Cholangioscopy in the digital era
Introduction and historical perspective
Cholangioscopy allows direct visualization and subsequent therapeutic manipulation of the biliary ductal system. As early as the 1950s, attempts at cholangioscopy have been described in the literature (1). Referred to as ‘choledoscopy’ in the early literature, this technique was initially conceived as a complementary method to cholangiography for the intraoperative evaluation of bile duct stones (2). In the 1970s, two different methods for per-oral cholangioscopy (POC) were separately described (3,4). The earliest platform used for cholangioscopy utilized a small ‘baby’ cholangioscope that is advanced through the working channel of a ‘mother’ duodenoscope. Urakami et al. were the first to report direct cholangioscopy using a forward viewing endoscope (4). Since their inception, ‘mother-baby’ cholangioscopy platforms have improved in image quality and added the ability to use narrow band imaging (NBI), which has enhanced our ability to differentiate malignant versus benign biliary lesions. The main disadvantages of the ‘mother-baby’ cholangioscopy platform include the need of two operators, the fragility of the baby cholangioscope and the high starting costs. In 2005, a novel catheter-based single-operator system cholangioscope (SpyGlass™, Boston Scientific Corp, Marlborough, MA, USA) was introduced (5). Since then, further advancement of endoscopic techniques and miniaturization of endoscopic tools has led POC to evolve into a readily-available, minimally invasive procedure for the evaluation and management of challenging biliary diseases. In this review, we discuss the technical evolution, clinical applications, limitations and safety of POC.
Cholangioscopy platforms & techniques
Percutaneous cholangioscopy
This technique, also referred to as percutaneous transhepatic cholangioscopy, is often reserved for situations when POC is not feasible or ineffective. This may include patients with altered gastrointestinal anatomy (Billroth II, Roux-en-Y) and patients with intra-hepatic or large biliary stones >1.5 cm (6). The procedure is initiated by performing percutaneous transhepatic cholangiography with biliary drainage. Serial dilatation and subsequent maturation of the percutaneous tract is obtained over several days with a percutaneous drainage catheter left in place. Once the tract is mature, the catheter is removed over a stiff guidewire and the cholangioscope is inserted into the mature tract over the guidewire with both endoscopic and fluoroscopic visualization (7). Subsequently, interventions including biopsy, electrohydraulic or laser lithotripsy (LL) can be performed depending on the indication for the procedure. The advantages of this approach include the ability to use a short cholangioscope (typically a ureteroscope) which in turn increases maneuverability and targeting of areas that may be difficult to reach via POC and the ability to easily perform multiple subsequent sessions once the percutaneous tract is established. Nevertheless, the significant limitations of this approach include its invasiveness as well as time required for tract maturation, which has made it a secondary choice to POC.
‘Mother-baby’ dual operator POC
Per-oral ‘mother-baby’ video-cholangioscopes are composed of a ‘mother’ duodenoscope and a ‘baby’ cholangioscope. The cholangioscope is advanced through the working channel of the dedicated therapeutic duodenoscope over a guidewire. This arrangement requires two operators; one to manipulate the controls of the “mother” duodenoscope and the other to manage the dedicated “baby” cholangioscope. One example of a current generation cholangioscope is the Olympus CHF-B260 (Olympus Co., Tokyo, Japan) where the distal cholangioscope diameter is 3.4 mm with an accessory channel diameter of 1.2 mm (8). The cholangioscope is advanced through the 4.2 mm accessory channel of the therapeutic duodenoscope. Sphincterotomy should be performed prior to insertion of the cholangioscope into the common bile duct due to its large outer diameter. Two-way tip deflection in the baby cholangioscope is possible and high definition and NBI functions are available for detailed examination of the biliary mucosa. A major advantage of the current generation mother-baby platform includes its excellent image quality and ability to use electronic enhancement (e.g., NBI). A number of disadvantages have limited its use including high operating cost, baby scope fragility, need for two skilled endoscopists to operate the scope, limitations in tip deflection capabilities, and suboptimal irrigation capability (9-11).
Single-operator POC
Introduced in 2005, the SpyGlass™ POC system (Boston Scientific Corp) was designed to overcome some of the limitations of the mother-baby platform. Similar to ‘mother-baby’ systems, the cholangioscope is advanced over a guidewire through the working channel of a therapeutic duodenoscope, although direct freehand cannulation after biliary sphincterotomy is possible due to the smaller 3.3 mm outer diameter and four-way tip deflection of this catheter-based system. The procedure is typically carried out alongside endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy to allow improved ductal access and drainage of the ductal system during irrigation (12). The original SpyGlass™ platform (now referred to as SpyGlass™ Legacy) was composed of a disposable delivery catheter (SpyScope™) through which a reusable fiberoptic probe (SpyGlass™ Direct Visualization Probe) was inserted. The reusable probe had an average expected use of 8–10 times but was fragile and in real-life lasted 3–4 times. The delivery catheter had an outer diameter of 3.3 mm, with an accessory channel diameter of 1.2 mm but importantly had a dedicated irrigation channel (5,12,13). The SpyGlass™ cholangioscope handle is designed to strap onto a therapeutic duodenoscope allowing single-operator use. The handle includes control knobs for four-way tip deflection, levers to lock position, controls for aspiration and irrigation and access to the working channel port. The single operator design is by far the most important advantage of the SpyGlass™ system but other important benefits include the ability to perform continuous irrigation with resultant improved visualization as well as four-way tip deflection for improved maneuverability. The early SpyGlass™ fiberoptic platform brought POC into the mainstream and made the procedure feasible in a busy everyday endoscopy practice (11). Nevertheless, the SpyGlass™ Legacy had some significant limitations related to fiberoptic probe durability, limited image quality and field of view, small therapeutic channel, and elaborate set up. In light of these shortcomings, a digital enhanced SpyGlass™ DS Direct Visualization System (Boston Scientific Corp) was recently introduced, with significant improvements including digital image quality, brighter light source, wider field of view, improved tip deflection, tapered tip, and easy “plug and play” set up (13).
