 2009;15:713-6. [Crossref] [PubMed]
- Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 2003;237:208-17. [Crossref] [PubMed]
- Glantzounis GK, Tokidis E, Basourakos SP, et al. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur J Surg Oncol 2017;43:32-41. [Crossref] [PubMed]
- Tustumi F, Ernani L, Coelho FF, et al. Preoperative strategies to improve resectability for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford) 2018;20:1109-18. [Crossref] [PubMed]
- Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386-94. [Crossref] [PubMed]
- Hayashi S, Baba Y, Ueno K, et al. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol 2007;48:721-7. [Crossref] [PubMed]
- Wang Z, Peng Y, Hu J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis b virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Vennarecci G, Grazi GL, Sperduti I, et al. ALPPS for primary and secondary liver tumors. Int J Surg 2016;30:38-44. [Crossref] [PubMed]
- Chia DKA, Yeo Z, Loh SEK, et al. ALPPS for hepatocellular carcinoma is associated with decreased liver remnant growth. J Gastrointest Surg 2018;22:973-80. [Crossref] [PubMed]
- Cai X, Tong Y, Yu H, et al. The ALPPS in the treatment of hepatitis b-related hepatocellular carcinoma with cirrhosis: a single-center study and literature review. Surg Innov 2017;24:358-64. [Crossref] [PubMed]
- D’Haese JG, Neumann J, Weniger M, et al. Should ALPPS be used for liver resection in intermediate-stage HCC? Ann Surg Oncol 2016;23:1335-43. [Crossref] [PubMed]
- Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 2014;260:829-36; discussion 36-8. [Crossref] [PubMed]
- Chan AC, Poon RT, Chan C, et al. Safety of ALPPS Procedure by the Anterior Approach for Hepatocellular Carcinoma. Ann Surg 2016;263:e14-6. [Crossref] [PubMed]
- Chan ACY, Chok K, Dai JWC, et al. Impact of split completeness on future liver remnant hypertrophy in associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in hepatocellular carcinoma: Complete-ALPPS versus partial-ALPPS. Surgery 2017;161:357-64. [Crossref] [PubMed]
- Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg 1991;214:221-8; discussion 228-9. [Crossref] [PubMed]
- Pichlmayr R, Weimann A, Oldhafer KJ, et al. Role of liver transplantation in the treatment of unresectable liver cancer. World J Surg 1995;19:807-13. [Crossref] [PubMed]
- Houben KW, McCall JL. Liver transplantation for hepatocellular carcinoma in patients without underlying liver disease: a systematic review. Liver Transpl Surg 1999;5:91-5. [Crossref] [PubMed]
- Mergental H, Adam R, Ericzon BG, et al. Liver transplantation for unresectable hepatocellular carcinoma in normal livers. J Hepatol 2012;57:297-305. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol 2016;65:1140-47. [Crossref] [PubMed]
- Longo L, de Freitas LBR, Santos D, et al. Sorafenib for Advanced Hepatocellular Carcinoma: A Real-Life Experience. Dig Dis 2018;36:377-84. [Crossref] [PubMed]
- Raoul JL, Kudo M, Finn RS, et al. Systemic therapy for intermediate and advanced hepatocellular carcinoma: Sorafenib and beyond. Cancer Treat Rev 2018;68:16-24. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol 2018;36:207. [Crossref]
- Chau I, Peck-Radosavljevic M, Borg C, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: Patient-focused outcome results from the randomised phase III REACH study. Eur J Cancer 2017;81:17-25. [Crossref] [PubMed]
- Raoul JL, Gilabert M, Adhoute X, et al. An in-depth review of chemical angiogenesis inhibitors for treating hepatocellular carcinoma. Expert Opin Pharmacother 2017;18:1467-76. [Crossref] [PubMed]
- Piscaglia F, Ogasawara S. Patient selection for transarterial chemoembolization in hepatocellular carcinoma: importance of benefit/risk assessment. Liver Cancer 2018;7:104-19. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Costentin CE, Ferrone CR, Arellano RS, et al. Hepatocellular carcinoma with macrovascular invasion: defining the optimal treatment strategy. Liver Cancer 2017;6:360-74. [Crossref] [PubMed]
- Cho YY, Lee M, Kim HC, et al. Radioembolization is a safe and effective treatment for hepatocellular carcinoma with portal vein thrombosis: a propensity score analysis. PLoS One 2016;11:e0154986. [Crossref] [PubMed]
- Ikeda M, Shimizu S, Sato T, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol 2016;27:2090-96. [Crossref] [PubMed]
- Kirstein MM, Voigtlander T, Schweitzer N, et al. Transarterial chemoembolization versus sorafenib in patients with hepatocellular carcinoma and extrahepatic disease. United European Gastroenterol J 2018;6:238-46. [Crossref] [PubMed]
- Kim GA, Shim JH, Yoon SM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol 2015;26:320-9.e6. [Crossref] [PubMed]
- Choi GH, Shim JH, Kim MJ, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology 2013;269:603-11. [Crossref] [PubMed]
- Wang G, Liu Y, Zhou SF, et al. Sorafenib combined with transarterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis and systematic review. Hepatol Int 2016;10:501-10. [Crossref] [PubMed]
- Cai R, Song R, Pang P, et al. Transcatheter arterial chemoembolization plus sorafenib versus transcatheter arterial chemoembolization alone to treat advanced hepatocellular carcinoma: a meta-analysis. BMC Cancer 2017;17:714. [Crossref] [PubMed]
- Zhang Y, Fan W, Wang Y, et al. Sorafenib with and without transarterial chemoembolization for advanced hepatocellular carcinoma with main portal vein tumor thrombosis: a retrospective analysis. Oncologist 2015;20:1417-24. [Crossref] [PubMed]
- Ha Y, Lee D, Shim JH, et al. Role of transarterial chemoembolization in relation with sorafenib for patients with advanced hepatocellular carcinoma. Oncotarget 2016;7:74303-13. [Crossref] [PubMed]
- de la Torre MA, Buades-Mateu J, de la Rosa PA, et al. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or sorafenib. Liver Int 2016;36:1206-12. [Crossref] [PubMed]
- Cho JY, Paik YH, Park HC, et al. The feasibility of combined transcatheter arterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma. Liver Int 2014;34:795-801. [Crossref] [PubMed]
- Peng Z, Chen S, Wei M, et al. Advanced recurrent hepatocellular carcinoma: treatment with sorafenib alone or in combination with transarterial chemoembolization and radiofrequency ablation. Radiology 2018;287:705-14. [Crossref] [PubMed]
- Giorgio A, Merola MG, Montesarchio L, et al. Sorafenib combined with radio-frequency ablation compared with sorafenib alone in treatment of hepatocellular carcinoma invading portal vein: a western randomized controlled trial. Anticancer Res 2016;36:6179-83. [Crossref] [PubMed]
- Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol 2018;4:661-9. [Crossref] [PubMed]
- Feng F, Jiang Q, Jia H, et al. Which is the best combination of TACE and Sorafenib for advanced hepatocellular carcinoma treatment? A systematic review and network meta-analysis. Pharmacol Res 2018;135:89-101. [Crossref] [PubMed]
- Finn RS, Zhu AX, Farah W, et al. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: A systematic review and meta-analysis. Hepatology 2018;67:422-35. [Crossref] [PubMed]
Cite this article as: Fernandes ES, Rodrigues PD, Álvares-da-Silva MR, Scaffaro LA, Farenzena M, Teixeira UF, Waechter FL. Treatment strategies for locally advanced hepatocellular carcinoma. Transl Gastroenterol Hepatol 2019;4:12.