
Ischemic postconditioning decreases iNOS gene expression but ischemic preconditioning ameliorates histological injury in a swine model of extended liver resection
Introduction
One of the most significant factors associated with poor outcomes in liver surgery is excessive intraoperative blood loss (1). The daunting task of mitigating blood loss during liver surgery has challenged liver surgeons, as the liver is innately richly vascularized. Since the introduction of porta hepatis occlusion by Pringle (2), the cornerstone among all operative strategies has been the temporary occlusion of blood flow to the liver (1,3). However, all implemented operative techniques intrinsically expose the liver to “warm ischemia”, which in turn incurs further histological damage and it has been associated with post-hepatectomy liver failure (4). This process is better known as ischemia/reperfusion (I/R) injury of the liver.
To ameliorate I/R injury, the technique of vascular occlusion has been refined through the introduction of pre- and postconditioning maneuvers. This refers to a brief period of ischemia and reperfusion; this maneuver could either precede or follow the main ischemic insult to the liver and referred to as either pre- or postconditioning respectively (5). The incorporation of this maneuver has been reported to heighten the threshold of sustainable warm ischemia through upregulation of the inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) genes, which in turn increase the levels of NO and prevent apoptosis (6).
There are available studies investigating the effect of either pre- or postconditioning on the expression of the iNOS and eNOS genes independently. However, to date there are no studies directly comparing the effect of each maneuver (i.e., pre- and postconditioning) on the expression of NOS genes in one setting, thus allowing the implication that the two maneuvers may be equivalent. Taking into account the above we designed an experimental prospective cohort study to compare the effect of pre- and postconditioning on iNOS and eNOS gene expression and the subsequent parenchymal injury. Both groups were also compared to a control group in the setting of an experimental large animal model of extended liver resection with prolonged warm ischemia time.
Methods
We designed a prospective experimental animal cohort study using female Landrace pigs weighing 25 to 30 kg. The study was performed at the Experimental and Research Unit of the Second Department of Surgery “Aretaieio” Hospital (National and Kapodistrian University of Athens, School of Medicine, Athens, Greece). The cohort consisted of three groups of 10 animals each: (I) sham group (SG); (II) preconditioning group (PrG); and (III) postconditioning group (PoG). The study protocol was in advance reviewed and approved by the Bioethics Committee of “Aretaieio” Hospital (institutional reference number: B-103/30-4-2015) and the Animal Research Veterinary Committee of the prefecture of Athens and was found in accordance with the National and European guidelines for ethical animal research and animal handling (regional reference number: ΔAKΠ1185).
The experimental model and study design
All animals underwent surgery under sterile conditions and endotracheal intubation. After achieving general anesthesia, through a right lateral cervical incision, surgical exposure and cutdown of the right internal carotid and internal jugular vein was performed for the insertion of arterial and venous catheters for invasive cardiovascular monitoring, fluid resuscitation and medication administration. A midline laparotomy was performed and urinary catheterization was made through a cystotomy. Subsequently the infrahepatic IVC was mobilized and the portal vein was skeletonized. A side-to-side portocaval (P-C) shunt was constructed with a running 6-0 prolene suture after partial occlusion of each vessel with Cooley clamps. Care was taken in order not to exceed more than 10 minutes of partial occlusion of the portal vein to avoid intestinal venous congestion. A functioning P-C shunt was necessary to avoid ischemic injury from venous congestion to the intestine during the subsequent liver transection and induced liver ischemia after porta hepatis occlusion (6).
At this point the subjects were randomly allocated to one of three groups: (I) sham group (SG)—the animals underwent occlusion of the hilum with patent P-C shunt to undergo a “left extended hepatectomy” (70% liver resection) as previously described (7). Following liver resection occlusion of the porta hepatis was maintained for a total of 90 min to induce ischemic injury to the liver (8). At the end of 90 min the hilar blood flow was released with simultaneous occlusion of the P-C shunt with a vascular clamp. (II) Preconditioning group (PrG)—the subjects prior to liver resection, underwent ischemic preconditioning by occluding the porta hepatis with patent P-C shunt for 10 min followed by 10 min reperfusion with clamped P-C shunt. After the preconditioning, the hepatectomy took place as described above. (III) Postconditioning group (PoG)—the subjects underwent the procedure as described for the SG. At the end of 90 minutes of warm ischemia, they underwent postconditioning by releasing the porta hepatis for 10 minutes with simultaneous occlusion of the P-C shunt followed by another 10 minutes of ischemia with patent P-C shunt. Following the postconditioning the blood flow to the liver was released and the P-C shunt was clamped. Care was taken in all animals to maintain minimal blood loss.