Direct POC (DPOC)
In DPOC, an UltraSlim endoscope (e.g., GIF XP190N, Olympus America, Center Valley, PA, USA) is directly advanced into the biliary system during standard upper endoscopy. A large sphincterotomy with or without papillary balloon dilation is required prior to DPOC (14). Advantages of DPOC include improved image definition compared to other systems, availability of electronic chromoendoscopy (e.g., NBI), a larger therapeutic 2.2 mm channel, and the fact that it obviates the need for a separate dedicated cholangioscopy platform (15). Preventing wide-spread adoption of DPOC is the fact that it is technically challenging to perform. Gastric looping, difficult biliary cannulation and instability of the endoscope within the bile duct are among the factors that increase the technical difficulty of the procedure (14-16). Furthermore, the relatively larger caliber UltraSlim endoscope when compared to other POC platforms, limits its ability to evaluate smaller bile ducts.
Diagnostic and interventional devices for cholangioscopy
All current cholangioscopy platforms allow the performance of diagnostic and therapeutic maneuvers. With DPOC, a standard “pediatric” forceps can be used to obtain tissue samples (e.g., Radial Jaw 4 Gastropediatric, Boston Scientific Corp, Marlborough, MA, USA). The smaller 1.2 mm accessory channel of the SpyGlass™ platform allows only passage of a dedicated mini forceps (SpyBite™ Boston Scientific Corp, Marlborough, MA, USA) (13).
Endoscopic intraductal lithotripsy under direct visualization via POC can be achieved by the passage lithotripsy devices. In electrohydraulic lithotripsy (EHL), sparks discharged under water generate high-frequency hydraulic pressure waves capable of fragmenting intraductal stones (17). The main advantages of the EHL platform are that the dedicated bipolar EHL generator (Northgate Technologies Inc, Elgin, IL, USA) is compact and comes at much lower cost compared to its laser counterpart. In LL, stones are fragmented by the initial formation of plasma on the stone surface, which then absorbs infrared light energy producing a strong shockwave that shatters the stone (18). Typically, a Holmium laser is utilized and capital equipment and disposables are available from multiple vendors. In general, the cost is substantially higher compared to EHL. Although per case rental agreements for LL are available, that adds to the complexity of arranging the use for a particular case. In the United States, most urology departments own and routinely use holmium laser platforms and the most feasible arrangement to perform a cholangioscopy-based LL is to utilize urology equipment if hospital logistics permit.
Clinical applications
Cholangioscopy may be used for either diagnostic or therapeutic purposes (Table 1). Diagnostic POC is most commonly used for the characterization of indeterminate biliary strictures and visualization of the extent of cholangiocarcinomas (19,20). Common therapeutic uses for POC include treatment of difficult biliary stones with intraductal lithotripsy (21) and palliative therapy of non-resectable biliary malignancies (22,23).
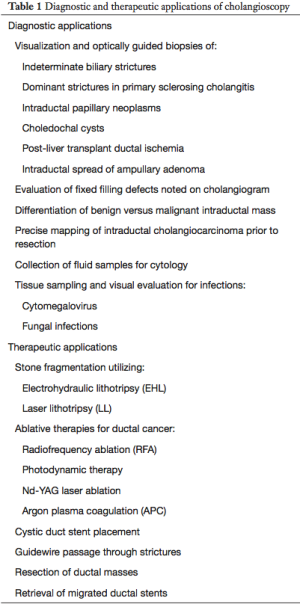
Full table
Difficult to remove bile duct stones
More than 85% of bile duct stones can be successfully extracted by ERCP with standard sphincterotomy with/without concomitant sphincteroplasty, followed by balloon or basket stone extraction (24). In select cases, the success rate of conventional ERCP stone extraction techniques may be limited by difficult scenarios. Factors making cases difficult include those with stones ≥15 mm in size, impacted stones, numerous stones, intrahepatic location, stones with a hard consistency, and difficult stone shape (e.g., piston shaped). Anatomical variants may also influence difficulty of cases and these include challenging size of the bile duct, shape of the bile duct (e.g., sigmoid shaped), low take-off of the cystic duct and/or presence of a periampullary diverticulum or stones proximal to a stricture (25,26). In such cases, direct visualization with POC allows accurate targeting of stones and decreases the risk of bile duct injury. It also allows for the use of EHL and LL probes, significantly improving procedural success.
Extrahepatic stones
Complete extrahepatic stone clearance using POC with intraductal lithotripsy is successful in 71–100% of patients (10,11,14,18,21,27-32). A large retrospective series using mother-baby POC by Arya et al. reported a stone clearance rate of 96% (89/93 patients, 61 complete, 28 partial) (21). A large multi-center prospective cohort study of 15 European and US centers using the first-generation single-operator SpyGlass™ system (SpyGlass Legacy) reported a ‘procedure-success rate’ of 92% in 66 patients undergoing therapeutic cholangioscopy. Procedural success, however, was defined as visualization and initiation of stone removal rather than complete stone clearance and only 71% of patients achieved complete clearance in the first session (33). Data on the latest single-operator SpyGlass DS™ system are encouraging. In a 2018 multi-center retrospective cohort study of 107 patients who underwent POC for biliary stones, clearance was successful in 91%. However, only 39.3% of cases achieved complete stone removal in the 1st session and an average of 3 procedures (range, 1–6) were required to achieve complete stone clearance (34). In the largest multicenter, retrospective cohort study of the SpyGlass DS™ to date, Brewer Gutierrez et al. reported a 97.3% complete stone clearance rate in 407 patients with difficult biliary stones at 22 tertiary centers in the United States, United Kingdom and Korea (35). Results with UltraSlim endoscopes (DPOC) are comparable, despite smaller size patient series. Moon et al. reported a clearance rate of 89% in 18 patients undergoing DPOC (14). Pooled data from combining all POC modalities were reported in a meta-analysis of 31 retrospective and prospective cohort studies with a pooled complete stone clearance rate of 88% [95% confidence interval (CI): 85–91%] (36).
Intrahepatic stones
The effectiveness of POC for intrahepatic stones is limited by the inability to advance the cholangioscope through narrow hepatic ducts and intrahepatic strictures (15). In a 36 patient case series of hepatolithiasis, Okugawa et al. reported complete intrahepatic stone removal rate of 64% using mother-baby POC (37). Clearance rates are higher with percutaneous cholangioscopy; complete clearance of hepatolithiasis was achieved in 88.4% of cases in a large 190 patient series (38). Recurrence is common after either approach however, with a reported recurrence rate of 22–50% (37,39).
Cholangioscopy decreases the need for mechanical lithotripsy
A major advantage of POC is that it decreases the need for mechanical lithotripsy (11,18,40). This was initially pointed out in a prospective cohort study of the SpyGlass™ Legacy system where 24/26 (92.3%) patients with stone disease achieved complete stone clearance and only 1 patient required mechanical lithotripsy (11). More recently, a 2:1 randomized trial compared cholangioscopy-guided LL to conventional therapy and found endoscopic stone clearance was achieved in 39/42 patients (93%) in the cholangioscopy-guided group compared to 12/18 patients (67%) in the conventional therapy group (40). Mechanical lithotripsy was allowed in both arms, however it was required significantly less frequently in the cholangioscopy-guided LL group (odds ratio 0.5). The benefits of reducing the need for mechanical lithotripsy may or may not justify the increased cost of POC based on local device cost and availability.
Indeterminate strictures
The term indeterminate stricture refers to biliary strictures where cross-sectional imaging is unrevealing with/without non-diagnostic brush cytology results on ERCP (41). While ERCP with brush cytology is the first line diagnostic modality for biliary strictures, it is far from perfect. Despite the high specificity of brush cytology (>95%), sensitivity remains an issue with a recent review of 16 studies showing a pooled sensitivity of 41.6%±3.2% for the detection of malignancy (42). POC offers a distinct advantage for the diagnosis and management of indeterminate strictures with direct visualization and the ability to perform cholangioscopic biopsy.
Visualization
While there is currently no standard classification system for visual diagnosis of ductal malignancy, multiple cholangioscopic findings suggestive of malignancy have been identified in the literature. These include intraductal masses and nodules, papillary/villous mucosal projections and tumor vessels (tortuous and dilated vessels) (20,43). Tumor vessel in particular appears to be the most agreed-upon characteristic visual finding. Kim et al. reported that visualization of a tumor vessel had a sensitivity of 61% for the detection of malignancy and combination with cholangioscopy-guided biopsy increased sensitivity to 96%. This finding was not present in any patient with a benign stricture (44). In a multi-center prospective cohort study, Chen et al. compared the sensitivity of ERCP impression to POC impression for the detection of biliary malignancy in 95 patients and found POC had a sensitivity of 77.8% compared to 51.1% for ERCP (33). A meta-analysis of 10 cohort studies examining POC visualization for diagnosis of malignancy showed a pooled visual accuracy, sensitivity and specificity of 89%, 93% and 85%, respectively (36). The authors noted a significantly reduced sensitivity of earlier single operator cholangioscopes compared to mother-baby platforms, due to their use of fiberoptic imaging (36). This issue appears to have been improved by newer generation single operator cholangioscopes that utilize digital imaging (SpyGlass DS™). Indeed, a prospective multi-center study of the SpyGlass DS™ cholangioscope in 44 patients with indeterminate strictures by Navaneethan et al. demonstrated POC visualization had a sensitivity and specificity of 90%, 95.8%, respectively (45).