All animals thereafter underwent temporary abdominal closure, with continuous cardiovascular monitoring under mechanical ventilation. Data collection was performed at the start of reperfusion (0 hours), 6 and 12 hours after reperfusion. Sample collection included liver tissue kept both in formalin and frozen to −80 °C, U&Es, LFTs and ABGs. At the end of data collection all animals were euthanized under general anesthesia and according to the European laws of animal handling (9).
Sample procession
Polymerase chain reaction (PCR)
Total RNA was extracted from liver tissue using TRIzol® Reagent (Invitrogen, USA). DNase treatment was performed using DNase I (Invitrogen, USA) to remove DNA traces, according to the manufacturer’s instructions. Subsequently, 1 µg of RNA was subjected to reverse transcription, using M-MLV reverse transcriptase and random hexamers (Invitrogen) according to the manufacturer’s instructions. The expression levels of eNOS and iNOS genes were accessed by real time PCR (Rotor Gene 6000™, Corbett Life Science). Briefly, 5 µL of cDNA were amplified with the use of the intercalating dye SYBR Green I using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, USA) at a final concentration 1x. Beta-actin, a constitutively expressed housekeeping gene, was also amplified under the same conditions and used to normalize reactions.
Real time PCR conditions were: initial denaturation at 95 °C for 10 minutes, followed by 45 cycles of 95 °C for 20 seconds, 57 °C for 20 seconds and 72 °C for 30 seconds. Relative quantification of gene expression was calculated by the 2−ΔΔCT method (10). RNA from the baseline controls is pooled and is used as a calibrator in qPCR equations.
To ensure the specificity of the reaction, the size of the PCR product for each gene was verified by 2.0% agarose gel electrophoresis. Additionally, sequencing of selected positive samples was performed on the DNA ABI PRISM® 3130 using the BigDye Terminator Cycle Sequencing kit (USA).
Histological assessment
Formalin-fixed liver tissue samples were embedded in paraffin and 5 µm sections were taken. A single experienced pathologist assessed the morphological changes “blindly” after staining with hematoxylin and eosin (H&E). The morphologic changes assessed were: subcapsular accumulation of neutrophils, sinusoidal congestion, sinusoidal infiltration by neutrophils, lobular inflammation, lobular necrosis, vacuolisation, portal inflammation, piecemeal necrosis and apoptosis. Apoptosis was assessed by morphological features of cell shrinkage, chromatin condensation and the formation of apoptotic bodies (11). Grading was performed on an ordinal scale of 0 to 4 where 0 represented the absence of morphological changes and 4 maximal intensity as previously described (11-13). A modified histological activity index (HAI) was post hoc calculated by adding the grade of lobular necrosis, apoptosis, portal inflammation and piecemeal necrosis to summarize the severity of parenchymal injury, as previously described (13).
Statistical analysis
Due to the sample size per group, a non-parametric analysis was performed, which is more robust to data distribution in small sample size datasets (14,15). Overall comparisons between groups were assessed with Friedman’s related samples two-way analysis of variance; Within specific time points differences were assessed with the Kruskal-Wallis test and pairwise comparisons were performed with either Mann-Whitney U test and Wilcoxon signed rank test or related samples sign test as appropriately for unrelated or related measurements respectively. For the above independent pairwise comparisons, the Bonferroni correction was applied to the P values to adjust for multiple comparisons (16). Calculations were performed with SPSS version 24.0 (17) and the report of results is in accordance with the ARRIVE guidelines (18).