Unfortunately, direct visualization during POC to diagnose indeterminate strictures has its limitations as well. Extrinsic compression may be explained by benign etiologies and in ductal disease such as primary sclerosing cholangitis (PSC), irregular patterns of biliary mucosa may not represent malignancy (10). Notably, POC is particularly more helpful for the evaluation of ‘intrinsic’ strictures (e.g., cholangiocarcinoma) but more limited in the diagnosis of malignancies causing ‘extrinsic’ strictures (such as pancreatic cancer, gallbladder cancer or metastatic disease). In a study by Chen et al., POC impression was accurate in 84% of intrinsic strictures (due to cholangiocarcinoma) compared to only 62% of extrinsic strictures (due to pancreatic cancer, gallbladder cancer or metastatic disease) (33). As such, cholangioscopy-directed biopsy is an essential supplement to endoscopic evaluation.
Cholangioscopy-directed biopsy
A distinct advantage of POC is the ability to obtain biopsies under direct vision from indeterminate strictures for histological analysis. Recent studies evaluating cholangioscopy-directed biopsy have demonstrated sensitivities ranging from 71% to 100% and specificities of 96.7% to 100% (10,11,29,43). The superiority of cholangioscopy-directed biopsy over standard ERCP cytology brushings was demonstrated in a 2012 prospective paired control design study of 26 patients with indeterminate strictures (46). Mini-forceps biopsy provided significantly better sensitivity (76.5% vs. 5.9%, P<0.0001) and overall accuracy (84.6% vs. 53.8%, P=0.0215) compared with standard cytology brushings (46). Pooled results from a 2016 meta-analysis of 13 studies of cholangioscopy-directed biopsy were similarly promising with a pooled cholangioscopy-directed biopsy accuracy, sensitivity and specificity of 79%, 69%, 94%, respectively (36). As with other endoscopic approaches, multiple biopsies increase diagnostic yield and allow higher sensitivity and specificity. In a study utilizing percutaneous cholangioscopy, Tamada et al. reported a positive diagnosis in 95% of patients when three biopsies were taken from the margin of the stenotic area rather than only the indeterminate stricture (47).
Ablative therapies
Ablative therapies are being increasingly utilized to improve cholestasis and quality of life in patients with intraductal cancer. These include modalities such as photodynamic therapy (48) and radiofrequency ablation (22). Photodynamic therapy involves administration of a photosensitizer intravenously that accumulates in tumor cells. This is followed by exposing the tumor to photocuring light cholangioscopically, which in turn generates a cytotoxic reaction, subsequent ischemia, necrosis and apoptosis of tumor cells (9). In a randomized trial of 32 patients with non-resectable bile duct cancer by Zoepf et al., patients randomized to photodynamic therapy had a median survival time of 21 months compared to 7 months in controls (P=0.0109) (49). On the other hand, radiofrequency ablation involves administration of temperature-dependent energy that induces thermal injury and subsequent localized tumor necrosis. Since this procedure is commonly performed using only fluoroscopic guidance, it has been associated with a high adverse event rate (50). Ogura et al. reported the feasibility and safety of the procedure using visual rather than fluoroscopic guidance. Tumor visualization and administration of radiofrequency ablation was performed in 12 patients with bile duct cancer using a single-operator cholangioscope. Technical success was 100% and only 1 patient developed post-operative cholangitis (51). Overall, both radiofrequency ablation and photodynamic therapy appear to be safe and feasible and provide significant potential for palliation in patients with non-resectable bile duct cancer. At this time photodynamic therapy is rarely used due to the complex logistics involved in the procedure and radiofrequency ablation is yet to establish a well-defined role in the therapeutic management algorithm of malignant biliary strictures.
PSC
Cholangioscopy has both diagnostic and therapeutic indications in patients with PSC. Aside from the high incidence of cholestasis and subsequent calculi formation, patients with PSC have an increased risk for the development of cholangiocarcinoma. Unfortunately, distinguishing between inflammatory versus malignant strictures in PSC is often challenging (52). Recently, IgG4-related sclerosing cholangitis is another disease process being increasingly identified that may closely mimic malignancy, but demonstrates good response to non-surgical treatment (53,54). This increases the need for definitive diagnosis of biliary strictures in this patient population. Importantly, initial experiences with orthotopic liver transplantation in patients with PSC and cholangiocarcinoma had poor results making the diagnosis of cholangiocarcinoma a contraindication to transplantation. However, this has recently changed making the need for tissue diagnosis in PSC patients with suspected cholangiocarcinoma even more important (55). A 2006 prospective cohort of 41 patients with PSC demonstrated clinical benefits for the use of POC in this patient population. Tissue sampling was performed in 33/41 (80%) of patients and allowed cholangiocarcinoma to be ruled out in 31. Stones were identified in 23/41 (56%) of patients of which 7/23 (30%) were missed on cholangiography and detected only by cholangioscopy (56). In a study of 33 patients with indeterminate strictures, Itoi et al. compared the visual characteristics of PSC to those of IgG4-related sclerosing cholangitis and cholangiocarcinoma. Scarring and pseudodiverticula were found significantly more often in PSC than IgG4-related sclerosing cholangitis. In contrast, dilated vessels were found significantly more often in IgG4-related sclerosing cholangitis than in patients with cholangiocarcinoma (57). Other modalities such as probe-based confocal laser endomicroscopy (58) and utilization of NBI (59) have been utilized to improve diagnostic yield of visualization during cholangioscopy but have not yet been established as part of routine practice.
Single-operator cholangioscopy systems have shown similar sensitivity, specificity and accuracy in diagnosis of cholangiocarcinoma in patients with PSC compared to controls (54,60). A meta-analysis of 21 studies evaluating different ERCP-based modalities for diagnosis of cholangiocarcinoma in PSC patients including bile duct brushings for cytology, fluorescence in situ hybridization, probe-based confocal laser endomicroscopy and single-operator cholangioscopy with targeted biopsies found single-operator cholangioscopy with biopsies to be the most accurate diagnostic modality with an overall accuracy of 96% (95% CI, 94–97%) (61). Despite of its multiple uses, cholangioscopy in patients with PSC is not without its challenges. Kalaitzakis et al. reported that cholangioscope insertion may be hampered by bile duct narrowing and that post-cholangioscopy cholangitis occurred more often in patients with PSC compared to controls (54).
Other clinical applications
Cholangioscopy allows endoscopists unparalleled access to the biliary ductal system and as a result different novel uses of this technology other than those described above have been reported in the literature (Table 1). This includes retrieval of migrated biliary stents (62,63), guidewire placement (64), gallbladder drainage via the cystic duct in selected cases of cholecystitis (65,66), evaluation of post liver transplant biliary strictures (67), evaluation and treatment of hemobilia (68) and even for access of difficult luminal GI strictures (69).
Safety
Cholangioscopy is a safe procedure with relatively few serious adverse events (12). Whether POC adds significant additional risks to those associated with standard ERCP remains controversial. A single center prospective case series reported that the adverse events associated with POC were in line with routine ERCP (11). At the same time a large retrospective study comparing the safety of 3,476 ERCPs to 402 cholangioscopies reported a significantly higher overall adverse event rate with cholangioscopy (7% vs. 2.9%, OR 2.50) and specifically a higher rate of post-procedure cholangitis (1.0% vs. 0.2%) (70). Authors of the study suggested that lengthier procedures in the cholangioscopy arm may have led to more intraluminal air insufflation and distension of the bile duct during irrigation, ultimately leading to a higher adverse event rate. Pooled data from systematic reviews and meta-analyses provides a broader perspective. A meta-analysis of 45 studies on cholangioscopy demonstrated a pooled adverse event rate of 7% (95% CI, 6–9%) and a pooled severe adverse event rate of 1% (95% CI, 1–2%) (36). These rates are comparable to those found in a systematic survey of prospective ERCP studies where the overall adverse event rate was approximately 6.85%, with a severe event rate of 1.67% (71). As a result, routine antibiotic prophylaxis is not recommended at the time of POC at this time if adequate biliary drainage is achieved at the end of the ERCP (72). Notably, some rare yet very serious and potentially fatal complications including air embolization may be seen more often with POC (70,73,74), therefore routine use of CO2 as an insufflation agent is strongly recommended.
Limitations and future directions
Initial cholangioscopy platforms were limited by requirement of two-operators, complex design, lack of 4-tip deflection, lack of a dedicated irrigation port and fragility of the actual cholangioscopes (12). Current systems have evolved and many of these limitations have been overcome, however, affordability, image quality, and small therapeutic channel remain significant limiting factors of current platforms. While affordability has markedly improved over the years, high capital costs for the processor, scopes and cost of repairs remain a limiting factor. A recent American Society of Gastrointestinal Endoscopy (ASGE) technology review reported start-up costs varying between $50,000 and $90,000 (15). In the United States, the lack of a dedicated Current Procedural Terminology (CPT) code for POC is another factor limiting widespread adoption of this technology (currently only an add-on code exists). Reimbursement is dependent on payer review and the outcome of such reviews are variable and payer dependent (15).