Results
Out of the 30 animals of the cohort, 7 experienced extreme hemodynamic instability during their follow up signified by either prolonged severe hypotension (<65 mmHg systolic blood pressure) despite fluid resuscitation, severe prolonged acidosis (Ph <7.2) and/or severe hyperlactatemia (>6 mmol/L); their measurements were excluded from analysis and were replaced with other subjects.
iNOS & eNOS gene expression
Table 1 presents the raw data for the iNOS and eNOS expression for each group. Overall the distribution of iNOS expression between groups and within time had significant differences (Friedman’s related samples two-way analysis of variance, P=0.021). More specifically, at 0 hour the SG had increased expression of iNOS (Kruskal-Wallis test, P=0.012) in comparison to the PoG only (Mann-Whitney U test, P<0.0005—Bonferroni correction). This difference was maintained at 6 hours (Kruskal-Wallis test, P=0.01; Mann-Whitney U test, P<0.0005—Bonferroni correction) but was eliminated by 12 hours (Kruskal-Wallis test, P=0.064). Specifically, within the SG there was a significant decrease of iNOS expression by 6 hours (related samples sign test P=0.002—Bonferroni correction), which remained stable until 12 hours. No significant differences were found at any time point between PrG and PoG. The distribution of eNOS expression between groups and within time points, failed to attend statistical significance although marginally (Friedman’s related samples two-way analysis of variance, P=0.093).
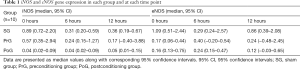
Full table
Liver function tests
The AST values presented significant differences when assessed overall (Friedman’s related samples two-way analysis of variance, P<0.0005). In detail, we did not detect any difference in AST values at any time point between groups as all had a similar increasing trend; as expected within each group and as time progressed, the AST values increased; specifically, both the SG and PrG increased significantly their AST levels by 6 hours (Wilcoxon Signed rank test, P=0.017 and P=0.012, respectively), but did not deteriorate further by 12 hours. Within PoG AST levels also increased by 6 hours (Wilcoxon Signed rank test P=0.018), but continued to deteriorate further until 12 hours (Wilcoxon signed rank test P=0.018) (Figure 1). In regards to ALP, the overall differences were again significant (Friedman’s related samples two-way analysis of variance, P<0.0005); similar results were obtained for the ALP kinetics, i.e., the SG and PrG increased their ALP levels by 6 hours but with no further significant increase (Wilcoxon signed rank test P=0.012 and P=0.025 respectively), however in the PoG ALP levels continued to increase until the 12 hours (Wilcoxon signed rank test, 0–6 hours P=0.018 and 6–12 hours P=0.028) (Figure 2). The ALT values also presented significant differences when assessed overall (Friedman’s related samples two-way analysis of variance, P=0.013). There were no significant differences between groups at any time point. In detail there was a significant increase of ALT values in the SG only (Friedman’s related samples two-way analysis of variance, P=0.05). In the PrG and PoG, although there was an increasing trend between time points, these differences did not attend statistical significance. No significant differences were identified in regard to the levels of bilirubin (Friedman’s related samples two-way analysis of variance, P=0.713).
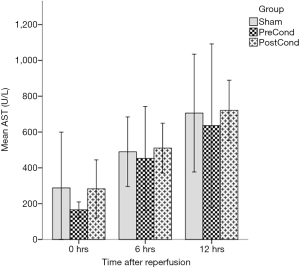
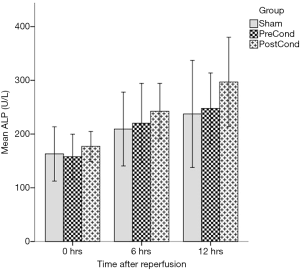
Histological assessment
Significant differences were found when assessed overall in the following variables: subcapsular accumulation of neutrophils, sinusoidal congestion and sinusoidal infiltration by neutrophils (Friedman’s related samples two-way analysis of variance, P=0.009, P<0.0005 and P=0.001 respectively). In detail, PrG had the lowest subcapsular accumulation of neutrophils, which gradually increased by 12 hours surpassing that of the PoG; the SG and PoG had a similar trend through time, although the PoG had a more significant decrease by 12 hours. The SG had significantly increased sinusoidal congestion at the baseline, which peaked by 6 hours followed by a mild decrease by 12 hours. Although by 6 hours the PrG and PoG followed the same trend, with the PrG group having significant lower values, by the 12 hours mark the PrG had return to the baseline values while the PoG maintained the same level of sinusoidal congestion. In terms of sinusoidal infiltration by neutrophils, although all groups increased by 6 hours their values, at 12 hours the PrG had returned to its baseline levels, being significantly decreased in comparison to both the SG and PoG.