In summary, cholangioscopy has indeed revolutionized biliary endoscopy and allowed for unprecedented access to the biliary tract both diagnostically and therapeutically. The field of cholangioscopy has developed significantly since early mother-baby platforms. Ease of use, endoscopic picture quality and types of accessories have also continued to improve and the latest single-operator digital cholangioscopes promise to expand the use of this technology beyond tertiary referral centers. The future of cholangioscopy will depend on further cutting-edge improvements in image quality, ease of use and the development of a wider array of accessories.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Roca J, Flichtentrei R, Parodi M. Progress in the radiologic study of the biliary tract in surgery; cholangioscopy and cholangiography; utilization of apparatus; preliminary note. Dia Med 1951;23:3420. [PubMed]
- Berci G. General surgery: endoscopic exploration (choledoscopy) of the biliary system at operation. West J Med 1977;126:388. [PubMed]
- Rösch W, Koch H, Demling L. Peroral cholangioscopy. Endoscopy 1976;8:172-5. [Crossref] [PubMed]
- Urakami Y, Seifert E, Butke H. Peroral Direct Cholangioscopy (PDCS) Using Routine Straight-view Endoscope: First Report. Endoscopy 1977;9:27-30. [Crossref] [PubMed]
- Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest Endosc 2007;65:303-11. [Crossref] [PubMed]
- Lee JH, Kim HW, Kang DH, et al. Usefulness of percutaneous transhepatic cholangioscopic lithotomy for removal of difficult common bile duct stones. Clin Endosc 2013;46:65-70. [Crossref] [PubMed]
- Ahmed O, Mathevosian S, Arslan B. Biliary interventions: Tools and techniques of the trade, access, cholangiography, biopsy, cholangioscopy, cholangioplasty, stenting, stone extraction, and brachytherapy. Semin Intervent Radiol 2016;33:283-90. [Crossref] [PubMed]
- Ishida Y, Itoi T, Okabe Y. Types of Peroral Cholangioscopy: How to Choose the Most Suitable Type of Cholangioscopy. Curr Treat Options Gastroenterol 2016;14:210-9. [Crossref] [PubMed]
- Franzini TA, Moura RN, de Moura EG. Advances in therapeutic cholangioscopy. Gastroenterol Res Pract 2016;2016. [Crossref] [PubMed]
- Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video){A figure is presented}. Gastrointest Endosc 2007;65:832-41. [Crossref] [PubMed]
- Draganov PV, Lin T, Chauhan S, et al. Prospective evaluation of the clinical utility of ERCP-guided cholangiopancreatoscopy with a new direct visualization system. Gastrointest. Endosc 2011;73:971-9. [Crossref] [PubMed]
- Judah JR, Draganov PV. The Use of SpyGlass Direct Visualization System in the Management of Pancreato Biliary Disease. In: Wu GY, Sridhar S. editors. Diagnostic and Therapeutic Procedures in Gastroenterology. Humana Press, 2011:195-209.
- Pereira P, Peixoto A, Andrade P, et al. Peroral cholangiopancreatoscopy with the SpyGlass® system: What do we know 10 years later. J Gastrointestin Liver Dis 2017;26:165-70. [PubMed]
- Moon JH, Ko BM, Choi HJ, et al. Direct peroral cholangioscopy using an ultra-slim upper endoscope for the treatment of retained bile duct stones. Am J Gastroenterol 2009;104:2729-33. [Crossref] [PubMed]
- Komanduri S, Thosani N, Abu Dayyeh BK, et al. Cholangiopancreatoscopy. Gastrointest Endosc 2016;84:209-21. [Crossref] [PubMed]
- Brauer BC, Chen YK, Shah RJ. Single-step direct cholangioscopy by freehand intubation using standard endoscopes for diagnosis and therapy of biliary diseases. Am J Gastroenterol 2012;107:1030-5. [Crossref] [PubMed]
- Seelhoff A, Schumacher B, Neuhaus H. Single operator peroral cholangioscopic guided therapy of bile duct stones. J Hepatobiliary Pancreat Sci 2011;18:346-9. [Crossref] [PubMed]
- Maydeo A, Kwek BEA, Bhandari S, et al. Single-operator cholangioscopy-guided laser lithotripsy in patients with difficult biliary and pancreatic ductal stones (with videos). Gastrointest Endosc 2011;74:1308-14. [Crossref] [PubMed]
- Shah RJ, Langer DA, Antillon MR, et al. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin Gastroenterol Hepatol 2006;4:219-25. [Crossref] [PubMed]
- Seo DW, Lee SK, Yoo KS, et al. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc 2000;52:630-4. [Crossref] [PubMed]
- Arya N, Nelles SE, Haber GB, et al. Electrohydraulic Lithotripsy in 111 Patients: A Safe and Effective Therapy for Difficult Bile Duct Stones. Am J Gastroenterol 2004;99:2330-4. [Crossref] [PubMed]
- Roque J, Ho SH, Reddy N, et al. Endoscopendoscopic ablation therapy for biliopancreatic malignancies. Clin Endosc 2015;48:15-9. [Crossref] [PubMed]
- Patel J, Rizk N, Kedia P, et al. Cholangioscopy-assisted photodynamic therapy for cholangiocarcinoma. Gastrointest Endosc 2015;81:1012-3. [Crossref] [PubMed]