The modified HAI score (Table 2) had overall significant differences in between and within groups (Friedman’s related samples two-way analysis of variance, P<0.0005); specifically, although the PrG was found to have a higher HAI at the start of reperfusion than PoG (Kruskal-Wallis test, P=0.004; pairwise P=0.009), it remained stabled through time and was significant lower by 12 hours than PoG (Kruskal-Wallis test, P<0.0005; pairwise P<0.0005). The SG had a significant increase by 6 hours (Friedman’s related samples two-way analysis of variance, P=0.018; pairwise P=0.014), which subsequently decreased by 12 hours, but never presented with significant differences in comparison to the other two groups. Finally, the PoG had a significant increase of the HAI by 6 hours, which continued to increase until 12 hours but not significantly (Friedman’s related samples two-way analysis of variance, P=0.001; pairwise P=0.037 0–6 hours and P=0.952 6–12 hours).
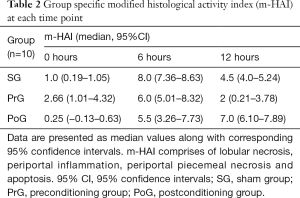
Full table
Discussion
The results of our study have shown that ischemic postconditioning reduced the expression of iNOS during the acute phase of I/R in a model of extended hepatectomy with prolonged warm ischemia until 6 hours following reperfusion in comparison to the sham operated animals. No difference in expression of either iNOS or eNOS was found between PrG or PoG. However ischemic preconditioning achieved to ameliorate the hepatocellular damage seen at 12 hours following reperfusion in comparison to both the sham group and the postconditioning group.
Different molecular pathways mediate the protective effect of pre- and postconditioning, where the physiologic differences extend far beyond the timing of the operative manoeuvres, i.e., before or after, the major ischemic insult. Ischemic preconditioning has been found to exert its protective effect through activation of nuclear transcription and subsequent upregulation of the production of iNOS (19); increased expression of iNOS in turn increases the production of NO which has been shown to decrease cell death mediated by mitochondrial permeability (20). However, excessive production of NO by overproduction of iNOS after prolonged ischemia has been found to be deleterious (21). Postconditioning protective effect is achieved through activation of signal transduction cascades mediated by autacoids, which in turn activate the RISK pathway and phosphorylation of eNOS which will increase production of NO (19,21).
Our data have shown that animals who did not underwent any liver conditioning (SG) had overexpression of iNOS; however, this difference attended statistical significance only in comparison to the PoG at the start of reperfusion up until 6 hours after reperfusion. This finding most probably signifies a deleterious effect of overproduction of NO during the early phase of I/R injury in the SG; this finding was further corroborated by the significant subcapsular accumulation of neutrophils at the start of reperfusion at the SG, which is a known early marker of hepatocellular injury (12). The PrG had increased expression of iNOS, although did not attended statistical significance in comparison to the PoG; however histological assessment did not produce significant evidence of I/R injury, suggesting that preconditioning may exert its protective effect through other molecular mechanisms as well. At this initial phase within 2 hours from the ischemic insult, the I/R injury is characterised predominantly by oxidative stress, where ROS directly cause hepatocellular injury (22). Finally, although marginally, we did not identify any alterations of the eNOS expression throughout the duration of reperfusion between groups; however eNOS has been shown that in pigs is predominantly expressed in heart and lungs rather than the liver in an endotoxin shock model (23).
Despite the unanimity of data in rats and mice that both pre- and postconditioning will have a beneficial effect to the liver I/R injury via modifications of the NO pathway (24-27), there are conflicting data in the literature in regard to iNOS and eNOS expression in pigs during the early hours of reperfusion. These conflicting data consist to either failure to identify significant differences in the expression of iNOS during the early hours of reperfusion following hepatectomy in pigs (28) or even reports of minimal protection of the ischemic preconditioning all together (29).