- Carr-Locke DL. Therapeutic role of ERCP in the management of suspected common bile duct stones. Gastrointest Endosc 2002;56:S170-4. [Crossref] [PubMed]
- Yasuda I, Itoi T. Recent advances in endoscopic management of difficult bile duct stones. Dig Endosc 2013;25:376-85. [Crossref] [PubMed]
- Williamson JB, Draganov PV. The Usefulness of spyglass choledochoscopy in the diagnosis and treatment of biliary disorders. Curr Gastroenterol Rep 2012;14:534-41. [Crossref] [PubMed]
- Sauer BG, Cerefice M, Swartz DC, et al. Safety and efficacy of laser lithotripsy for complicated biliary stones using direct choledochoscopy. Dig Dis Sci 2013;58:253-6. [Crossref] [PubMed]
- Piraka C, Shah RJ, Awadallah NS, et al. Transpapillary Cholangioscopy-Directed Lithotripsy in Patients With Difficult Bile Duct Stones. Clin Gastroenterol Hepatol 2007;5:1333-8. [Crossref] [PubMed]
- Kalaitzakis E, Webster GJ, Oppong KW, et al. Diagnostic and therapeutic utility of single-operator peroral cholangioscopy for indeterminate biliary lesions and bile duct stones. Eur J Gastroenterol Hepatol 2012;24:656-64. [Crossref] [PubMed]
- Patel SN, Rosenkranz L, Hooks B, et al. Holmium-yttrium aluminum garnet laser lithotripsy in the treatment of biliary calculi using single-operator cholangioscopy: A multicenter experience (with video). Gastrointest Endosc 2014;79:344-8. [Crossref] [PubMed]
- Sepe PS, Berzin TM, Sanaka S, et al. Single-operator cholangioscopy for the extraction of cystic duct stones (with video). Gastrointest Endosc 2012;75:206-10. [Crossref] [PubMed]
- Tsuyuguchi T, Sakai Y, Sugiyama H, et al. Long-term follow-up after peroral cholangioscopy-directed lithotripsy in patients with difficult bile duct stones, including Mirizzi syndrome: An analysis of risk factors predicting stone recurrence. Surg Endosc 2011;25:2179-85. [Crossref] [PubMed]
- Chen YK, Parsi MA, Binmoeller KF, et al. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest Endosc 2011;74:805-14. [Crossref] [PubMed]
- Turowski F, Hügle U, Dormann A, et al. Diagnostic and therapeutic single-operator cholangiopancreatoscopy with SpyGlassDS™: results of a multicenter retrospective cohort study. Surg Endosc 2018;32:3981-8. [Crossref] [PubMed]
- Brewer Gutierrez OI, Bekkali NLH, Raijman I, et al. Efficacy and Safety of Digital Single-Operator Cholangioscopy for Difficult Biliary Stones. Clin Gastroenterol Hepatol 2018;16:918-26.e1. [Crossref] [PubMed]
- Korrapati P, Ciolino J, Wani S, et al. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: a systematic review and meta-analysis. Endosc Int Open 2016;4:E263-75. [Crossref] [PubMed]
- Okugawa T, Tsuyuguchi T, Sudhamshu KC, et al. Peroral cholangioscopic treatment of hepatolithiasis: Long-term results. Gastrointest Endosc 2002;56:366-71. [Crossref] [PubMed]
- Cheng YF, Lee TY, Sheen-Chen SM, et al. Treatment of complicated hepatolithiasis with intrahepatic biliary stricture by ductal dilatation and stenting: Long-term results. World J Surg 2000;24:712-6. [Crossref] [PubMed]
- Lee SK, Seo DW, Myung SJ, et al. Percutaneous transhepatic cholangioscopic treatment for hepatolithiasis: An evaluation of long-term results and risk factors for recurrence. Gastrointest Endosc 2001;53:318-23. [Crossref] [PubMed]
- Buxbaum J, Sahakian A, Ko C, et al. Randomized trial of cholangioscopy-guided laser lithotripsy versus conventional therapy for large bile duct stones (with videos). Gastrointest Endosc 2018;87:1050-60. [Crossref] [PubMed]
- Xu MM, Kahaleh M. Recent developments in choledochoscopy: Technical and clinical advances. Clin Exp Gastroenterol 2016;9:119-24. [PubMed]
- Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res 2013;184:304-11. [Crossref] [PubMed]
- Woo YS, Lee JK, Oh SH, et al. Role of SpyGlass peroral cholangioscopy in the evaluation of indeterminate biliary lesions. Dig Dis Sci 2014;59:2565-70. [Crossref] [PubMed]
- Kim HJ, Kim MH, Lee SK, et al. Tumor vessel: A valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc 2000;52:635-8. [Crossref] [PubMed]
- Navaneethan U, Hasan MK, Kommaraju K, et al. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: a multicenter clinical experience (with video). Gastrointest Endosc 2016;84:649-55. [Crossref] [PubMed]
- Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: A prospective, long-term follow-up study. Gastrointest Endosc 2012;75:347-53. [Crossref] [PubMed]
- Tamada K, Kurihara K, Tomiyama T, et al. How many biopsies should be performed during percutaneous transhepatic cholangioscopy to diagnose biliary tract cancer? Gastrointest Endosc 1999;50:653-8. [Crossref] [PubMed]
- Choi HJ, Moon JH, Ko BM, et al. Clinical feasibility of direct peroral cholangioscopyguided photodynamic therapy for inoperable cholangiocarcinoma performed by using an ultra-slim upper endoscope (with videos). Gastrointest Endosc 2011;73:808-13. [Crossref] [PubMed]