A significant experimental difference between studies undertaken in pigs and rodents (e.g., mice and rats) is the need of venous decompression of the intrabdominal viscera in pigs during the occlusion of the porta hepatis; this can be achieved either by a portocaval shunt (28) or veno-venous bypass (29,30). Pigs are unable to tolerate continuous prolonged occlusion of the portal vein as they develop quickly significant intestinal congestion with subsequent severe haemodynamic instability (30). Portocaval shunting however has been shown to decrease the availability of arginine, which is the substrate for NO production (31) and also increase the uptake of arginine in the brain (32). Furthermore it has been shown that during the revascularisation of the pig liver there is increase of L-arginase (33), an enzyme that catalyses L-arginine to L-ornithine and urea (21). Indeed, it has been shown that arginine availability strongly affects the iNOS protein expression both in a translational manner, i.e., protein synthesis as well as posttranslational manner, i.e., protein stability (34,35). Furthermore, more recent published evidence further strengthens the knowledge that iNOS and arginase expression are linked and thus altering NO production (36). It follows that in our study, where a portocaval shunt is necessary to be established early to avoid hemodynamic instability due to bowel congestion, may have led to depletion of arginine via overproduction of arginase; thus impeding with our ability to detect significant alterations of the iNOS expression beyond the acute phase of reperfusion.
Surprisingly we were unable to identify significant changes of the eNOS expression across groups or between time points. It has been shown that the expression of eNOS in rats is significantly attenuated after the super acute phase (minutes), while the iNOS exhibits a more gradual increase in the hours to follow the I/R insult (37), a trend that was not clearly seen in our data. Interestingly there has been evidence that in a model of endothelial porcine cells treated with lipopolysaccharide (LPS), the common stimulus (LPS) did not affect the production of iNOS and eNOS in the same manner, i.e., increase; on the contrary a significant increase of iNOS expression was found in conjunction with a significant decrease of eNOS expression in the same cells (38).
However, despite the significant low expression of iNOS in the postconditioning group in comparison to the sham group, the hepatocellular injury in this group was found to gradually deteriorate by 12 hours. In contrast the preconditioning group was found to gradually improve the hepatocellular injury observed by 12 hours following reperfusion. This inverse trend between the two groups was further corroborated by the observed trend kinetics of the AST and ALP, which are surrogate markers of hepatocellular injury and the PrG was found to have consistently the lowest levels of AST. Evidently, the hepatocellular injury continues to progress in the aftermath of the ischemic insult, independently of the iNOS and eNOS expression in hepatocytes. The current study is the first to directly compare the hepatocellular injury observed following two protective manoeuvres (pre- and postconditioning), in the setting of extended liver resection and I/R injury following prolonged warm ischemia and our results suggested that preconditioning may exert a more potent protective effect than postconditioning; it is also evident that much more complex pathways are activated during hepatic I/R injury which appear to be active during both the early and late phase of reperfusion. To date, only few studies have advocated independently the protective role on the hepatocyte of either preconditioning (30) or postconditioning (26,27) in both animal models and humans (39); however none has compared directly the effect of pre- to postconditioning. Furthermore, recent studies have concluded that during I/R injury there is over expression of iNOS and/or eNOS and their inhibition protects from I/R injury (40), therefore pointing towards a fine balance between beneficial and detrimental effects of the bioavailability of NO.
The results of this study were limited due to the small sample size; however as this study is a translational study with no direct clinical application, we maintained the number of animal participants to the minimum required to obtain meaningful results in accordance with the 3R’s policy (Replacement, Reduction and Refinement) of the European Union (9). To overcome this limitation we used only a non-parametric analysis, which is more resilient to the effect of distribution of data on statistical results (14). Furthermore, in hind sight, it would have been a helpful insight to have directly measure the levels of NO in this study. As it became evident the expression of iNOS and eNOS may have potentially been affected by the availability of substrates, thus a direct measurement of the end-product of the activation of NOS, could have further helped as to delineate the effects of pre- and postconditioning on NO production and the concomitant hepatocellular injury.
In conclusion the results of this study have shown that postconditioning decreased more effectively the expression of iNOS gene expression than preconditioning in comparison to animals who did not underwent any form of conditioning. However, preconditioning was the most effective manoeuvre to ameliorate hepatocellular injury at 12 hours after reperfusion, indicating that more complex mechanisms apart from NO bioavailability maybe involved. Further studies are needed to delineate the exact association of genetic and molecular events during the acute phase of I/R injury of the liver.
Acknowledgements
The authors would like to thank Dr. Eftychia Aravidou veterinarian, for her assistance with the animal processes.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was in advance reviewed and approved by the Bioethics Committee of “Aretaieio” Hospital (Institutional reference number: B-103/30-4-2015) and the Animal Research Veterinary Committee of the prefecture of Athens and was found in accordance with the National and European guidelines for ethical animal research and animal handling (Regional reference number: ΔAKΠ1185).
References
- Eeson G, Karanicolas PJ. Hemostasis and Hepatic Surgery. Surg Clin North Am 2016;96:219-28. [Crossref] [PubMed]
- Pringle JH. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg 1908;48:541-9. [Crossref] [PubMed]
- Chouillard EK, Gumbs AA, Cherqui D. Vascular clamping in liver surgery: physiology, indications and techniques. Ann Surg Innov Res 2010;4:2. [Crossref] [PubMed]
- van den Broek MA, Olde Damink SW, Dejong CH, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int 2008;28:767-80. [Crossref] [PubMed]
- Gurusamy KS, Gonzalez HD, Davidson BR. Current protective strategies in liver surgery. World J Gastroenterol 2010;16:6098-103. [Crossref] [PubMed]
- Mao Y, Wang J, Yu F, et al. Ghrelin protects against palmitic acid or lipopolysaccharide-induced hepatocyte apoptosis through inhibition of MAPKs/iNOS and restoration of Akt/eNOS pathways. Biomed Pharmacother 2016;84:305-13. [Crossref] [PubMed]
- Hori T, Yagi S, Okamua Y, et al. How to successfully resect 70% of the liver in pigs to model an extended hepatectomy with an insufficient remnant or liver transplantation with a small-for-size graft. Surg Today 2014;44:2201-7. [Crossref] [PubMed]
- Quan D, Wall WJ. The safety of continuous hepatic inflow occlusion during major liver resection. Liver Transpl Surg 1996;2:99-104. [Crossref] [PubMed]
- The European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union 2010:L276/33-79.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007;47:598-607. [Crossref] [PubMed]
- Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, et al. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993;55:1265-72. [Crossref] [PubMed]
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-9. [Crossref] [PubMed]
- Bland JM, Altman DG. Analysis of continuous data from small samples. BMJ 2009;338:a3166. [Crossref] [PubMed]
- Altman DG, Bland JM. Parametric v non-parametric methods for data analysis. BMJ 2009;338:a3167. [Crossref] [PubMed]
- Boffetta P, McLaughlin JK, La Vecchia C, et al. False-positive results in cancer epidemiology: a plea for epistemological modesty. J Natl Cancer Inst 2008;100:988-95. [Crossref] [PubMed]
- Norussis M. SPSS 16.0 guide to data analysis. New Jersey: Prentice Hall, 2008.
- Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [Crossref] [PubMed]
- Selzner N, Boehnert M, Selzner M. Preconditioning, postconditioning, and remote conditioning in solid organ transplantation: basic mechanisms and translational applications. Transplant Rev (Orlando) 2012;26:115-24. [Crossref] [PubMed]
- Kaizu T, Ikeda A, Nakao A, et al. Donor graft adenoviral iNOS gene transfer ameliorates rat liver transplant preservation injury and improves survival. Hepatology 2006;43:464-73. [Crossref] [PubMed]
- Abu-Amara M, Yang SY, Seifalian A, et al. The nitric oxide pathway--evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver Int 2012;32:531-43. [Crossref] [PubMed]
- Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol 2003;18:891-902. [Crossref] [PubMed]
- Doursout MF, Oguchi T, Fischer UM, et al. Distribution of NOS isoforms in a porcine endotoxin shock model. Shock 2008;29:692-702. [PubMed]
- Young SB, Pires AR, Boaventura GT, et al. Effect of ischemic preconditioning and postconditioning on liver regeneration in prepubertal rats. Transplant Proc 2014;46:1867-71. [Crossref] [PubMed]
- Zhang WX, Yin W, Zhang L, et al. Preconditioning and postconditioning reduce hepatic ischemia-reperfusion injury in rats. Hepatobiliary Pancreat Dis Int 2009;8:586-90. [PubMed]
- Guo JY, Yang T, Sun XG, et al. Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J Biomed Sci 2011;18:79. [Crossref] [PubMed]
- Guo Y, Yang T, Lu J, et al. Rb1 postconditioning attenuates liver warm ischemia-reperfusion injury through ROS-NO-HIF pathway. Life Sci 2011;88:598-605. [Crossref] [PubMed]
- Athanasopoulos P, Mastoraki A, Papalois A, et al. Expression of Inflammatory and Regenerative Genes in a Model of Liver Ischemia/Reperfusion and Partial Hepatectomy. J Invest Surg 2016;29:67-73. [Crossref] [PubMed]
- Schulz R, Walz MK, Behrends M, et al. Minimal protection of the liver by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol 2001;280:H198-207. [Crossref] [PubMed]
- Shimoda M, Iwasaki Y, Sawada T, et al. Protective effect of ischemic preconditioning against liver injury after major hepatectomy using the intermittent pringle maneuver in swine. Pathobiology 2007;74:42-9. [Crossref] [PubMed]
- Sharma V, Ten Have GA, Ytrebo L, et al. Nitric oxide and L-arginine metabolism in a devascularized porcine model of acute liver failure. Am J Physiol Gastrointest Liver Physiol 2012;303:G435-41. [Crossref] [PubMed]
- Rao VL, Audet RM, Butterworth RF. Increased nitric oxide synthase activities and L-[3H]arginine uptake in brain following portacaval anastomosis. J Neurochem 1995;65:677-8. [PubMed]
- Langle F, Steininger R, Waldmann E, et al. Improvement of cardiac output and liver blood flow and reduction of pulmonary vascular resistance by intravenous infusion of L-arginine during the early reperfusion period in pig liver transplantation. Transplantation 1997;63:1225-33. [Crossref] [PubMed]
- El-Gayar S, Thuring-Nahler H, Pfeilschifter J, et al. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J Immunol 2003;171:4561-8. [Crossref] [PubMed]
- Mori M, Gotoh T. Regulation of nitric oxide production by arginine metabolic enzymes. Biochem Biophys Res Commun 2000;275:715-9. [Crossref] [PubMed]
- Lou Y, Zhang G, Geng M, et al. TIPE2 negatively regulates inflammation by switching arginine metabolism from nitric oxide synthase to arginase. PLoS One 2014;9:e96508. [Crossref] [PubMed]
- Miyake T, Yokoyama Y, Kokuryo T, et al. Endothelial nitric oxide synthase plays a main role in producing nitric oxide in the superacute phase of hepatic ischemia prior to the upregulation of inducible nitric oxide synthase. J Surg Res 2013;183:742-51. [Crossref] [PubMed]
- Bernardini C, Greco F, Zannoni A, et al. Differential expression of nitric oxide synthases in porcine aortic endothelial cells during LPS-induced apoptosis. J Inflamm (Lond) 2012;9:47. [Crossref] [PubMed]
- de Rougemont O, Lehmann K, Clavien PA. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transplantation 2009;15:1172-82. [Crossref] [PubMed]
- Zhang YQ, Ding N, Zeng YF, et al. New progress in roles of nitric oxide during hepatic ischemia reperfusion injury. World J Gastroenterol 2017;23:2505-10. [Crossref] [PubMed]
Cite this article as: Kontis E, Pantiora E, Melemeni A, Tsaroucha A, Karvouni E, Polydorou A, Vezakis A, Fragulidis GP. Ischemic postconditioning decreases iNOS gene expression but ischemic preconditioning ameliorates histological injury in a swine model of extended liver resection. Transl Gastroenterol Hepatol 2019;4:5.