- Zoepf T, Jakobs R, Arnold JC, et al. Palliation of nonresectable bile duct cancer: Improved survival after photodynamic therapy. Am J Gastroenterol 2005;100:2426-30. [Crossref] [PubMed]
- Wadsworth CA, Westaby D, Khan SA. Endoscopic radiofrequency ablation for cholangiocarcinoma. Curr Opin Gastroenterol 2013;29:305-11. [Crossref] [PubMed]
- Ogura T, Onda S, Sano T, et al. Evaluation of the safety of endoscopic radiofrequency ablation for malignant biliary stricture using a digital peroral cholangioscope (with videos). Dig Endosc 2017;29:712-7. [Crossref] [PubMed]
- Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660-78. [Crossref] [PubMed]
- Ghazale A, Chari ST, Zhang L, et al. Immunoglobulin G4-Associated Cholangitis: Clinical Profile and Response to Therapy. Gastroenterology 2008;134:706-15. [Crossref] [PubMed]
- Kalaitzakis E, Sturgess R, Kaltsidis H, et al. Diagnostic utility of single-user peroral cholangioscopy in sclerosing cholangitis. Scand J Gastroenterol 2014;49:1237-44. [Crossref] [PubMed]
- Sapisochín G, Fernández de Sevilla E, Echeverri J, et al. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J Hepatol 2015;7:2396-403. [Crossref] [PubMed]
- Awadallah NS, Chen YK, Piraka C, et al. Is There a Role for Cholangioscopy in Patients with Primary Sclerosing Cholangitis? Am J Gastroenterol 2006;101:284-91. [Crossref] [PubMed]
- Itoi T, Kamisawa T, Igarashi Y, et al. The role of peroral video cholangioscopy in patients with IgG4-related sclerosing cholangitis. J Gastroenterol 2013;48:504-14. [Crossref] [PubMed]
- Heif M, Yen RD, Shah RJ. ERCP with probe-based confocal laser endomicroscopy for the evaluation of dominant biliary stenoses in primary sclerosing cholangitis patients. Dig Dis Sci 2013;58:2068-74. [Crossref] [PubMed]
- Azeem N, Gostout CJ, Knipschield M, et al. Cholangioscopy with narrow-band imaging in patients with primary sclerosing cholangitis undergoing ERCP. Gastrointest Endosc 2014;79:773-9.e2. [Crossref] [PubMed]
- Arnelo U, von Seth E, Bergquist A. Prospective evaluation of the clinical utility of single-operator peroral cholangioscopy in patients with primary sclerosing cholangitis. Endoscopy 2015;47:696-702. [Crossref] [PubMed]
- Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther 2016;44:1139-51. [Crossref] [PubMed]
- Sejpal DV, Vamadevan AS, Trindade AJ. Removal of an embedded, migrated plastic biliary stent with the use of cholangioscopy. Gastrointest Endosc 2015;81:1482-3. [Crossref] [PubMed]
- Ikeura T, Shimatani M, Takaoka M, et al. Reintervention for an occluded metal stent under the guidance of peroral direct cholangioscopy by using an ultra-slim enteroscope. Gastrointest Endosc 2015;81:226-7. [Crossref] [PubMed]
- Parsi MA. Peroral cholangioscopy-assisted guidewire placement for removal of impacted stones in the cystic duct remnant. World J Gastrointest Surg 2009;1:59. [Crossref] [PubMed]
- Tyberg A, Zerbo S, Kahaleh M, et al. Digital cholangioscopy-assisted gallbladder drainage: Seeing is accessing. Endoscopy 2015;47. [Crossref] [PubMed]
- Gutkin E, Hussain SA, Kim SH. The successful treatment of chronic cholecystitis with SpyGlass cholangioscopy-assisted gallbladder drainage and irrigation through self-expandable metal stents. Gut Liver 2012;6:136-8. [Crossref] [PubMed]
- Parsi MA, Guardino J, Vargo JJ. Peroral cholangioscopy-guided stricture therapy in living donor liver transplantation. Liver Transpl 2009;15:263-5. [Crossref] [PubMed]
- Komaki Y, Kanmura S, Funakawa K, et al. A case of hereditary hemorrhagic telangiectasia with repeated hemobilia arrested by argon plasma coagulation under direct peroral cholangioscopy. Gastrointest Endosc 2014;80:528-9. [Crossref] [PubMed]
- Yang D, Draganov PV, Gupte AR, et al. Beyond the scope: using the single-operator digital cholangioscope to access an afferent loop obstruction. Gastrointest Endosc 2016;84:861. [Crossref] [PubMed]
- Sethi A, Chen YK, Austin GL, et al. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: A single-center experience. Gastrointest Endosc 2011;73:251-6. [Crossref] [PubMed]
- Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: A systematic survey of prospective studies. Am J Gastroenterol 2007;102:1781-8. [Crossref] [PubMed]
- Khashab MA, Chithadi KV, Acosta RD, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2015;81:81-9. [Crossref] [PubMed]
- Donepudi S. Air embolism complicating gastrointestinal endoscopy: A systematic review. World J Gastrointest Endosc 2013;5:359. [Crossref] [PubMed]
- Chavalitdhamrong D, Donepudi S, Pu L, et al. Uncommon and rarely reported adverse events of endoscopic retrograde cholangiopancreatography. Dig Endosc 2014;26:15-22. [Crossref] [PubMed]
Cite this article as: Ayoub F, Yang D, Draganov PV. Cholangioscopy in the digital era. Transl Gastroenterol Hepatol 2018;3:82.


